ABSTRACT
Objective
Anthracyclines and cytarabine have comprised standard induction therapy for acute myeloid leukemia (AML) for decades. Low overall survival of AML is due to non-remission or relapse after remission. Hypomethylating agent (HMA) decitabine combined with low-dose chemotherapy or other targeted agents has shown promising effect for AML in clinical trials, especially in t(8;21) acute myeloid leukemia. We previously investigated histone deacetylase inhibitor (HDACi) chidamide could regulate Wnt/β-catenin signaling pathway in leukemia cell lines.
Methods
Adult patients with de novo or relapsed/refractory AML who were treated with chidamide and decitabine in combination with chemotherapy (chidamide group, n = 23) or only decitabine combination with chemotherapy (decitabine group, n = 17) were analyzed.
Results
Chidamide group represented higher complete response rate (82.6% and 52.9%, p = 0.0430, vs. decitabine group), progression-free survival and overall survival rates (p = 0.0088 and p = 0.0139, respectively), especially for patients with de novo AML. Hematological toxicity and infections were the most common adverse events (AEs) in both groups, and they were manageable by supportive treatments.
Conclusions
This HDACi- and HMA-based protocol is an effective and tolerable therapy for patients with AML. The comprehensive mechanism and effects of chidamide in combination with decitabine are worth to be further explored in AML.
Introduction
Acute myeloid leukemia (AML, not acute promyelocytic leukemia [non-APL]), which is a most common adult hematological malignancy, is considered to be probably caused by abnormal genetic or epigenetic regulation of hematopoietic stem cells [Citation1,Citation2]. Anthracyclines and cytarabine have comprised the standard AML induction therapy for decades. More than 80% of young patients with AML could achieve complete responses (CRs) [Citation3], whereas only 24–29.5% of adult patients could achieve 5-year overall survival due to non-remission or relapse after remission [Citation4–7]. Further exploration is strongly encouraged to increase overall response rate (ORR), as well as to seek opportunities for consolidation treatments or hematopoietic stem cell transplantation (HSCT).
Genomic and epigenetic alterations participate in the occurrence and progression of hematological malignancies [Citation8–11]. Hypomethylating agent (HMA) decitabine works by binding to DNA methyltransferase [Citation12], thus specifically inducing cell cycle arrest, activating tumor suppressor genes, and inhibiting the proliferation of tumor cells [Citation13,Citation14]. Mutations such as DNMT3A, TET2 are related to functions of DNA methylation, and HMA has shown efficacy for patients with mutation mentioned above [Citation15]. Decitabine has been approved as a favorable agent by the Food and Drug Administration for clinical treatments of AML (combined with venetoclax for patients intolerant for intense chemotherapy), myelodysplastic syndromes. Clinical trials related to decitabine combined with chemotherapy (such as FLAG, DA and CAG) for newly diagnosed or relapsed/refractory AML (R/R AML) achieved better outcomes [Citation16–18].
Histone deacetylases (HDACs) are essential for maintaining the balance of histone acetylation, which induces gene transcriptional repression through chromatin condensation, and the overexpression of HDACs is implicated in oncogenesis [Citation19,Citation20]. HDAC inhibitors (HDACis) have emerged as new therapeutic options that modulate anti-tumor mechanisms and exert anti-tumor effects synergistically with other therapeutic protocols [Citation21–23]. Chidamide (CS055/HBI-8000) is a subtype-selective HDACi with the ability to inhibit HDAC1, HDAC2, HDAC3, and HDAC10. Chidamide could target leukemia cells by inhibiting Wnt/β-catenin, ERK1/2 and JAK2/STAT3 signaling pathway [Citation24–26], which could also synergize with decitabine to inhibit the proliferation of AML cells and significantly enhance the cytotoxicity in AML cells [Citation27–30]. We conducted a retrospective study on chidamide and decitabine combined with chemotherapy in adults with AML and evaluated the efficacy and safety of this therapeutic regimen.
Materials and methods
Patients and treatments
We retrospectively analyzed treatment efforts on de novo or relapsed adult AML (non-APL) patients treated between December 2016 and October 2019 in the Department of Hematology, First Affiliated Hospital of Harbin Medical University, who have undergone a complete inductive course with evaluable efficacy. The diagnosis was made according to 2016 World Health Organization AML criteria [Citation31]. Due to the fact that none of these patients were given FLT3 inhibitors, FLT-3 mutation was still seemed as adverse prognosis, and risk stratification was conducted according to the 2017 European Leukemia Net (ELN) recommendations for diagnosis and management of adults with AML [Citation32]. This study was approved by ethics committees of the First Affiliated Hospital of Harbin Medical University (Approval No.201609).
The regimens were chidamide and decitabine combined with chemotherapy (based on anthracyclines and cytarabine, shown in Sup Table 1,2). Therapeutic protocols: (1) Chidamide group: oral chidamide given at 30 mg twice a week for 2 weeks starting on day 1, decitabine administered at 20 mg/(m2·day) via intravenous infusion on days 1–5, and combination chemotherapy; or (2) Decitabine group: decitabine administered at 20 mg/(m2·day) via intravenous infusion on days 1–5 and combination chemotherapy. Both protocols were followed by consolidation therapies and symptomatic therapy and supportive care were adequately provided.
Assessment of efficacy and safety
Treatment responses and outcomes were evaluated at the completion of each cycle on the basis of NCCN clinical practice guidelines [Citation33]. The primary efficacy evaluation included CR, CR with incomplete blood count recovery (CRi), partial response (PR), and ORR. ORR was calculated as CR+CRi+PR. The secondary efficacy evaluation included progression-free survival (PFS), duration of response (DOR), and overall survival (OS). MRD and gene mutations were measured by flow cytometry (FCM) and next-generation sequencing, respectively.
The incidence and severity of adverse events (AEs) were monitored and recorded by the investigators throughout the process. Toxicities were evaluated and graded using the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0 and compared between two groups. Post-therapy follow-up and detailed examinations of patients with AML were performed every 3 months until November 20, 2019.
Statistical analysis
All statistical analyses were conducted using SAS software version 9.3. When data followed a normal distribution, Student’s t-test was performed to compare quantitative variables between both groups. Otherwise, quantitative variables were compared using the rank sum test. PFS and OS were estimated using Kaplan–Meier method, and differences were compared by applying the log-rank test. The hazard ratio (HR) was estimated using Cox proportional-hazards model, and survival outcomes were presented as HRs with 95% confidence intervals (CIs), with statistical significance evaluated at 0.05 alpha level.
Results
Patient characteristics
We enrolled 40 AML patients between December 2016 and October 2019 in this study, including 26 men and 14 women with a median age of 46.2 ± 15.7 years. Twenty-three patients (23/40, 57.5%) were treated with chidamide and decitabine combined with chemotherapy (chidamide group), and 17 patients (17/40, 42.5%) received decitabine and chemotherapy (decitabine group). Baseline characteristics of patients in both groups are listed in Sup Table 3. In total, 22 patients were newly diagnosed with AML (22/40, 55.0%), and 18 patients with R/R AML (18/40, 45.0%). Five patients (5/40, 12.5%) were intermediate-risk and 35 patients (35/40, 87.5%) were adverse risk according to the 2017 ELN recommendations. Of 18 patients with R/R AML, 6 (6/18, 33.3%) had received other salvage therapeutic regimens (e.g. FLAG, CAG, HMA) for R/R AML before the study. Excluding sex, age, and disease status, there were no significant differences in baseline characteristics between two groups (p > 0.05). Among 32 patients with accessible cytogenetic analysis, 16 patients were evaluated as intermediate risk, and other 16 patients were adverse risk (detailed in Sup Table 3). Gene mutations, including FLT3-ITD (12/33, 36.4%), DNMT3A (8/33, 24.2%), NPM1 (7/33, 21.2%), CEBPA (7/33, 21.2%), IDH2 (6/33, 18.2%), NRAS (6/33, 18.2%), TET2 (5/33, 15.2%), KRAS (5/33,15.2%), RUNX1 (5/33, 15.2%), GATA2 (3/33, 9.1%), and ASXL1 mutations (2/33, 6.1%), were detected in 33 patients (Sup Fig. 1a). FLT3-ITD, NPM1, NRAS, DNMT3A, CEBPA, IDH2, and RUNX1 mutations were mainly identified in patients with newly diagnosed AML, whereas FLT3-ITD, DNMT3A, TET2, CEBPA, NPM1, IDH2, and GATA2 mutations were more common in patients with R/R AML (Sup Fig. 1b). FLT3-ITD, DNMT3A, NPM1, CEBPA, IDH2, NRAS, TET2, and RUNX1 mutations were present in both groups before treatment (Sup Fig. 1c).
Treatment responses
Chidamide group (n = 23) included 9 patients with newly diagnosed AML and 14 patients with R/R AML. CR or CRi was reported in 19 patients in this group (19/23, 82.6%), including CRs in 16 patients (16/23, 69.6%, 6 newly diagnosed patients [6/9, 66.7%] and 10 relapsed/refractory patients [10/14, 71.4%]) and CRis in 3 patients (3/23, 13.0%, 2 newly diagnosed patients [2/9, 22.2%] and 1 relapsed/refractory patient [1/14, 7.1%]). No patients displayed a PR, and 4 patients (4/23, 17.4%) experienced treatment failure. ORR in this group was 82.6% (19/23). Of 19 patients with CRs/CRis, 3 patients achieved CR after two cycles of treatment, whereas the other 16 patients achieved CR/CRi after one cycle. Fifteen patients (15/23, 65.2%) were MRD negative (MRD−, <1 × 10−4) according to FCM after one or two cycles of treatment. Thirteen patients with newly diagnosed AML and four patients with R/R AML were investigated in decitabine group (n = 17). CRs or CRis were observed in nine patients in decitabine group (9/17, 52.9%), including CRs in three patients with newly diagnosed AML (3/13, 23.1%), three patients with R/R AML (3/4, 75.0%) and CRis in three patients with newly diagnosed AML (3/13, 23.1%). Two patients (2/17, 11.8%) had PRs, and treatment failure was observed in six patients (6/17, 35.3%). Of all nine patients with CRs/CRis, one patient achieved a CR after two cycles of treatment, whereas the other eight patients achieved a CR/CRi after one cycle. ORR was 64.7% (11/17), and seven patients (7/17, 41.2%) achieved MRD− (Sup Table 4). Twenty patients (13 in chidamide group and 7 in decitabine group) who achieved CRs/CR were continued to receive consolidation chemotherapy.
Patients in chidamide group exhibited higher CR/CRi rates (82.6% vs. 52.9%, p = 0.0430) and improved DOR (4.9 months vs. 1.1 months, p = 0.0274) than decitabine group. Median durations of complete responses before relapse were 7.0 months (range: 2.9–11.9 months) and 2.9 months (range: 0.9–8.8 months) in chidamide and decitabine groups, respectively (p = 0.1853, Sup Table 4). ORR was not significantly different between groups (p = 0.2743). Patients with de novo AML showed higher CR/CRi rates (88.9% vs. 46.2%, p = 0.0405) and prolonged median DOR (7.5 months vs. 5.7 months) in chidamide group. However, there were no significant differences in CR/CRi rates in patients with R/R AML (78.6% vs. 75.0%, p = 0.8796). Extramedullary infiltration lesions disappeared in two patients and significantly declined in one patient in chidamide group, whereas no obvious improvement of extramedullary infiltration lesions was noted in decitabine group. Thirteen patients in both groups experienced disease progression over the study period.
Genetic abnormality and responses
Genetic abnormality might predict the response to treatment in patients with AML. Chidamide combined with decitabine and chemotherapy seemed to overcome adverse effects of karyotypes leading to CR/CRi in 8/8 patients all with complex karyotype, while 2/8 patients in decitabine group (complex karyotype in 2 CR/CRi patients, one with −7 and 5 with complex karyotype in 6 PR or NR patients, Sup Table 3). While gene mutations affected prognosis at the same time. Patients with DMNT3A, IDH2, and TET2 gene mutations generally experienced disease remission in both groups, as presented in Sup Figure 2a–b. In chidamide group, 66.7% of patients (4/6) with FLT3-ITD mutations alone or combined with other gene mutations achieved CR/CRi. Two patients exhibited treatment failure, including one patient with FLT3-ITD, NRAS, PTPN11, CEBPA, and WT1 gene mutations and one with an FLT3-ITD mutation alone, but it is worth noted that extramedullary disease was reduced after one cycle of treatment. Meanwhile, some satisfactory responses were observed after chidamide treatment in patients with FLT3-ITD, AXSL1, and DNA methylation-related gene mutations (e.g. DNMT3A, IDH2, TET2). In decitabine group, CRs were observed in 83.3% of patients (5/6) with FLT3-ITD gene mutations, and patients with DNMT3A, IDH2, or TET2 gene mutations achieved disease remission. However, one patient with a TP53 gene mutation displayed an unfavorable response to decitabine regimen. A majority of patients with NPM1 gene mutations achieved CR/CRi (66.7% [2/3] in chidamide group vs. 75.0% [3/4] in decitabine group). Patients with RUNX1 gene mutations achieved CR/CRi after one or two cycles of treatment, but a short DOR and poor prognosis were observed in both groups. Chidamide in combination with decitabine and chemotherapy produced better effects on NRAS and KRAS gene mutations than regimen without chidamide, and 66.7% of patients (4/6) with at least one of two aforementioned gene mutations achieved remission. Variations of gene mutations before and after treatment were also delineated in 25 patients (Sup Fig. 3).
Survival analysis
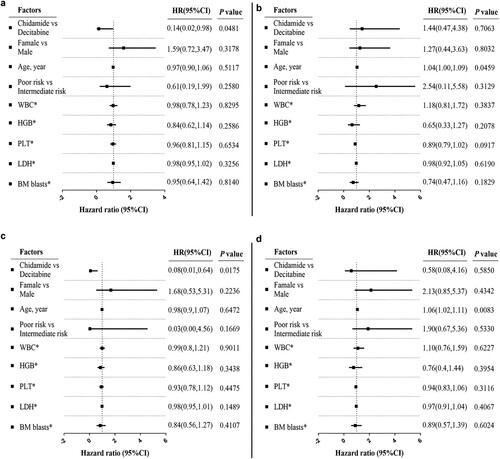
In total, eight patients in chidamide group and three patients in decitabine group were still alive at the last follow-up, and the cumulative mortality rates in these groups were 65.2% (15/23) and 82.4% (14/17), respectively. Median OS was 9.7 months in chidamide group, versus 8.5 months in decitabine group (log-rank test, p = 0.0684, (a)). Patients with newly diagnosed AML who received chidamide exhibited an OS improvement (log-rank test, p = 0.0450, (b)), whereas no significant OS improvement was noted in patients with R/RAML in either group ((c)). Median PFS was 6.4 months in chidamide group compared with 2.6 months in decitabine group (log-rank test, p = 0.0242, (d)). Median PFS among patients with newly diagnosed AML was 12.9 months in chidamide group, versus 2.0 months in decitabine group (log-rank test, p = 0.0135, (e)), whereas no significant difference in PFS was noted among patients with R/R AML (7.2 months vs. 3.3 months; log-rank test, p = 0.5124, (f)). OS and PFS benefits were analyzed and compared using Cox proportional-hazards model (Sup Tables 4 and 5). OS and PFS among patients with newly diagnosed AML were significantly improved in chidamide group ((a,c)). However, few survival benefits were observed among patients with R/R AML because of the limited sample size ((b,d)). It was also estimated that sex, age, and adverse risk stratification were crucial factors.
Figure 1. Kaplan–Meier analysis of survival in two treatment groups and MRD status. (a) OS analysis among all patients in two therapeutic groups. (b, c) OS analysis separately in patients with newly diagnosed acute myeloid leukemia (AML) and R/R AML. (d) PFS analysis among all patients in therapeutic groups. (e, f) PFS analysis separately in patients with newly diagnosed acute myeloid leukemia (AML) and R/RAML. (g) OS analysis according to MRD status in two therapeutic groups. MRD− was associated with significantly improved OS (p < 0.0001). (h, i) OS analysis in chidamide and decitabine groups, respectively. MRD− had a positive effect on OS among patients in decitabine group (p = 0.0014), but there was no significant difference in OS in chidamide group (p = 0.1128). (j) PFS analysis according to MRD status in two therapeutic groups. MRD− was associated with significantly improved PFS (p < 0.0001). (k, l) PFS analysis in chidamide and decitabine groups, respectively. Median PFS was significantly longer among patients with AML and MRD−than among those without MRD− in two groups (p = 0.0220 and p = 0.0023, respectively). Differences were compared by applying log-rank test. Statistical significance was set at p < 0.05.
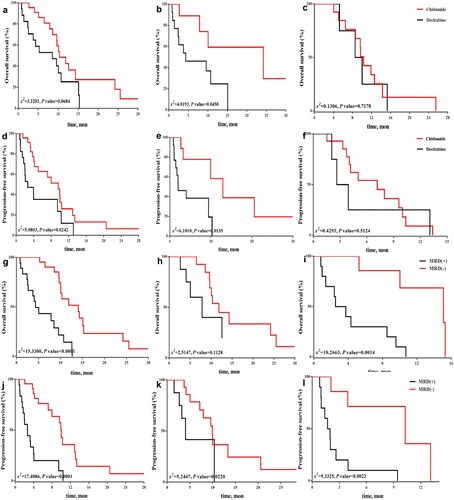
Figure 2. Overall survival (OS) and progression-free survival (PFS) analysis by acute myeloid leukemia (AML) subgroup (newly diagnosed and R/R AML) using Cox proportional-hazards model. (a, b) OS analysis in newly diagnosed and R/R AML subgroups. OS and PFS were more significantly improved by chidamide combination regimen than by decitabine combination regimen among patients with newly diagnosed AML. (c, d) PFS analysis in newly diagnosed and R/RAML subgroups. Few survival benefits were observed among patients with R/RAML in chidamide group.
In this study, the incidence of MRD− was higher in chidamide group, but no significant difference was observed (65.2% vs. 41.2%, p = 0.1308, ). We found that MRD− could significantly improve OS and PFS (both p < 0.0001, (g,j)). MRD− had a positive effect on OS among patients in decitabine group (log-rank test, p = 0.0014), but no significant difference was recorded in chidamide group (log-rank test, p = 0.1128, (h,i)). Median PFS among patients with MRD− (9.9 months in chidamide group vs. 10.3 months in decitabine group) was significantly longer than that among patients who did not achieve MRD− (p = 0.0220 and p = 0.0023, respectively, (k,l)).
Table 1. Treatment responses in two therapeutic groups.
Safety and toxicities
AEs of any grade are presented in . Hematological toxicities were most common all-cause AEs reported in both groups. Of note, grade 3 or 4 hematological AEs occurred in nearly all patients in both groups. The incidence of grade 3 or higher febrile neutropenia was slightly higher in decitabine group (64.7% vs. 47.8%). The most common grade 1 or 2 non-hematological AE was infection (including pneumonitis, sepsis, and anal mucositis). A higher incidence of sepsis was observed in chidamide group (30.4% [7/23] vs. 5.9% [1/17]); however, serious sepsis caused 1 death in decitabine group (1/17, 5.9%). However, there were no significantly differences in myelosuppression time (15.57 ± 5.36 vs.15.18 ± 5.29, p = 0.8209). The high rates of sepsis in chidamide group might be related with more frequencies of severer neutropenia (grade 3 or higher), but more were mild (grade 2 or less). Other severe (grade 3 or higher) non-hematological AEs occurred more frequently in decitabine group. Most patients tolerated treatment, and the majority of AEs were managed and cured using symptomatic and supportive treatments, such as transfusions, subcutaneous injection of granulocyte colony-stimulating factor (G-CSF), anti-inflammatory treatment, and correction of electrolyte disturbance. During one or two cycles of treatment, no patients died in chidamide group, whereas three patients (3/17, 17.6%) died in decitabine group (one case each of severe infection, tumor lysis syndrome, and possible cerebral hemorrhage).
Table 2. Adverse events in two therapeutic groups.
Discussion
Achieving remission is crucial for patients with newly diagnosed or R/R AML to increase ORR and enhance survival benefits in patients, as well as for consolidation treatments or HSCT. Epigenetic therapy has widely attracted attention for the treatment of cancers in recent years. Hypomethylating agents (HMAs) such as decitabine and azacitidine were used for elder AML or adverse risk MDS patients. Interestingly, regimens of HMA combined with DA, HAAG, CAG and FLAG have shown better effect in R/R or de novo AML in clinical trials [Citation16–18,Citation34,Citation35]. Hypomethylating agents or low dose- cytarabine combined with BCL-2 inhibitor has become promising regimen for AML patients intolerant for intensive chemotherapy, while there were also limitations in treating resistance or AML relapse [Citation36]. Chidamide has shown significant inhibition of AML cell lines and exerted synergistic effects with HMAs [Citation37,Citation38]. A phase II study on chidamide combined with decitabine and CAG in patients with relapsed/refractory acute myeloid leukemia has shown tolerated and effective outcomes [Citation39]. Chidamide also has been reported to overcome venetoclax plus azacitidine regimen-resistant AML [Citation40], and increase the sensitivity of refractory or relapsed AML cells to anthracyclines [Citation41]. Therefore, chidamide combined with HMA and chemotherapy seems to have great potential for AML patients especially resistant to conventional chemotherapy or targeted therapy.
In this study, we analyzed 18 patients with R/R AML to compare efficacy between two salvage regimens. The CR/CRi rate was 78.6% in chidamide group, which was higher than that in decitabine group, and median OS (9.7 months) and PFS (6.4 months) were also prolonged. Compared with results in decitabine group, CR/CRi rates and DOR of patients with newly diagnosed AML were significantly improved in chidamide group, and extramedullary disease disappeared in two patients. Moreover, OS and PFS among patients with newly diagnosed AML were also significantly prolonged with chidamide combination regimen. Although R/R AML patients in chidamide group had no significant improvement in OS and PFS, results in those patients displayed an increased CR/CRi rates, which provided an opportunity for those preparing for bridging HSCT. However, in this study, it is a pity that none of these patients chose to conduct HSCT due to financial reasons. During treatments, hematological toxicities were the most common AEs reported in both groups. Grade 3 or higher hematological AEs were observed in almost all patients in both groups. Non-hematological AEs, especially several infections, gastrointestinal disorders, general disorders, and metabolic disorders, occurred more frequently in decitabine group. Most AEs could be managed using symptomatic and supportive treatments. Hence, chidamide-based regimens might be regarded as effective and tolerable therapeutic protocols.
Genetic abnormalities are closely correlated with risk categories, treatment options, and prognosis in AML with evolution of whole-genome sequencing technology. Chidamide regimen could overcome karyotype adverse conditions with higher CR/CRi rates in our study, while less effective for certain genetic mutations. TP53 mutations are associated with complex karyotypes, monosomal karyotypes, and some aneuploid karyotypes, which are suggestive of unfavorable prognoses in AML [Citation42]. Patients with RUNX1-mutant AML generally have poor outcomes [Citation9]. ASXL1 mutations are more common in elderly patients with AML, and they are negatively correlated with long-term survival [Citation43]. In our study, frequently mutated genes included FLT3-ITD, DNMT3A, NPM1, CEBPA, IDH2, NRAS, TET2, KRAS, RUNX1, GATA2, and ASXL1. Patients with DNA methylation-related gene mutations (DMNT3A, IDH2, and TET2) generally achieved remission under HMA- and HDACi-based treatment. FLT3-ITD, ASXL1, RUNX1, and GATA2 gene mutations are considered poor risk factors for AML. Although some satisfactory responses were observed in patients with FLT3-ITD, AXSL1, and DNA methylation-related gene mutations in chidamide group, the combination of an HDACi and HMA exerted significant effects on patients with NRAS and KRAS gene mutations compared with the effects of HMA monotherapy. In this study, MRD− rate was higher in chidamide group than in decitabine group, which was correlated with improved PFS and OS survival. However, the efficacy of combination of a HDACi and HMA should be examined using greater numbers of clinical samples for clarification and these findings in this cohort need to be replicated in a larger one.
There were several limitations in our study: (1) no significant differences of OS and FPS for R/R AML were detected in both groups, which should be re-evaluated if samples and follow-up time are sufficient; (2) did not evaluation HDACi and HMA combination with other targeted drugs, such as BCL2 inhibitor and FLT-3 inhibitors, which could be encouraging. Further prospective or retrospective studies of targeted drug combinations will help improve outcomes and reduce toxicity for AML patients.
Conclusion
The combination of an HDACi, HMA, and chemotherapy is an effective therapy with tolerable toxicities in adults with intermediate- and adverse- risk AML in this cohort, especially for those with newly diagnosed disease. There remain challenges and opportunities for AML treatments, and further explorations to clarify mechanisms of HDACis and HMAs in AML will be critical.
Supplemental Material
Download TIFF Image (4.7 MB)Supplemental Material
Download TIFF Image (29.3 MB)Supplemental Material
Download TIFF Image (25.1 MB)Supplemental Material
Download MS Word (183 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Short NJ, Rytting ME, Cortes JE. Acute myeloid leukaemia. Lancet. 2018 Aug 18;392(10147):593–606.
- Venney D, Mohd-Sarip A, Mills KI. The impact of epigenetic modifications in myeloid malignancies. Int J Mol Sci. 2021 May 9;22(9):5013.
- Dombret H, Gardin C. An update of current treatments for adult acute myeloid leukemia. Blood. 2016 Jan 7;127(1):53–61.
- Kantarjian HM, Kadia TM, DiNardo CD, et al. Acute myeloid leukemia: treatment and research outlook for 2021 and the MD Anderson approach. Cancer. 2021 Apr 15;127(8):1186–1207.
- Pulte D, Jansen L, Brenner H. Changes in long term survival after diagnosis with common hematologic malignancies in the early 21st century. Blood Cancer J. 2020 May 13;10(5):56.
- Shallis RM, Wang R, Davidoff A, et al. Epidemiology of acute myeloid leukemia: recent progress and enduring challenges. Blood Rev. 2019 Jul;36:70–87.
- Klepin HD, Rao AV, Pardee TS. Acute myeloid leukemia and myelodysplastic syndromes in older adults. J Clin Oncol. 2014 Aug 20;32(24):2541–2552.
- Cancer Genome Atlas Research N, Ley TJ, Miller C, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013 May 30;368(22):2059–2074.
- Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016 Jun 9;374(23):2209–2221.
- Nakamura S, Yokoyama K, Shimizu E, et al. Prognostic impact of circulating tumor DNA status post-allogeneic hematopoietic stem cell transplantation in AML and MDS. Blood. 2019 Jun 20;133(25):2682–2695.
- Carbonell D, Suarez-Gonzalez J, Chicano M, et al. Next-generation sequencing improves diagnosis, prognosis and clinical management of myeloid neoplasms. Cancers. 2019 Sep 13;11(9):1364.
- Neri F, Rapelli S, Krepelova A, et al. Intragenic DNA methylation prevents spurious transcription initiation. Nature. 2017 Mar 2;543(7643):72–77.
- Shenker N, Flanagan JM. Intragenic DNA methylation: implications of this epigenetic mechanism for cancer research. Br J Cancer. 2012 Jan 17;106(2):248–253.
- Lund K, Cole JJ, VanderKraats ND, et al. DNMT inhibitors reverse a specific signature of aberrant promoter DNA methylation and associated gene silencing in AML. Genome Biol. 2014 Aug 30;15(8):406.
- Zeidan AM, Fenaux P, Gobbi M, et al. Prospective comparison of outcomes with azacitidine and decitabine in patients with AML ineligible for intensive chemotherapy. Blood. 2022 Jul 21;140(3):285–289.
- Li L, Zhang X, Yu H, et al. Low-dose hypomethylating agent decitabine in combination with aclacinomycin and cytarabine achieves a better outcome than standard FLAG chemotherapy in refractory/relapsed acute myeloid leukemia patients with poor-risk cytogenetics and mutations. Onco Targets Ther. 2018;11:6863–6870.
- Scandura JM, Roboz GJ, Moh M, et al. Phase 1 study of epigenetic priming with decitabine prior to standard induction chemotherapy for patients with AML. Blood. 2011 Aug 11;118(6):1472–1480.
- Xu Q, Li Y, Jing Y, et al. Epigenetic modifier gene mutations-positive AML patients with intermediate-risk karyotypes benefit from decitabine with CAG regimen. Int J Cancer. 2020 Mar 1;146(5):1457–1467.
- Barneda-Zahonero B, Parra M. Histone deacetylases and cancer. Mol Oncol. 2012 Dec;6(6):579–589.
- Singh AK, Bishayee A, Pandey A. Targeting histone deacetylases with natural and synthetic agents: an emerging anticancer strategy. Nutrients. 2018 Jun 6;10(6):731.
- Quintas-Cardama A, Santos FPS, Garcia-Manero G. Histone deacetylase inhibitors for the treatment of myelodysplastic syndrome and acute myeloid leukemia. Leukemia. 2011 Feb;25(2):226–235.
- Suraweera A, O'Byrne KJ, Richard DJ. Combination therapy with histone deacetylase inhibitors (HDACi) for the treatment of cancer: achieving the full therapeutic potential of HDACi. Front Oncol. 2018;8:92.
- Autin P, Blanquart C, Fradin D. Epigenetic drugs for cancer and microRNAs: a focus on histone deacetylase inhibitors. Cancers. 2019 Oct 10;11(10):1530.
- Zhao L, Lv C, Sun L, et al. Histone deacetylase inhibitor chidamide regulates the Wnt/beta-catenin pathway by MYCN/DKK3 in B-ALL. Invest New Drugs. 2021 Aug;39(4):961–970.
- Liu J, Lv N, Zhou L, et al. Chidamide inhibits t(8;21) AML cell proliferation and AMK1/ETO and C-KIT expression by inhibiting ERK1/2 signaling pathway. Transl Cancer Res. 2020 Feb;9(2):827–839.
- Zhao S, Guo J, Zhao Y, et al. Chidamide, a novel histone deacetylase inhibitor, inhibits the viability of MDS and AML cells by suppressing JAK2/STAT3 signaling. Am J Transl Res. 2016;8(7):3169–3178.
- Wouters BJ, Delwel R. Epigenetics and approaches to targeted epigenetic therapy in acute myeloid leukemia. Blood. 2016 Jan 7;127(1):42–52.
- Blagitko-Dorfs N, Schlosser P, Greve G, et al. Combination treatment of acute myeloid leukemia cells with DNMT and HDAC inhibitors: predominant synergistic gene downregulation associated with gene body demethylation. Leukemia. 2019 Apr;33(4):945–956.
- Yao Y, Zhou J, Wang L, et al. Increased PRAME-specific CTL killing of acute myeloid leukemia cells by either a novel histone deacetylase inhibitor chidamide alone or combined treatment with decitabine. PLoS One. 2013;8(8):e70522.
- Mao J, Li S, Zhao H, et al. Effects of chidamide and its combination with decitabine on proliferation and apoptosis of leukemia cell lines. Am J Transl Res. 2018;10(8):2567–2578.
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016 May 19;127(20):2391–2405.
- Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017 Jan 26;129(4):424–447.
- Tallman MS, Wang ES, Altman JK, et al. Acute myeloid leukemia, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Network. 2019 Jun 1;17(6):721–749.
- Zhu JF, Dai HP, Zhang QQ, et al. Efficacy and safety of decitabine combined with HAAG (homoharringtonine, aclarubicin, low-dose cytarabine and G-CSF) for newly diagnosed acute myeloid leukemia. Front Oncol. 2022;12:998884.
- Liu J, Liu X, Jia J, et al. Patients with AML-MRC benefit from decitabine in combination with low-dose G-CSF, cytarabine and aclarubicin: a single center cohort study. Leuk Res Rep. 2022;18:100354.
- Dhakal P, Bates M, Tomasson MH, et al. Acute myeloid leukemia resistant to venetoclax-based therapy: what does the future hold? Blood Rev. 2022 Dec 1;59:101036.
- Zhang B, Pei Z, Wang H, et al. Chidamide and decitabine in combination with a HAG priming regimen for acute myeloid leukemia with TP53 mutation. Acta Med Okayama. 2022 Feb;76(1):63–70.
- Li Z, Zhang J, Zhou M, et al. Epigenetic therapy with chidamide alone or combined with 5-azacitidine exerts antitumour effects on acute myeloid leukaemia cells in vitro. Oncol Rep. 2022 Apr;47(4):66.
- Wang L, Luo J, Chen G, et al. Chidamide, decitabine, cytarabine, aclarubicin, and granulocyte colony-stimulating factor (CDCAG) in patients with relapsed/refractory acute myeloid leukemia: a single-arm, phase 1/2 study. Clin Epigenetics. 2020 Sep 1;12(1):132.
- Wang BR, Wan CL, Liu SB, et al. A combined histone deacetylases targeting strategy to overcome venetoclax plus azacitidine regimen resistance in acute myeloid leukaemia: three case reports. Front Oncol. 2021;11:797941.
- Wang H, Liu YC, Zhu CY, et al. Chidamide increases the sensitivity of refractory or relapsed acute myeloid leukemia cells to anthracyclines via regulation of the HDAC3 -AKT-P21-CDK2 signaling pathway. J Exp Clin Cancer Res. 2020 Dec 9;39(1):278.
- Metzeler KH, Herold T, Rothenberg-Thurley M, et al. Spectrum and prognostic relevance of driver gene mutations in acute myeloid leukemia. Blood. 2016 Aug 4;128(5):686–698.
- Paschka P, Schlenk RF, Gaidzik VI, et al. ASXL1 mutations in younger adult patients with acute myeloid leukemia: a study by the German-Austrian acute myeloid leukemia study group. Haematologica. 2015 Mar;100(3):324–330.
