ABSTRACT
Purpose:
Therapeutic regimens and outcome of acute myeloid leukaemia (AML) patients substantially improved over the past decades. However, AML in older patients is still widely understudied and therapeutic standards are far less well defined. This study provides a retrospective analysis of a cohort of AML patients above 65 years of age treated at a single university centre in Germany.
Methods:
Treatment regimens including intensive chemotherapy with or without subsequent allogenic stem cell transplantation (allo-SCT), hypomethylating agent (HMA) or low-dose cytarabine (LD-AraC) based therapy or best supportive care (BSC) were evaluated and compared to patient-specific variables, comorbidities indices such as Haematopoietic Cell Transplantation-specific Comorbidity Index (HCT-CI) or Charlson Comorbidity Index (CCI), or Eastern Cooperative Oncology Group (ECOG) performance status to assess their potential impact on outcome.
Results:
229 patients ≥ 65 years with newly diagnosed AML were included in this study. Patients received either intensive chemotherapy (IT) without (n = 101, 44%), or followed by allo-SCT (n = 27, 12%), HMA (n = 29, 13%), LD-Ara-C (n = 16, 7%) or best supportive care (BSC) only (n = 56, 24%). Of interest, ECOG performance status predicted overall survival in patients treated with IT, and combinatorial assessment of ECOG and HCT-CI was particularly useful to predict outcome in this subgroup of patients.
Conclusion:
Subsets of AML patients above 65 years of age benefit from intensive chemotherapy and allogenic stem cell transplantation. Combined assessment of ECOG scores and HCT-CI might help to objectively identify suitable patients, and this concept should be further investigated in a prospective manner in future studies.
Key points
Selected subsets of AML patients may profit from intensive chemotherapy and allogenic stem cell transplantation.
Combined analysis of ECOG performance status and HCT-CI might help to predict outcome in elderly AML patients.
Introduction
Acute myeloid leukaemia (AML) is a complex disease with considerable genetic and clinical heterogeneity [Citation1]. Incidence rates have been steadily increasing and median age at the time of first diagnosis is often found to be above 70 years [Citation2]. While therapeutic options and supportive care for AML patients substantially improved over the past decades, overall prognosis of old patients largely remained poor [Citation3]. Today there is a striking discrepancy in prognosis of young versus old patients, with estimated rates of cure ranging from 70% for patients younger than 30, as compared to 2% for individuals older than 75 years [Citation2]. Induction chemotherapy (IT) following classical ‘7 + 3’ regimens represents a standard therapeutic approach for fit AML patients [Citation4], but in elderly patients IT more often results in longer hospitalization or impaired survival [Citation5]. Allogenic haematopoietic stem cell transplantation (allo-SCT) offers the best chance for cure for young patients with intermediate or high-risk AML, but in older patients its role is not well established and thus it is infrequently used in this important subset of individuals. Therefore, older patients are more likely to receive less intensive treatment options with more favourable toxicity profiles such as hypomethylating agents (HMA), although subsets of these patients might in fact benefit from more intensive therapy [Citation6]. Low-dose cytarabine (LD-AraC) or best supportive care only are frequently discussed as valid alternative options for unfit older patients [Citation7].
The choice of therapy best suited in individual cases cannot be made based on patient age alone and to date there is no single surrogate marker, scoring system or generally accepted consensus algorithm that would allow to select patients of older age for intensive chemotherapy versus less intensive treatment options.
This study presented here provides a retrospective analysis of acute myeloid leukaemia patients above 65 years of age at the time of diagnosis treated at a single university hospital in Germany. The main emphasis will be to document, firstly, which therapeutic regimens were applied in this cohort of patients, secondly, what outcomes were assessed for each therapeutic option, and thirdly, if any predictive biomarker of clinical score can be identified based on the patients’ clinical records, that might predict outcome and might help in clinical decision making in order to determine the particular therapy regimen best suited for each individual patient.
Materials and methods
Study population
Patients≥65 years who were diagnosed with de novo AML or sAML at the University Hospital Bonn from 01/2006 until 12/2013 were included in this retrospective analysis, patients with acute promyelocytic leukaemia were excluded.
Data collection was performed using a standard questionnaire, which contained variables necessary for the analysis of endpoints as described below.
Therapy regimens administered (including intensive chemotherapy, hypomethylating agents (HMA), low-dose cytarabine (LD-AraC) or best supportive care) were documented.
Patients undergoing HMA therapy were only included in this analysis, if HMA was administered as first-line therapy. Also, patients that were treated within clinical trials were excluded from this analysis.
Extramedullary disease was diagnosed based on clinical or radiological examination, histological verification was not carried out in every case.
AML treatment protocols
Patients who were decided by the physicians to be fit enough for intensive chemotherapy (IT) received one or two induction cycles (most 3 + 7 regime) and usually three consolidation cycles (high-dose cytarabine) or salvage treatment for refractory AML. Some of the older patients also received allogeneic transplantation if the ELN risk score 2017 was intermediate or high, or as salvage treatment.
If no intensive treatment was decided, patients received azacitidine (HMA, 75 mg/m2/day for seven consecutive days by s. c. injection every four weeks), or low-dose AraC (LD-AraC, 20 mg/m2 for 10 days consecutive days by s. c. or i. v. injection every four weeks) or best supportive care only (BSC).
Scoring systems
The Eastern Cooperative Oncology Group (ECOG) Performance Status measures disease impact on patients’ daily life. It shows the level of functioning, daily activity and physical ability [Citation8].
The Charlson Comorbidity Index (CCI) predicts the 10-year survival in patients with different comorbidities [Citation9].
The Haematopoietic Cell Transplantation – specific Comorbidity Index (HCT-CI) was developed to identify comorbidities before allogenic stem cell transplantation to evaluate the risk of the allogeneic transplantation. It is based on the CCI, but includes organ function tests such as blood tests, echocardiography or body plethysmography [Citation10].
Almost all patients were diagnosed within the AMLSG-study group (German-Austrian Acute Myeloid Leukemia Study Group) with central cytogenetic diagnostic. Because of the long time period of this retrospective analysis at the point of the first data collection 2006 molecular profiling was not standard of care even within this study group and only karyotyping was done. Since 2008 Oktober FLT3 and NPM1 mutation analysis was routinely included in the diagnostic work-up of the study group, and later on analysis of inv(16), t(8/21), CEBPA mutation and t(11;23) were added. Therefore, not all cases included in this work can be stratified according to ELN risk score.
Definitions of endpoints
Remission status was assessed using the following criteria: complete remission (CR): less than 5% blast cells in the bone marrow, absence of any previously detected cytogenetic abnormalities, absolute neutrophil count >1000/µl and platelet count >100,000/µl, independence from red cell transfusion and no extramedullary manifestation. Partial remission (PR): 5–25% blasts, but more than 50% reduction of blasts in the bone marrow, absolute neutrophil count >1000/µl and platelet count >100,000/µl, independence from red cell transfusion.
Overall survival (OS) served as primary endpoint. OS defines the time from diagnosis to death from any cause. Leukaemia-related death was not documented. Secondary endpoints included OS in patients subgroups defined by baseline patients characteristics and AML characteristics: Age, gender, ECOG, CCI, HCT-CI, ELN risk score, secondary or therapy related AML.
Ethical considerations
All study investigators were staff of Department III of Internal Medicine at the University Hospital Bonn. Because of the retrospective manner no interventions were performed as part of the study. Patient care, data collection and analyses were performed by site personnel using current techniques of privacy assurance. In the state Northrine-Westphalia, Germany therefore, neither approval by an Ethics Committee nor patient consent is necessary.
Statistical analyses
The demographics and baseline characteristics of the patients were reported by descriptive statistics by using median and range. The patients in each range were counted using N and percentage. OS was determined using Kaplan Maier analysis. OS analyses were done by log-rank tests. HR and 95% CI were estimated by using Cox regression models. HR < 1 shows a benefit for one group over another. All variables which were tested significant in univariate analyses were included in multivariate analyses (forward calculated). A two-sided p-value <0.05 was considered statistically significant. Surviving patients were censored at the end of June 2016. Patients who were lost to follow-up were censored on the day of last physician contact.
Statistical analyses were performed by SPSS Statistics (IBM Corp., Armonk, NY) version 27 for MAC OS.
Results
Study population
Two hundred twenty-nine AML patients ≥65 years were included in this analysis, 56% (n = 128) of these received IT. Baseline characteristics of all patients are summarized in . The median age for this cohorte was 74 years. 26 patients (11%) had a favourable, 77 patients (34%) had an intermediate, 64 patients (28%) had an adverse ELN risk. Sadly, for 62 patients (27%) there was no ELN risk due to lack of data. Consolidating or salvage allogeneic Haematopoietic stem cell transplantation was only done in a very selected proportion of IT patients (IT + allo-SCT 21%, n = 27/128 or 12% of all 229 patients). 13% (n = 29) received Azacitidine (HMA), 7% (n = 16) had LD-AraC and 26% (n = 56) no cytoreductive therapy (BSC) ().
Table 1. Patient characteristics.
In the IT cohort, 59% of the patients were male (n = 76) with a median age of 71 years. In this subgroup, 72% (n = 92) were judged as ECOG = 0-1. HCT-CI = 0-2 was documented in 54/128 (42%). Likewise, CCI = 2 was observed in 52/128 (41%) ().
In the subcohort treated with HMA (n = 29), more than half of the patients were male (55%, n = 16), and 45% (n = 13) were female, with a median age of 77 years. 56% (n = 16) of the HMA group had an ECOG = 0–1. Here, HCT-CI = 0–2 was documented in 10/29 (35%). CCI = 2 was observed in 8/29 (28%) ().
In the group treated with low-dose AraC (n = 16), more than half of the patients were female (62%, n = 10) and 38% (n = 6) were male, with a median age of 75 years. 31% of the LD-AraC group had ECOG = 0–1 (n = 5) ().
Of note, 75% of patients in the HMA-treated cohort had secondary or therapy-related AML.
Relapse rates
Relapse was only seen in the IT group, in which patients could achieve a complete remission with the treatment. Overall, 26% (n = 34) of patients treated with induction chemotherapy with or without subsequent allogenic SCT showed a relapse within the follow-up period. Eight patients received allogenic SCT due to relapse and five (19%) patients relapsed after allogenic SCT with a median time to relapse of 186 (range:149–365) days (). For patients treated with induction chemotherapy but without consolidating allogenic stem cell transplantation relapse was documented in 29/101 (29%) of cases with a median time to relapse of 243 (range: 90–869) days.
Table 2. Relapse rates.
Survival and early mortality with respect to therapy regimen applied
Median overall survival of all patients included in this analysis was 4.9 months. Median OS survival was statistically significant for patients treated with induction chemotherapy with or without subsequent allogenic SCT (8.2 months) as compared to those treated with HMA (5.0 months, HR of death 1.5 (0.97–2.3), p = 0.056), LDAC 0.4 months (HR of death 2.5 (1.4–2.3), p = 0.001) and BSC 1.4 months (HR of death: 3.6 (2.5–5.2), p = 0.000). For patients treated with IT followed by allogenic SCT median overall survival was 28.9 months, as compared to 5.9 months for patients who received induction therapy without subsequent consolidating allogenic SCT. 1-year OS rates for patients treated with induction therapy followed by allogenic SCT, induction therapy without subsequent SCT or HMA-based therapy were 74%, 23%, and 24%, while 2-year OS rates were 48%, 3%, and 7%, respectively (). The complete remission rate after induction chemotherapy was 61%.
Figure 1. Kaplan-Mayer analysis of overall survival depends on therapeutic regimen applied. Patients who were judged eligible for and treated with induction chemotherapy followed by allogenic stem cell transplantation showed a significantly better outcome as patients without consolidation with allogenic stem cell transplantation. Not surprisingly, prognoses were worst for those patients who only received palliative treatment with low-dose AraC or best supportive care.
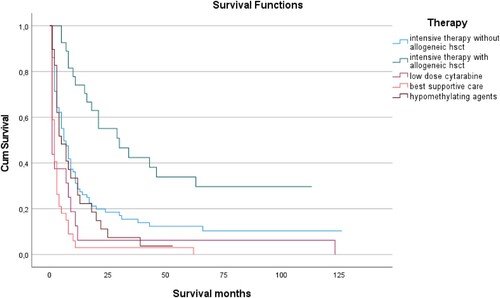
In univariate Cox Regression analyses, induction therapy alone without allogenic stem cell transplantation versus induction therapy followed by allogenic stem cell transplantation showed a HR of 2.5 (1.5–4.2) with respect to shorter overall survival (p < 0.01), HMA-based therapy versus induction therapy followed by allogenic stem cell transplantation had a HR of 2.9 (1.6–5.4; p < 0.01), low dose AraC versus induction therapy followed by allogenic stem cell transplantation had a HR of 4.9 (2.5–9.7; p < 0.01) and best supportive care versus induction therapy followed by allogenic stem cell transplantation showed a HR of 7.3 (4.2–13; p < 0.01). Similarly, upon Cox Regression analyses HMA-based therapy versus induction therapy applied without subsequent allogenic SCT had a HR of 1.2 (0.8–1.8; NS), while low dose AraC versus induction therapy without subsequent allogenic SCT showed a HR of 1.9 (1.1–3.4: p < 0.01), and administration of best supportive care as compared to induction therapy without subsequent allogenic SCT resulted in a HR of 2.9 (2.1–4.3; p < 0.01).
Early (i.e. 4- and 8-week) mortality in the IT-allogenic SCT group was 11% and 24%, respectively, which was only slightly higher compared to the HMA cohort (11% and 18%). In the IT+ allogenic SCT group there was no 4- and 8-week mortality due to time on therapy. In contrast, LDAC and BSC resulted in clearly higher early mortality rates (LDAC 57% and 62.5% and BSC 49% and 68%, respectively).
Impact of aetiology on survival
In patients with tAML who received induction therapy followed by allogenic stem cell transplantation (n = 7), median OS was not reached, whereas for patients treated with induction therapy without subsequent allogenic stem cell transplantation (n = 14), median OS was 10.6 months, and patients who received HMA therapy only (n = 3) had a median OS of 3.1 months (NS). Patients with sAML who received induction therapy followed by allogenic stem cell transplantation (n = 11) had a median OS of 20.3 months, whereas those treated with induction therapy without allogenic SCT (n = 39) had a median OS of 8.3 months and patients treated with HMA therapy (n = 10) had a median OS of 7.6 months (NS).
Baseline ECOG, CCI or HCT-CI predict outcome in AML patients
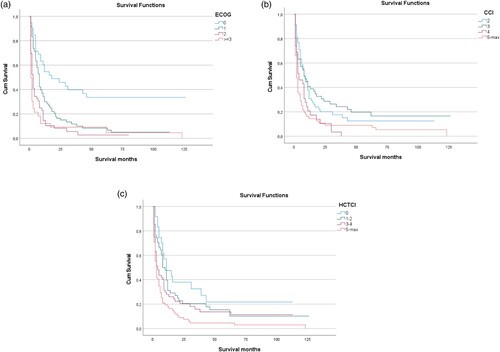
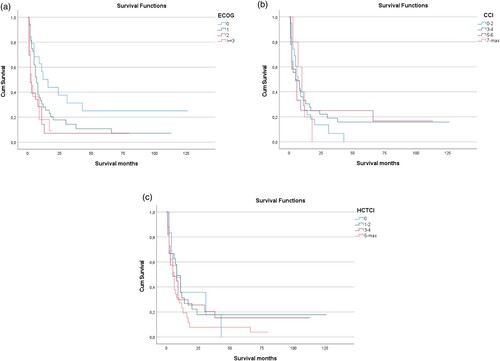
Patients with ECOG score of 0 or 1 had an improved median OS of 8.2 months and those with ECOG ≥2 had a dismal outcome with a median OS of 1.6 months (p < 0.001; (a)). Similar to the ECOG score and not surprisingly, cytogenetic risk categories were found to be associated with overall survival in the cohort of AML patients analyzed here: favourable, intermediate or adverse ELN risk groups had a median OS of 16.8, 7.8 or 5.9 months, respectively (p < 0.001; data not shown). Interestingly, non-disease-specific variables, such as patients comorbidities scored by the HCT-CI or the CCI are also significantly linked to OS. Patients with a CCI of 2 had a median OS of 8 months, patients with a CCI of 3 7.6 months, patients with a CCI of 4 3.3 months and patients with a CCI >4 1.7 months ((b)). Patients with a HCT-CI of 0 had a median OS of 10.9 months, with HCT-CI of 1–2 7.7 months, with HCT-CI of 3–4 3.3 months and with an HCT-CI of ≥ 5 2.3 months (p < 0.001; (c)).
Figure 2. Effect of predictive scores on overall survival (OS) of AML patients. (A) ECOG core predicted overall survival as depicted by means of Kaplan-Mayer analysis, with patients with an ECOG score of 0 showing a significantly better median OS as compared to all other scores. (B) Likewise, CCI predicted overall survival, with higher CCI scores being associated with shorter median OS. (C) Thirdly, in the population analyzed here, HCT-CI correlated with OS as well. Median OS was best for HCT-CI of 0 and was shortest for HCT-CI of 5 or above.
Impact of ELN-risk score and treatment on overall survival (OS)
Patients with favourable ELN risk score AML treated with induction therapy reached a significantly longer median OS of 42.4 months vs. 3.1 months for patients treated with HMA-based therapy (p = 0.030). Among these patients with favourable risk score, those who were treated with induction therapy followed by allogenic stem cell transplantation (n = 7), median OS was not reached, versus 10.6 months for those treated with induction therapy but without subsequent stem cell transplantation (n = 14; p = 0.14).
In the subgroup of patients with intermediate risk, median OS was 20.3 months for induction therapy followed by allogenic SCT (n = 11), as compared to 8.3 months for induction therapy without SCT (n = 39) (P = 0.012), and 7.6 months for HMA-based therapy only (n = 10; p = 0.5).
Patients with adverse risk according to the ELN model profited from allogenic SCT following induction therapy (n = 7), with median OS of 17.6 months, while induction therapy without subsequent allogenic SCT (n = 28) led to a median OS of 5.7 months, and outcome in this subgroup was worst in cases treated with HMA-based therapy only (n = 11) with median OS of only 3.7 months (p = 0.021), underscoring the importance of allogenic stem cell transplantation in this scenario.
The 11 patients with favourable ELN risk were transplanted after a relapse or due refractory disease. The patients with adverse ELN risk sometimes were not fit enough for transplantation or the patients rejected the treatment.
Prognostic value of ECOG, CCI and HCT-CI in AML patients treated with induction chemotherapy
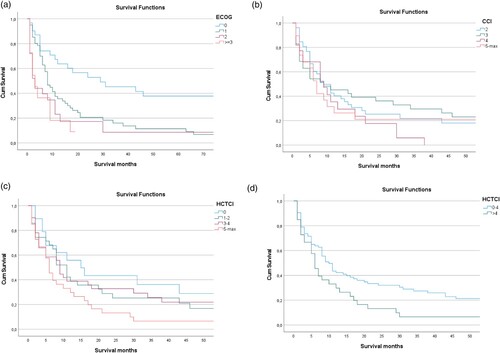
Of interest, ECOG score at baseline predicted outcome of AML patients treated with induction chemotherapy irrespective of subsequent allogenic stem cell transplantation, with median OS of 832, 231, 62, 73 months for baseline ECOG of 0, 1, 2, or above, respectively (p < 0.001; (a)). On the other hand, neither CCI nor HCT-CI were associated to differential survival in this subset of patients ((a,b)). Of interest, however, patients with favourable ECOG score of 0 or 1 at baseline showed particularly improved outcome with median overall survival of 358 months, when at the same time HCT-CI was also in a favourable range between 0 and 4, while HCT-CI > 4 in patients with ECOG 0 or 1 showed a median OS of only 191 months (p = 0.01), suggesting that combinatorial assessment of these scoring systems might confer additional predictive value in this setting ((d)).
Figure 3. Correlation of overall survival in AML patients treated with induction chemotherapy irrespective of consolidating allogenic stem cell transplantation with prognostic scores. (A) In the subgroup of AML patients treated with induction chemotherapy ECOG score correlated with overall survival, with patients with an ECOG score of 0 or 1 showing significantly longer median OS as compared to patients with ECOG sore 3 or 4, respectively. (B) On the other hand, neither CCI nor (C) HCT-CI predicted overall survival in this subgroup of AML patients. (D) In the subgroup of AML patients treated with induction chemotherapy with favourable ECOG score of 0 or 1, overall survival was markedly enhanced for low HCT-CI of 0–4 as compared to HCT-CI >5 (p = 0.01).
Most of the transplanted patients in our analysis (92%) had an ECOG score <2, and more than half of this patients had an HCT-CI <3. Likewise, beneficial ECOG scores predicted enhanced outcome with longer median overall survival in patients receiving allogenic stem cell transplantation after induction chemotherapy () as well as in cases treated with induction chemotherapy but without consolidating allogenic stem cell transplantation ((a)), while neither CCI ((b)) nor HCT-CI ((c)) were found to predict outcome in these cases.
Figure 4. ECOG predicts survival of AML patients undergoing allogenic stem cell transplantation. Median OS after consolidation allogenic stem cell transplantation was significantly better for patients with an initial ECOG score of 0 as compared to those with an ECOG score of 1 at the time of first diagnosis.
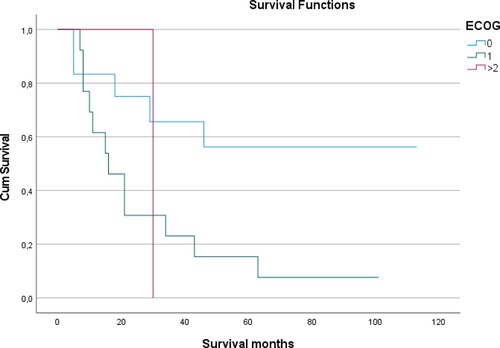
Figure 5. ECOG predicts survival of AML patients treated with induction chemotherapy without subsequent allogenic stem cell transplantation. (A) In AML patients treated with induction chemotherapy that did not receive consolidation therapy with allogenic stem cell transplantation, ECOG score at baseline predicted outcome with patients with an initial ECOG core of 0 showed significantly better median overall survival than those with higher scores. As opposed to this observation, not correlation with OS was found for (B) CCI or (C) HCT-CI scores, respectively.
Multivariate analysis
The multivariate analysis also included only those values that were found to be significant in the univariate analysis (). In the multivariate analysis for the elderly patients, the HCT-CI, ECOG, ELN-risk and age were found to be significant ().
Table 3. Kaplan-Meier analysis for median OS for patients ≥65 years.
Table 4. Multivariate analysis for patients ≥ 65 years.
Furthermore, we performed a cox regression with time dependent covariates. We performed the analysis with different Cut-Points (0.5, 1, 1.5, 2 years) and it showed a good sensitivity for all Cut-points. The HR (years < 1) for IT+ allo HSCT is 0.224; p = 0.001 and the HR (years > 1) for IT+ allo is 0.840; p = 0.673. We have shown that the IT + allo HSCT has especially in the first year a significant advantage compared to IT − allo HSCT. After 1 year there is still an advantage, but it is not significant anymore.
Discussion
Despite considerable progress in treatment and outcome of patients suffering from acute myeloid leukaemia over the past decades, the choice of therapy for elderly patients is a matter of ongoing discussion and outcome is often unsatisfactory. Nevertheless, the fraction of elderly patients has increasingly moved into the focus of clinical attention particularly in Western industrialized countries and is likely to gain more clinical relevance in the future due to epidemiological developments with overall increasing fractions of older and often otherwise fit patients [Citation11]. Although frequently excluded from standard-defining large prospective clinical trials, there is an increasing notion that at least some of these older patients may also benefit from intensive chemotherapy and potentially from allogenic stem cell transplantation, but clearly defined selection criteria telling which individual patients are likely to benefit from intensive therapy regimens and which might on the other hand might profit more from palliative therapies are widely lacking.
Here, a cohort of AML patients above 65 years of age at the time of initial diagnosis treated at a single large university hospital in Germany was retrospectively evaluated to examine, which therapeutic options were chosen and what outcome was documented. Moreover, our study aimed to identify potential predictors of outcome in this cohort that might in the future help to inform what subsets of patients could be most eligible for intensive therapy.
Although not surprising, it is a noteworthy finding that in the entire cohort of AML patients older than 65 years analyzed here those that were treated with induction chemotherapy followed by allogenic stem cell transplantation showed the most favourable outcome by far in terms of overall survival as compared to all other forms of therapy applied in this setting. While there is obviously a strong selection bias in a retrospective cohort analysis as presented here, where patients were selected for intensive therapy based on the treating physicians’ judgment of overall fitness and eligibility. Our data strongly support the concept, that allogenic stem cell transplantation must not be ruled out from the beginning purely based on a patient's age, but that in older patients, too, intensive therapy including stem cell transplantation should be carefully evaluated by a team of experienced physicians in an individualized, case by case manner. This finding is in line with previous observations by others [Citation12]. The upper age limit for an allogenic stem cell transplantation steadily increased in recent years, and even transplantation in the 8th decade is no longer unheard of [Citation13]. Farag et al. reported that patients between 60 and 70 years who received allogeneic stem cell transplantation had a significantly lower rate of relapse and longer leukaemia-free survival than patients with conventional chemotherapy and thus without transplantation [Citation14]. 3-year overall survival of transplanted patients in this study was 37%, which is in line with outcome of transplanted patients seen in our data set presented here with a 3-year overall survival of 37% as well.
The role of HMA-based therapy as compared to other regimens in older AML patients was addressed in the pivotal AZA-AML-001 study [Citation15]. In this study, no significant difference in response rates was found between HMA (azacitidine) and other conventional care regimens, including induction therapy, low-dose cytarabine or best supportive care. Median overall survival was not different for patients treated with azacitidine (10.5 months) as compared to individuals that received other regimens (6.5 months; HR = 0.85 (0.69–1.03); p = 0.1009). However, it is important to note that patients with an ECOG >2 were not included in this analysis, thus not fully reflecting the patient clientele typically seen in routine clinical practice.
In line with these observations another study reported by Falantes et al. that also included patients with ECOG scores ≥2 found a median OS of 9 months for HMA therapy in older AML patients [Citation16]. The median OS for HMA-based therapy in our analysis presented here was shorter as compared to the data reported by Falantes et al. as well as in the AZA-AML-001 trial, which might be due to a higher fraction of patients with unfavourable ECOG >2 and overall higher age of patient. The median age in the cohort presented here was 77 years while it was 75 years for the cohorts reported by Falantes et al. and in the AZA-AML-001 study, respectively. In line with the results of the AZA-AML-001 trial in our cohort median OS for HMA-based therapy was significantly better than for treatment with low-dose cytarabine or best supportive care. Pleyer et al. reported a median overall survival of 9,6 months for HMA-based therapy with an overall response rate of 48%, although in this report did not include a comparison to other conventional care regimens [Citation17].
Of interest and as opposed to results from to the AZA-AML-001 trial, in the current analysis reported here there was a significantly better median overall survival when intensive therapy included allogeneic stem cell transplantation, underscoring the notion that allogenic stem cell transplantation may be a viable therapeutic option in appropriately selected elderly patients with a chance for long-time survival.
High-risk cytogenetic features are well described adverse prognostic factors for the outcome of AML patients [Citation4,Citation18]. Ramos et al. previously showed that this adverse prognostic value also persists in patients treated HMA therapy [Citation19]. Of interest this observation could also be confirmed in our current analysis were median OS under HMA therapy was shorter in high risk versus low or intermediate ELN risk. Moreover, Dombret et al. found that treatment with azacitidine in patients with high-risk genetic features lead to a better outcome than other non-intensive options [Citation15], which is in line with our observations presented her, where patients with an intermediate or adverse ELN risk had a trend towards longer median OS with HMA-based therapy as compared to low-dose cytarabine.
Curiously in our cohort, in patients with t-AML, induction therapy led to significantly better median OS as compared to HMA therapy, even if it was not followed by allogenic stem cell transplantation. This finding is in contrast to Dumas et al. [Citation20], where t-AML patients showed longer median OS with HMA than with induction therapy. However, these results should be interpreted with caution, since case numbers were small in this subset of patients in our data. In patients with sAML, on the other hand, our results were in line with previously reported data, showing longer median survival upon HMA treatment as compared to induction therapy [Citation20].
Also, not entirely surprising is the fact, that ECOG performance status at baseline as well as comorbidity scores CCI and HCT-CI, respectively, were found to be strong predictors of outcome in terms of overall survival in the analysis presented here. Nevertheless, it is a striking finding in our analysis that irrespective of other factors and of treatment modalities received, AML patients with ECOG 0 had showed dramatically improved median overall survival as compared to those with ECOG 1 or higher, suggesting that meticulous assessment following strictly standardized routine of this simple parameter for each and every individual patient as part of routine baseline clinical evaluation can in fact confer highly relevant information to judge further prospects for a given individual. Of note and in line with previous observations, careful assessment of comorbidities in a standardized manner was also found to be a strong predictor of outcome in the cohort of AML patients discussed here. Although a study by Pleyer et al. did not report formal scoring systems and rather just counted comorbidities, of interest this analysis also found that outcome was worse when the patient had more than three comorbidities [Citation17].
Focusing only on HMA-treated patients in our analyses, individuals with higher ECOG ≥ 2 appeared to profit less, which is in line with previous reports by others [Citation19].
Patients with a good performance status (ECOG 0 or 1) profited from intensive induction chemotherapy with prolonged overall survival as compared to other therapies only when allogenic stem cell transplantation was added, thus clearly advocating for consideration of this strategy in older patients with favourable ECOG score.
To the best of our knowledge it is another novel finding of our study, that AML patients above 65 years had a particularly beneficial outcome with better overall survival upon intensive induction chemotherapy if they presented with a beneficial ECOG score of 0 or 1 and at the same time carried a low HCT-comorbidity index between 0 and 4. Moreover, low ECOG scores of 0 or 1 predicted better overall survival in patients treated with induction chemotherapy as well as with induction therapy plus subsequent allogenic stem cell transplantation. For neither of the comorbidity scores alone analyzed here, CCI or HCT-CI, respectively, such a correlation with survival after intensive therapy was observed.
Therefore, based on our analysis an impact of acute myeloid leukaemia being judged as secondary or therapy-associated on overall survival was not found, possibly due to the low patient numbers.
A major shortcoming of this analysis presented here, apart from what has already been discussed above, is the fact that several novel treatment modalities for old or frail AML patients such as venetoclax plus HMA combinations, IDH2 inhibitors, midostaurin in FLT3 mutated AML, or CPX-351 entered routine clinical practice only in recent years which are thus not compared in this cohort [Citation21–24]. Due to these development, therapy of acute myeloid leukaemia has become increasingly complex in recent years [Citation25]. Of note, a recent phase 3 clinical trial including AML patients deemed to be unfit for induction chemotherapy due to limiting comorbidities or old age above 75 years showed dramatically improved outcome for venetoclax plus azacitidine combination as compared to azacitidine monotherapy with median overall survival of 14.7 versus 9.6 months (p < 0.001) and CR rates of 36.7% versus 17.9% (p < 0.001), respectively [Citation26]. A comparison of this data with our HMA treatment cohort showed an extraordinary better and clinical very relevant difference with favouring venetoclax plus azacitidne (14.7 vs 5 months). Even when patients received induction therapy without allogeneic stem cell transplantation in our group the med. OS was notably shorter (5.9 months) in comparison with the data from Di Nardo et al with venetoclax plus azacitidine. Therefore venetoclax plus azacitidine is the new standard of care for unfit patients.
Of note, a recent phase 3 clinical trial including AML patients deemed to be unfit for induction chemotherapy due to limiting comorbidities or old age above 75 years showed dramatically improved outcome for venetoclax plus azacitidine combination as compared to azacitidine monotherapy with median overall survival of 14.7 versus 9.6 months (p < 0.001) and CR rates of 36.7% versus 17.9% (p < 0.001), respectively [Citation26]. Venetoclax plus azacitidine is the new standard of care for unfit patients. In fact, overall survival reported here was well in the range described in previous trials studying ‘7 + 3’ induction chemotherapy on eligible, otherwise ‘fit’ patients underlining the potential benefit of this particular protocol particularly for older patients [Citation27].
AML cases carrying t(8;21), resulting in formation of RUNX1-RUNX1T1 fusion genes, or inv(16)/t(16;16), leading to formation of CBFB-MYH11 fusion genes are generally referred to as core-binding factor (CBF) AML and show particular sensitivity to high-dose cytarabine. Patients with CBF AML that were 60 years or older that were treated with induction followed by consolidation chemotherapy achieved a complete response in 88% of cases with a 5-year overall survival rate of 31% based on a retrospective analysis, suggesting that suitable CBF AML patients should be offered intensive chemotherapy [Citation28,Citation29]. Combination of intensive chemotherapy with the anti-CD33 antibody–drug conjugate gemtuzumab ozogamicin (GO) has been shown to improve overall survival in CBF AML [Citation29]. Of note, in a meta-analysis of five randomized trials this advantage was not affected by patient age [Citation30].
FLT3 inhibitors midostaurin and gilterinib have been FDA approved for the treatment of FLT3-mutated AML, and older patients aged 61–70 years have been shown to benefit from addition of midostaurin to IC in terms of improved event-free survival in a phase 2 trial [Citation31].
In IDH1 mutated AML patients that were ineligible for IC, combination therapy with the IDH1 inhibitor ivosidenib plus azacitidine (AZA) improved event-free survival, clinical response rate and overall survival as compared to AZA monotherapy (Pau Montesinos ASH 2021: 697 AGILE: A Global, Randomized, Double-Blind, Phase 3 Study of Ivosidenib + Azacitidine Versus Placebo + Azacitidine in Patients with Newly Diagnosed Acute Myeloid Leukaemia with an IDH1 Mutation).
The Hedgehog inhibitor glasdegib improved CR rate (17% vs. 2%) and overall survival (median 8.8 vs. 4.9 months) in combination with LDAC as compared to LDAC alone, and has been approved for the treatment of newly diagnosed AML patients above 75 years of age or unfit for intensive chemotherapy [Citation32].
Taken together, in line with previous observations by others, the analysis presented here strongly support the notion that certain subsets of patients above 65 years of age may well benefit from intensive chemotherapy and allogenic stem cell transplantation. Combined assessment of ECOG performance status and HCT-CI could be a valuable tool to support identification of patients likely to benefit from such an approach.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Karin Mayer and Michael Serries. The first draft of the manuscript was written by Georg Feldmann and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi:10.1182/blood-2016-03-643544.
- Juliusson G, Lazarevic V, Hörstedt A-S, et al. Acute myeloid leukemia in the real world: why population-based registries are needed. Blood. 2012;119(17):3890–3899. doi:10.1182/blood-2011-12-379008.
- Sorensen JT, Gerald K, Bodensteiner D. Holmes FF effect of age on survival in acute leukemia. 1950–1990. Cancer. 1993;72(5):1602–1606.
- Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447. doi:10.1182/blood-2016-08-733196.
- Kantarjian H, Ravandi F, O'Brien S, et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood. 2010;116(22):4422–4429. doi:10.1182/blood-2010-03-276485.
- Almeida AM, Ramos F. Acute myeloid leukemia in the older adults. Leuk Res Rep. 2016;6:1–7. doi:10.1016/j.lrr.2016.06.001.
- Burnett AK, Milligan D, Prentice AG, et al. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007;109(6):1114–1124. doi:10.1002/cncr.22496.
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655. doi:10.1097/00000421-198212000-00014.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi:10.1016/0021-9681(87)90171-8.
- Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. doi:10.1182/blood-2005-05-2004.
- Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–385. doi:10.3322/caac.21565.
- Appelbaum FR. Effectiveness of allogeneic hematopoietic cell transplantation for older patients with acute myeloid leukemia. Best Pract Res Clin Haematol. 2021;34(4):101320. doi:10.1016/j.beha.2021.101320.
- Appelbaum FR. Impact of allogeneic hematopoietic cell transplantation on the outcome of older patients with acute myeloid leukemia. Best Pract Res Clin Haematol. 2017;30(4):320–326. doi:10.1016/j.beha.2017.09.004.
- Farag SS, Maharry K, Zhang M-J, et al. Comparison of reduced-intensity hematopoietic cell transplantation with chemotherapy in patients age 60-70 years with acute myelogenous leukemia in first remission. Biol Blood Marrow Transplant. 2011;17(12):1796–1803. doi:10.1016/j.bbmt.2011.06.005.
- Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with 30% blasts. Blood. 2015;126(3):291–299. doi:10.1182/blood-2015-01-621664.
- Falantes J, Pleyer L, Thepot S, et al. Real life experience with frontline azacitidine in a large series of older adults with acute myeloid leukemia stratified by MRC/LRF score: results from the expanded international E-ALMA series (E-ALMA+). Leuk Lymphoma. 2018;59(5):1113–1120. doi:10.1080/10428194.2017.1365854.
- Pleyer L, Burgstaller S, Girschikofsky M, et al. Azacitidine in 302 patients with WHO-defined acute myeloid leukemia: results from the Austrian Azacitidine Registry of the AGMT-Study Group. Ann Hematol. 2014;93(11):1825–1838. doi:10.1007/s00277-014-2126-9.
- Dohner H, Dolnik A, Tang L, et al. Cytogenetics and gene mutations influence survival in older patients with acute myeloid leukemia treated with azacitidine or conventional care. Leukemia. 2018;32(12):2546–2557. doi:10.1038/s41375-018-0257-z.
- Ramos F, Thepot S, Pleyer L, et al. Azacitidine frontline therapy for unfit acute myeloid leukemia patients: clinical use and outcome prediction. Leuk Res. 2015;39(3):296–306. doi:10.1016/j.leukres.2014.12.013.
- Dumas P-Y, Bertoli S, Berard E, et al. Azacitidine or intensive chemotherapy for older patients with secondary or therapy-related acute myeloid leukemia. Oncotarget. 2017;8(45):79126–79136. doi:10.18632/oncotarget.15988.
- DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7–17. doi:10.1182/blood-2018-08-868752.
- Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722–731. doi:10.1182/blood-2017-04-779405.
- Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454–464. doi:10.1182/bloodadvances.2020002904.
- Lancet JE, Uy GL, Cortes JE, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018;36(26):2684–2692. doi:10.1016/S2352-3026(21)00145-9.
- Urbino I, Secreto C, Olivi M, et al. Evolving therapeutic approaches for older patients with acute myeloid leukemia in 2021. Cancers (Basel). 2021;13(20). doi:10.3390/cancers13205075.
- DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617–629. doi:10.1056/NEJMoa2012971.
- Foran JM. Can venetoclax-based therapy replace 7+3 induction in fit older adults with AML? Best Pract Res Clin Haematol. 2021;34(4):101335. doi:10.1016/j.beha.2021.101335.
- Prébet T, Boissel N, Reutenauer S, et al. Acute myeloid leukemia with translocation (8;21) or inversion (16) in elderly patients treated with conventional chemotherapy: a collaborative study of the French CBF-AML intergroup. J Clin Oncol. 2009;27(28):4747–4753. doi:10.1200/JCO.2008.21.0674.
- Bazinet A, Kadia TM. Changing paradigms in the treatment of acute myeloid leukemia in older patients. Clin Adv Hematol Oncol. 2022;20(1):37–46.
- Hills RK, Castaigne S, Appelbaum FR, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014;15(9):986–996. doi:10.1016/S1470-2045(14)70281-5.
- Schlenk RF, Weber D, Fiedler W, et al. Midostaurin added to chemotherapy and continued single-agent maintenance therapy in acute myeloid leukemia with FLT3-ITD. Blood. 2019;133(8):840–851. doi:10.1182/blood-2018-08-869453.
- Cortes JE, Heidel FH, Hellmann A. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 2019;33(2):379–389. doi:10.1038/s41375-018-0312-9.
