ABSTRACT
Objectives
Primary mediastinal large B-cel l lymphoma (PMBCL) is a rare subtype of B-cell lymphoma that is not yet fully understood. This population-based study aimed to assess the latest survival and treatment strategies for patients with PMBCL.
Methods
The study used the dataset from the Surveillance, Epidemiology, and End Results Program registry to retrospectively analyze adult patients diagnosed with PMBCL between 2001 and 2018. The primary outcome measures included overall survival (OS) and disease-specific survival (DSS).
Results
Among the 814 identified cases, the study revealed a 5-year OS rate of 86.7% and a 5-year DSS rate of 88.2% after a median follow-up of 54 months. Cox regression analysis indicated that age over 60 years, pre-2010 diagnosis, non-White ethnicity, advanced stage, and absence of chemotherapy significantly reduced both OS and DSS. It also found that chemotherapy has remained the primary therapeutic protocol for PMBCL over the last 20 years, whereas the utilization of surgery and radiation declined significantly. Patients diagnosed with PMBCL between 2010 and 2018 had a significantly reduced mortality risk (∼50%) compared to those diagnosed between 2001 and 2009. Notably, in the era of rituximab’s widespread usage, recipients of radiotherapy exhibited a poorer OS rate than non-recipients.
Conclusion
Survival outcomes for patients with PMBCL have significantly improved in the current era, possibly due to the evolving treatment paradigm. The value of radiotherapy in PMBCL is still debated and requires further prospective evaluation.
Introduction
Primary mediastinal large B-cell lymphoma (PMBCL) is a rare and aggressive lymphoma that originates from the thymic and accounts for approximately 2% to 4% of all non-Hodgkin lymphomas [Citation1,Citation2]. Although it shares a similar histology with diffuse large B-cell lymphoma (DLBCL) and a genetic profile with classic Hodgkin lymphoma, PMBCL is recognized as a distinct entity in the 2016 World Health Organization classification [Citation3,Citation4]. Compared to DLBCL, PMBCL has a substantially better prognosis, with a 5-year survival rate of around 85% [Citation5]. However, the most optimal therapy for PMBCL has yet to be determined, given the current lack of long-term follow-up data and prospective head-to-head trials [Citation6]. An anthracycline-based polychemotherapy regimen combined with a CD20 monoclonal antibody is the backbone of PMBCL treatment at present [Citation7,Citation8]. There is limited evidence available on the role of consolidation radiotherapy in the modern era of PMBCL treatment, which has led to controversy surrounding its efficacy and safety. Given the recent release of the Surveillance, Epidemiology, and End Results (SEER) database with extended follow-up and broader population coverage, we conducted an evaluation of the latest survival rates and treatment strategies for PMBCL.
Materials and methods
Data collection
We collected data from the custom SEER database [Incidence-SEER Research Plus Data, 18 Registries, November 2020 Sub (2000–2018)], which compiles information regarding cancer prevalence, treatment, and survival from population-based cancer registries and currently represents ∼28% of cancer cases in the U.S. population. In our study, we identified PMBCL cases using the International Classification of Diseases for Oncology 3 histology code 9679. The data we collected for our study included various significant variables, namely patient ID, year of diagnosis, age of onset, gender, race, primary site, Ann Arbor stage, coexisting malignancy, treatment parameters, survival duration, causes of death, and diagnostic confirmation. We excluded individuals under 18 and those with missing histological evidence or relevant information. The specific screening process is shown in Figure S1.
Statistical methods
We used the National Cancer Institute (NCI) SEER*Stat version 8.3.9.2 and R software version 4.2.1 for statistical analyses. The designated end-points of this study were overall survival (OS), measured from the time of diagnosis until death or last follow-up, and disease-specific survival (DSS), measured from the date of diagnosis until PMBCL-related death or last follow-up. To ensure the reliability of our analysis, we randomly divided the dataset into two parts: a training set (75%) and a validation set (25%) for cross-validation. The Chi-squared test, with Yates correction if necessary, or the Fisher exact test were used for categorical variables as appropriate. Univariate and multivariate Cox regression analyses were used to identify the independent risk factors of survival. We confirmed that the proportional hazards assumption was met for Cox regression models, by using the Schoenfeld residuals test. To avoid multicollinearity, we ensured that the variance inflation factors remained below 4. Forest plots were employed to illustrate the hazard ratio (HR) along with 95% confidence intervals (CI) of the summary outcomes. Through multivariate Cox regression, we calculated coefficients for each variable to establish a risk score formula. We then assigned individual risk scores for each patient and categorized them into low- and high-risk groups based on the median score. The log-rank test was employed to compare Kaplan-Meier curves. To reassess the effect of radiotherapy on survival while controlling for all remaining variables, we conducted propensity score-matched (PSM) analyses using the 1:1 nearest neighbor technique with a caliper of 0.1. Statistical significance was defined as P < 0.05, and all tests were two-sided.
Ethical statement
We received authorization from the NCI SEER program to utilize these data for analysis. Institutional approval or patient consent was not required. All procedures adhered to the Declaration of Helsinki principles. The authors take full responsibility for the accuracy and integrity of the work, and are committed to thoroughly investigating and resolving any related issues.
Results
Patient characteristics
A total of 814 PMBCL cases were identified from records between 2001 and 2018. The median age of onset was 36 years old (range, 18–94). Females showed a predominance (F: M ratio of 1.62:1). The majority of the cohort (77.5%) were White, with extranodal sites being most common (77.5%). Most patients presented with stage I-II lesions (76.9%). In era 1 (2001–2009), 224 patients were diagnosed, while era 2 (2010–2018) saw 590 diagnoses. Regarding intervention strategies, approximately 11.3% of patients received surgical procedures, 34.9% underwent radiotherapy, and 96.6% received chemotherapy. There was no significant difference in the distribution of patient and treatment characteristics between the training and validation set ().
Table 1. Patient and treatment characteristics.
Table S1 presents the patient and treatment characteristics comparison by period. The percentage of extranodal PMBCL was significantly higher in era 2 than in era 1 (83.2% vs. 62.5%, P < 0.001). Additionally, we observed a significant decrease in the rates of surgery (from 20.1% to 8%) and radiotherapy (from 56.2% to 26.8%) over time.
Survival for the whole cohort
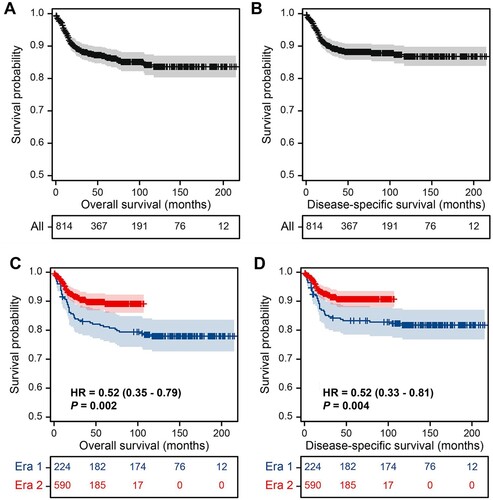
The median follow-up for the overall cohort was 54 months. During follow-up, there were 96 deaths (11.8%), with a median time to death of 14 months. Of these, 81 cases resulted in death from PMBCL. Neither median OS nor DSS was reached. The OS rates were 86.7% (95% CI, 83.8–89.1) at 5 years and 83.6% (95% CI, 79.9–86.7) at 10 years (A); the DSS rates were 88.2% (95% CI, 85.5–90.5) at 5 years and 86.8% (95% CI, 83.5–89.5) at 10 years (B). Patients diagnosed with PMBCL in era 2 had a significantly reduced mortality risk (∼50%) compared to those diagnosed in era 1 (C and D). In era 1, the 5-year OS and DSS were 81.2% (95% CI, 76.2–86.5%) and 83.3% (95% CI, 78.5–88.3%), while in era 2, they improved to 89.1% (95% CI, 86.0–92.3%) and 90.7% (95% CI, 87.9–93.5%), respectively.
Prognostic factor and risk stratification
In the training set (n = 610), Cox regression analyses revealed that age over 60 years, diagnosis between 2001 and 2009, non-White ethnicity, advanced Ann Arbor stage, and lack of chemotherapy were all significantly associated with shorter OS (A). The risk score for each patient was calculated using the following formula: risk score = 1.95 × age of diagnosis (coded 1 = over 60 years; coded 0 = less than or equal to 60 years) + 0.68 × year of diagnosis (coded 1 = era 1; coded 0 = era 2) + 0.62 × race (coded 1 = non-White; coded 0 = White) + 0.62 × Ann Arbor stage (coded 1 = III–IV; coded 0 = I–II) – 1.63 × chemotherapy (coded 1 = yes; coded 0 = no). The patients were divided into low- and high-risk groups based on their median risk score (B). A statistically significant difference was observed in OS between the groups (C, P < 0.001). The high-risk group had a 3.83-fold higher risk of all-cause death than the low-risk group (95% CI, 1.96–7.47). Similar results were observed for DSS, as shown in . In multivariate analysis, age, year of diagnosis, race, Ann Arbor stage, and chemotherapy were also independently significant prognostic factors for DSS (A). All variables were coded in the same manner as described previously. The risk-score calculation formula is as follows: risk score = 1.88 × age of diagnosis + 0.58 × year of diagnosis + 0.71 × race + 0.93 × Ann Arbor stage – 1.67 × chemotherapy (A,B). The high-risk group had an HR of 4.15 (95% CI, 1.97–8.72) compared to the low-risk group (C, P < 0.001).
Figure 2. Analysis of independent prognostic factors, risk scoring, and risk stratification for OS. (A) Univariate and multivariate Cox regression models for OS in the training set; (B) Distribution of risk scores and survival status of patients in the training set; (C) OS curves for low- and high-risk groups in the training set; (D) OS curves for low- and high-risk groups in the validation set. Abbreviations: HR, hazard ratio; OS, overall survival.
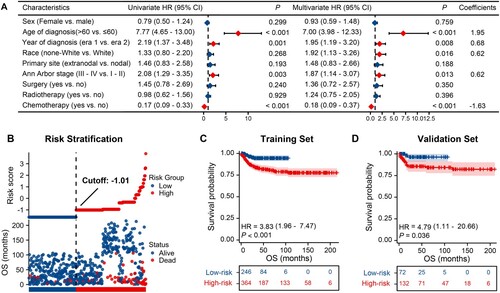
Figure 3. Analysis of independent prognostic factors, risk scoring, and risk stratification for DSS. (A) Univariate and multivariate Cox regression models for DSS in the training set; (B) Distribution of risk scores and survival status of patients in the training set; (C) DSS curves for low- and high-risk groups in the training set; (D) DSS curves for low- and high-risk groups in the validation set. Abbreviations: HR, hazard ratio; DSS, disease-specific survival.
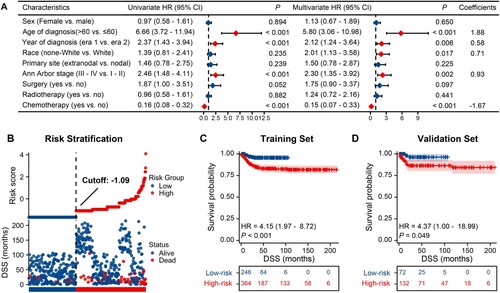
Based on the risk score, patients in the validation groups (n = 204) were divided into low- and high-risk groups, and then survival was compared. Similar to the results obtained from the training cohort, patients in the high-risk group had a significantly shorter OS (HR = 4.79, P = 0.036, D) and DSS (HR = 4.37, P = 0.049, D) than those in the low-risk group.
The value of radiotherapy
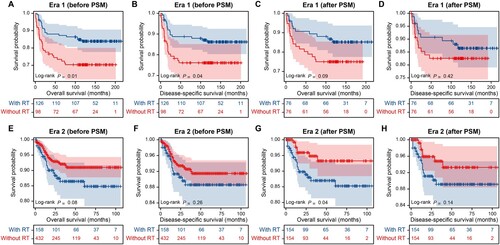
Cox regression analysis indicated no significant correlations between radiotherapy and survival outcomes, in either the univariate or multivariate models. A subanalysis using the PSM model was performed to further validate the prognostic value of radiotherapy. This resulted in 76 matched pairs in era 1 and 366 pairs in era 2, with all other covariates well-balanced (Tables S2 and S3). Significant OS and DSS benefits from radiotherapy were observed before matching for patients in era 1 (A and B, P < 0.05); however, propensity matching attenuated these associations (C and D, P > 0.05). Following matching, radiotherapy recipients in era 2 showed a worse OS than non-recipients, while no significant difference was observed in DSS (E–H).
Figure 4. The impact of radiotherapy on survival in patients with primary mediastinal large B-cell lymphoma. (A) OS before PSM in era 1; (B) DSS before PSM in era 1; (C) OS after PSM in era 1; (D) DSS after PSM in era 1; (E) OS before PSM in era 2; (F) DSS before PSM in era 2; (G) OS after PSM in era 2; (H) DSS after PSM in era 2. Abbreviations: PSM, propensity score matching; RT, radiotherapy; OS, overall survival; DSS, disease-specific survival.
Discussion
The present study has a population-based design and a large study population, which is similar in age, gender, and disease stage to prior studies [Citation9–11]. The 5-year survival rate for PMBCL patients is approximately 85%, which is consistent with previous studies’ findings [Citation11–13]. Additionally, our research identified that the number of cases increased significantly during era 2. Recent studies by Zhou H et al. reported similar findings, revealing an increase in the incidence of PMBCL over the years (from 0.05/1,000,000 in 1975 to 2.38/1,000,000 in 2018) [Citation14]. This growing trend may be attributed to increased exposure risks to lymphomagenesis-associated factors and improved diagnostic capabilities in identifying this subtype of lymphoma. As a result, further research into PMBCL is warranted.
Our study found substantial ethnic survival disparities in PMBCL, with White patients demonstrating better OS than patients of other races, consistent with previous findings [Citation12,Citation15]. In addition, our data revealed that White patients had a significantly better DSS compared to individuals of other races, even after adjusting for other confounding factors. Various factors may contribute to these survival disparities, including differences in disease biology, host pharmacogenetics, hospital-related factors, access to healthcare opportunities, and socioeconomic status.
Chemotherapy was the dominant treatment strategy in the past two decades. While the SEER database lacks detailed data on drug use, it is widely known that chemotherapy regimens for PMBCL patients have varied over time. Initially, first-generation (CHOP and CHOP-like) to third-generation (MACOP-B, VACOP-B, ProMACECytaBOM) chemotherapy regimens were used to treat this entity [Citation16]. However, retrospective data showed that the superiority of V/MACOP-B over CHOP disappeared once rituximab was added [Citation17]. More recently, DA-EPOCH has emerged as the preferred frontline chemotherapy, since it seems to decrease the need for radiation when combined with rituximab [Citation11,Citation18]. However, there are no published results from prospective, randomized trials comparing these chemotherapy strategies. Rituximab was approved by the US FDA in 2006, implying that its utilization increased between 2010 and 2018 relative to 2001–2009. The significance of the diagnosis year in prognosis is therefore reasonable. Although there is a lack of detailed data on immunochemotherapy in the database, the robust and persistent improvement in survival further supports the potential benefits of the modern treatment paradigm.
Prior to the advent of rituximab, there was a general consensus that radiotherapy could benefit patients with PMBCL [Citation19–22] However, as we shift to the contemporary era of rituximab, concerns are increasing over the efficacy and safety of radiotherapy as a therapeutic approach for PMBCL [Citation9,Citation18]. While there is currently no clear evidence advocating for the elimination of radiotherapy, its use in the real world has been steadily decreasing. Our study found that the proportion of American patients receiving radiotherapy dropped from 56.2% to 26.8% between two periods. In Sweden, only 17% of patients received radiotherapy during the era of rituximab [Citation13]. Although retrospective, our study supports the findings of Giri S et al. and Wästerlid T et al. that radiotherapy does not provide additional survival benefits in the era of rituximab [Citation9,Citation13]. Furthermore, our study revealed that patients who received radiotherapy had a worse OS than those who did not receive it after 2010. This may be due to the potential toxicities of radiotherapy such as cardiopulmonary issues and secondary malignancies [Citation23]. Differences in radiotherapy indications between different eras could also play a role. Historical use of mediastinal radiotherapy was routine, but now it is guided by 18F-fluorodeoxyglucose positron emission tomography (PET) scans, and only patients with PET-positive scans receive it [Citation24,Citation25]. This suggests that contemporary-era radiotherapy recipients likely have inadequate chemotherapy responses, which itself portends a worse prognosis. We must acknowledge that these factors cannot be entirely corrected for through the propensity score analysis as there may be multiple drivers that are not recorded in the SEER register. Comparing treatments retrospectively is always challenging, thus we eagerly anticipate the results of an ongoing randomized controlled trial aimed to evaluate the role of consolidation mediastinal radiotherapy after immunochemotherapy in patients newly diagnosed with PMBCL [Citation26].
There is currently no risk score system specifically designed for PBMCL available. Risk stratification with the international prognostic index is of limited value in PMBCL due to the young age and limited stage of the disease at presentation. In this study, we utilized clinical variables from the SEER database to categorize patients into low- and high-risk groups, and found a significant difference between the two groups. However, the limited number of variables included in the analysis is an inherent flaw in database studies, and further research is necessary to clarify this point.
Our retrospective study had some limitations. First, despite using several analytical techniques to control for confounders, unmeasured confounding factors may remain. Second, our findings have applicability to diverse populations in the US, yet it remains unclear whether the results will hold true in other demographic groups. Last, the database lacked detailed clinical information regarding treatment and information about disease recurrence or progression. Therefore, future prospective multicenter studies are needed to validate our findings.
To summarize, our study indicates that the survival outcomes of patients with PMBCL have improved over time. These improvements may be attributed in part to the development of novel therapies. The current usage rate of radiotherapy has decreased, and its survival advantages for PMBCL patients have become less significant. Further prospective research is necessary to gain a more thorough understanding of the optimal treatment strategies for PMBCL.
Author contributions
Lin Quan: Conceptualization, Methodology, Writing-Original Draft; Zhen He: Methodology, Validation, Visualization, Writing-Original Draft; Xiaoling Zuo: Methodology, Validation, Writing-Original Draft; Lei Cao: Software, Funding acquisition, Writing-Review & Editing; Yi Wang: Validation, Resources, Writing-Review & Editing; Hongyu Dai: Validation, Data Curation, Writing-Review & Editing; Wei Wu: Formal analysis, Data Curation, Writing-Review & Editing; Xiao Shi: Formal analysis, Resources, Writing-Review & Editing; Hailing Liu: Writing-Review & Editing, Supervision, Project administration. All authors approved the final submitted paper.
Ethics approval statement
We received authorization from the NCI SEER program to utilize these data for analysis. Institutional approval or patient consent was not required. All procedures adhered to the Declaration of Helsinki principles.
Geolocation information
Nanjing 210029, Jiangsu, China.
Data availability statement
The dataset generated and analyzed during the current study is available in the National Cancer Institute’s SEER, https://seer.cancer.gov/data/access.html.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Primary mediastinal (thymic) large B-cell lymphoma. Available from: https://seer.cancer.gov/seertools/hemelymph/51f6cf56e3e27c3994bd5318/
- Steidl C, Gascoyne RD. The molecular pathogenesis of primary mediastinal large B-cell lymphoma. Blood. 2011;118(10):2659–2669. doi:10.1182/blood-2011-05-326538
- Teras LR, DeSantis CE, Cerhan JR, et al. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66(6):443–459. doi:10.3322/caac.21357
- Savage KJ, Monti S, Kutok JL, et al. The molecular signature of mediastinal large B-cell lymphoma differs from that of other diffuse large B-cell lymphomas and shares features with classical Hodgkin lymphoma. Blood. 2003;102(12):3871–3879. doi:10.1182/blood-2003-06-1841
- Savage KJ, Al-Rajhi N, Voss N, et al. Favorable outcome of primary mediastinal large B-cell lymphoma in a single institution: the British Columbia experience. Ann Oncol. 2006;17(1):123–130. doi:10.1093/annonc/mdj030
- Zinzani PL, Broccoli A, Casadei B, et al. The role of rituximab and positron emission tomography in the treatment of primary mediastinal large B-cell lymphoma: experience on 74 patients. Hematol Oncol. 2015;33(4):145–150. doi:10.1002/hon.2172
- Fakhri B, Ai W. Current and emerging treatment options in primary mediastinal B-cell lymphoma. Ther Adv Hematol. 2021;12:20406207211048959. doi:10.1177/20406207211048959
- Ahmed Z, Afridi SS, Shahid Z, et al. Primary mediastinal B-cell lymphoma: a 2021 update on genetics, diagnosis, and novel therapeutics. Clin Lymphoma Myeloma Leuk. 2021;21(11):e865–e875. doi:10.1016/j.clml.2021.06.012
- Giri S, Bhatt VR, Pathak R, et al. Role of radiation therapy in primary mediastinal large B-cell lymphoma in rituximab era: a U.S. population-based analysis. Am J Hematol 2015;90(11):1052–1054. doi:10.1002/ajh.24172
- Zhou H, Xu-Monette ZY, Xiao L, et al. Prognostic factors, therapeutic approaches, and distinct immunobiologic features in patients with primary mediastinal large B-cell lymphoma on long-term follow-up. Blood Cancer J. 2020;10(5):49. doi:10.1038/s41408-020-0312-7
- Chan EHL, Koh LP, Lee J, et al. Real world experience of R-CHOP with or without consolidative radiotherapy vs DA-EPOCH-R in the first-line treatment of primary mediastinal B-cell lymphoma. Cancer Med. 2019;8(10):4626–4632. doi:10.1002/cam4.2347
- Jackson MW, Rusthoven CG, Jones BL, et al. Improved survival with radiation therapy in stage I-II primary mediastinal B cell lymphoma: a surveillance, epidemiology, and End results database analysis. Int J Radiat Oncol Biol Phys. 2016;94(1):126–132. doi:10.1016/j.ijrobp.2015.09.017
- Wästerlid T, Hasselblom S, Joelsson J, et al. Real-world data on treatment and outcomes of patients with primary mediastinal large B-cell lymphoma: a Swedish lymphoma register study. Blood Cancer J. 2021;11(5):100. doi:10.1038/s41408-021-00491-7
- Zhou H, Liu Q, Lu S, Zou L. Primary mediastinal/thymic diffuse large B-cell lymphoma: a population-based study on incidence and survival. Ann Hematol 2023. Epub ahead of print. doi:10.1007/s00277-023-05225-2
- Liu PP, Wang KF, Xia Y, et al. Racial patterns of patients with primary mediastinal large B-cell lymphoma. Medicine. 2016;95(27):e4054. doi:10.1097/MD.0000000000004054
- Martelli M, Ferreri AJ, Johnson P. Primary mediastinal large B-cell lymphoma. Crit Rev Oncol Hematol 2008;68(3):256–263. doi:10.1016/j.critrevonc.2008.07.020
- Avigdor A, Sirotkin T, Kedmi M, et al. The impact of R-VACOP-B and interim FDG-PET/CT on outcome in primary mediastinal large B cell lymphoma. Ann Hematol 2014;93(8):1297–1304. doi:10.1007/s00277-014-2043-y
- Dunleavy K, Pittaluga S, Maeda LS, et al. Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. N Engl J Med. 2013;368(15):1408–1416. doi:10.1056/NEJMoa1214561
- Todeschini G, Secchi S, Morra E, et al. Primary mediastinal large B-cell lymphoma (PMLBCL): long-term results from a retrospective multicentre Italian experience in 138 patients treated with CHOP or MACOP-B/VACOP-B. Br J Cancer. 2004;90(2):372–376. doi:10.1038/sj.bjc.6601460
- Zinzani PL, Martelli M, Bertini M, et al. Induction chemotherapy strategies for primary mediastinal large B-cell lymphoma with sclerosis: a retrospective multinational study on; 426, previously untreated patients. Haematologica. 2002;87(12):1258–1264.
- Zinzani PL, Martelli M, Bendandi M, et al. Primary mediastinal large B-cell lymphoma with sclerosis: a clinical study of 89 patients treated with MACOP-B chemotherapy and radiation therapy. Haematologica. 2001;86(2):187–191.
- Martelli MP, Martelli M, Pescarmona E, et al. MACOP-B and involved field radiation therapy is an effective therapy for primary mediastinal large B-cell lymphoma with sclerosis. Ann Oncol. 1998;9(9):1027–1029. doi:10.1023/A:1008412009667
- Martelli M, Ferreri A, Di Rocco A, et al. Primary mediastinal large B-cell lymphoma. Crit Rev Oncol Hematol 2017;113:318–327. doi:10.1016/j.critrevonc.2017.01.009
- Hayden AR, Tonseth P, Lee DG, et al. Outcome of primary mediastinal large B-cell lymphoma using R-CHOP: impact of a PET-adapted approach. Blood. 2020;136(24):2803–2811. doi:10.1182/blood.2019004296
- Freitas AC, Carvalho IP, Esteves S, et al. End of treatment FDG-PET in primary mediastinal B-cell lymphoma treated with R-chemotherapy: prognostic indicator and implications for consolidation radiotherapy. Eur J Haematol 2022;108(2):118–124. doi:10.1111/ejh.13715
- Ceriani L, Barrington S, Biggi A, et al. Training improves the interobserver agreement of the expert positron emission tomography review panel in primary mediastinal B-cell lymphoma: interim analysis in the ongoing international extranodal lymphoma study group-37 study. Hematol Oncol. 2017;35(4):548–553. doi:10.1002/hon.2339