ABSTRACT
Objectives and Methods
This single-center retrospective study was performed to evaluate the safety and efficacy of FMS-like tyrosine kinase 3 (FLT3) inhibitors before and after allogeneic hematopoietic cell transplantation (HCT) in relapsed/refractory patients with FLT3-mutation positive acute myeloid leukemia (AML).
Results
Ten patients who met the eligibility criteria were included. Eight of them achieved hematological remission at HCT, within a median span of 79 days (range: 43–197). In post-HCT, patients started maintenance therapy (MT; median time-to-start 79 days, range: 43–197), and the median duration of MT was 390 days (range: 67–815). Grade 3 hematological adverse events (AEs) were found in two patients, and non-hematological AEs were found in five patients. Nine patients underwent either dose reduction, discontinuation of therapy, or a switch to another FLT3 inhibitor due to AEs. Disease relapse occurred in one patient during MT. At the time of the last follow-up, seven patients are alive and disease-free, while three have died due to infection or transplant complications.
Conclusion
In relapsed/refractory FLT3 mutation-positive AML, the use of FLT3 inhibitors can lead to high response rates and provide a safe bridge from HCT to MT. If sufficient attention is paid to safety, this therapy is expected to prevent disease relapse even with reduced dosages.
Background
FMS-like tyrosine kinase 3(FLT3) mutation-positive acute myeloid leukemia (AML) is an independent poor prognostic factor with high relapse and mortality rates after chemotherapy and allogeneic hematopoietic stem cell transplantation (HCT) [Citation1–4].
In relapsed/refractory FLT3 mutation-positive AML, FLT3 inhibitors such as gilteritinib and quizartinib have been shown to induce high remission rates and increase the patient bridging rate to HCT [Citation5–8] Furthermore, several studies have suggested a clinical significance to post-transplant maintenance therapy with FLT3 inhibitors including sorafenib, midostaurin, and quizartinib [Citation9–15]. Both gilteritinib and quizartinib are now approved by medical insurance companies and available for the treatment of relapsed/refractory FLT3 mutation-positive AML in Japan. However, real-world data on these inhibitors are still insufficient, particularly for patient management before and after HCT. We performed a single-center retrospective analysis to evaluate the efficacy and safety of FLT3 inhibitors, including gilteritinib and quizartinib, before and after HCT in patients with relapsed/refractory FLT3 mutation-positive AML.
Methods
Our study cohort included patients with FLT3 mutation-positive AML who were diagnosed with primary induction failure or post-remission relapse. A small number of patients who used FLT3 inhibitors in the first-line chemotherapies were excluded because the FLT3 inhibitors are approved for relapsed or refractory FLT3 mutation-positive AML in Japan. Maintenance therapy was defined as the initiation of FLT3 inhibitors at the time of hematological remission and negative Wilms’ Tumor-1 (WT1 < 50 copy/µgRNA) in the post-transplant phase. Patients who used FLT3 inhibitors during first-line chemotherapies were excluded. Adverse events that occurred after the start of FLT3 inhibitor use were evaluated according to the Common Terminology Criteria for Adverse Events version 5.0. The study was approved by the ethics committee of the Kanagawa Cancer Center (No.2020-11).
Results
10 patients with relapsed/refractory FLT3 mutation-positive AML who met the eligibility criteria were recruited between April 2018 and September 2022. Patient characteristics and treatment profiles are shown in . The clinical courses of the 10 patients are shown in . The median age was 36 (15–54) years at the time of diagnosis of relapsed/refractory FLT3 mutation-positive AML. Five patients had prior histories of HCT and used FLT3 inhibitors for their disease relapses after their previous HCTs, and the others used FLT3 inhibitors for primary induction failure. Eight patients harbored the FLT3-ITD mutation, one had the FLT3-TKD mutation, and one had the FLT3-ITD/TKD mutation. Except for one patient, who was diagnosed with AML following myelodysplastic syndrome just before HCT, all patients used FLT3 inhibitors for bridging to HCT. Four used gilteritinib, three took quizartinib, and two were on both gilteritinib and quizartinib. Grade 3 or 4 hematological adverse events (HAEs) including neutropenia or thrombocytopenia were found in three patients, along with other Grade 3 adverse events including infection, pneumonia, and hepatic disorder. At the time of HCT, complete hematological remission was obtained in eight of the patients, and negative WT1 in six. Positive WT1 was found four patients, including two who were in non-hematological remission. The median duration from the administration of FLT3 inhibitors to complete remission was 32 days (range: 25–104).
Figure 1. Clinical course of the 10 study patients before and after hematopoietic stem cell transplantation. Gilteritinib and quizartinib doses (mg/body) are indicated numerically. AEs were grade 3 or higher and were indicated by letters such as ‘SOS/VOD’. 
 (red): diagnosis;
(red): diagnosis;  : CR indicates complete remission;
: CR indicates complete remission;  : NR, non-remission;
: NR, non-remission;  (yellow): HCT, allogeneic hematopoietic cell transplantation;
(yellow): HCT, allogeneic hematopoietic cell transplantation;  (blue): gilteritinib; fx8(green): quizartinib;
(blue): gilteritinib; fx8(green): quizartinib;  (orange): AE, adverse event;
(orange): AE, adverse event; 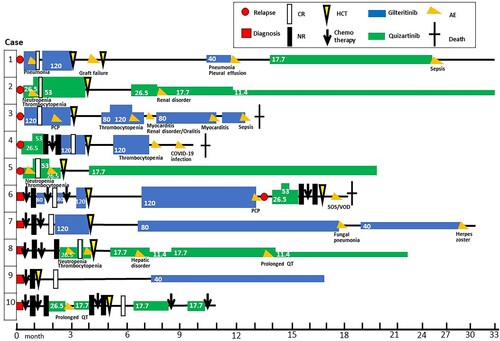
Table 1. Patient characteristics from diagnosis to transplantation.
The profiles of post-transplant maintenance therapies in our patients are shown in . The median time from HCT to initiation of FLT3 inhibitor therapy was 79 days (43–197 days). Two patients had the standard dose of gilteritinib (120 mg/day) at the start of maintenance therapy, while the other patients were started on a reduced dose. The median duration for continuing the starting dose of FLT3 inhibitors was 120 days (28–493), and the median duration of maintenance therapy was 390 days (range: 67–815). Grade 3 HAEs including neutropenia or thrombocytopenia were found in two patients. Grade 4 sepsis was found in two patients, and other non-hematological adverse events included pleural effusion, renal disorder, myocarditis with heart failure, hepatic disorder, and prolonged QT. Dose reductions of FLT3 inhibitors were done in four patients, due to adverse events including renal dysfunction, thrombocytopenia, fungal pneumonia, prolonged QT. Two patients were discontinued due to infection. Switching from gilteritinib to quizartinib was done in one patient due to pneumonia and plural effusion. Except for one patient, who developed oral graft-versus-host disease (GVHD), no exacerbation of acute or chronic GVHD was observed.
Table 2. Profile of maintenance therapy.
Disease relapse with clonal evolution occurred in one patient after 206 days of maintenance therapy. The other nine cases remained in hematological remission with negative WT1 throughout the follow-up period. At the time of writing, seven patients are alive and disease-free and two patients have stopped maintenance therapy due to both sufficient treatment duration and adverse events. Three patients have died during the maintenance therapy, either due to sepsis, pneumonia, or sinusoidal obstructive syndrome after a subsequent HCT.
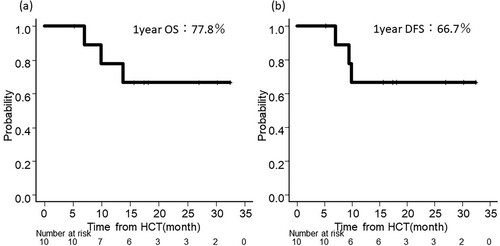
Patient outcomes in this cohort are shown in . The median survival from the date of HCT was 511 days (range: 160–987 days). The overall survival and disease-free survival rate at one-year post-HCT were 77.8% and 66.7%, respectively ((a,b)). Overall survival and disease-free survival rate at one year from the start of maintenance therapy were 64.8% and 66.7%, respectively.
Discussion
A single-center retrospective analysis was performed on 10 patients with relapsed/refractory FLT3 mutation-positive AML who were treated with FLT3 inhibitors. Although the cohort size was small and heterogeneous, the safety and efficacy of FLT3 inhibitors for bridging to HCT and post-transplant maintenance therapy were appropriately evaluated in detail. These data may be useful for future clinical management of patients with relapsed/refractory FLT3 mutation-positive AML.
In the pre-transplant treatment, this study supported the data of previous studies. The use of FLT3 inhibitors before HCT has been reported to be safe and effective for bridging to HCT [Citation6,Citation8,Citation14,Citation15]. Post hoc analyses of the QuANTUM-R and ADMIRAL trials, which examined the efficacy and safety of gilteritinib and quizartinib before and after allogeneic transplantation in relapsed and refractory FLT3 mutation-positive AML, showed higher CR rates and better survival rates in the FLT3 inhibitor monotherapy arm, demonstrating the efficacy and safety of FLT3 inhibitors as bridge to HCT and maintenance therapy after HCT [Citation14,Citation15].
Despite relapsed/refractory FLT3 mutation-positive AML, the hematological remission rate is high when FLT3 inhibitor therapy is used, and the therapy is expected to obtain a negative WT1 result as well. Therefore, it follows that hematological remission with negative WT1 is one of the treatment goals at time of HCT. Since the median time to achieve hematological remission with FLT3 inhibitors was 32 days, it may be possible to coordinate HCT within a few months. Often, in relapsed/refractory AML, it is difficult to maintain disease remission. Because of this, in our center, umbilical cord blood has been largely selected for its fast and simple donor coordination. In the selection of a conditioning regimen, with an emphasis on safety, a reduced conditioning regimen was used even for younger patients or those who were not in remission.
In terms of the safety for bridging therapy with FLT3 inhibitors, myelosuppression and other severe adverse events were found, particularly for the induction therapy. Along with the disease activity, careful attention should be paid to adverse events during induction therapy. For the management of these adverse events, dose reduction or cessation of FLT3 inhibitors is reasonable and should be considered. Nevertheless, most patients can receive FLT3 inhibitor monotherapies as outpatients. This type of therapy provides good disease control and performance status and may have both mental and physical advantages over intensive chemotherapy.
There are currently no definite criteria to start maintenance therapy after HCT. In AML, MRD monitoring by flow cytometry is not commercially available in Japan. Instead, WT1 monitoring is used as an indicator of MRD. Therapeutic intervention in patients with positive WT1 is defined as preemptive therapy, clearly differentiated from maintenance therapy, which is a therapeutic intervention in patients with negative MRD [Citation16]. Meanwhile, several significant criteria for initiating FLT3 inhibitors include transfusion independence, adequate hematopoietic recovery, good performance, and absence of active GVHD or severe organ damage. As for the timing and the duration of the maintenance therapy, no consensus has been reached so far. According to a review summarizing post-transplant maintenance therapy with FLT3 inhibitors, several post-transplant maintenance trials with sorafenib, midostaurin, and gilteritinib started maintenance therapy between 28 and 100 days post-transplant, and continued for periods from four months up to 24 months [Citation17–21]. According to the previous studies, the maximum duration of maintenance therapy was set at two years [Citation17–21]. Therefore, a sufficient treatment duration period of 2 years is considered reasonable. In this study, the median time from HCT to starting maintenance therapy, and duration of maintenance therapy, were 79 and 390 days, respectively. A few patients were delayed in starting treatment because of transplant-related complications and terminated the treatment early because of disease relapse or sufficient treatment duration [Citation17–21].
Significant findings were obtained regarding the safety of maintenance therapy. According to the previous reports, the continuation rate due to adverse events of an FLT3 inhibitor has been a clinical issue. In the SORMAIN trial, 48.8% of patients required dose reduction and 22% required discontinuation of FLT3 inhibitors due to adverse events [Citation13]. Similarly, 63% of patients required dose reduction in the Radius trial [Citation9]. This profile is similar to the results of a meta-analysis of post-transplant FLT3 inhibitor maintenance therapy [Citation20,Citation21]. Unlike chemotherapy, allografted patients have reduced organ capacity and performance. For safety reasons, most patients used reduced doses of FLT3 inhibitors, and only a few used the standard dose. Although high-grade AEs may have been reasonably avoided, even with a reduced dose, several adverse events including organ disorders and hematological toxicity occurred. Therefore, several patients required further dose reduction or discontinuation of FLT3 inhibitors. Nevertheless, long-term administration of the FLT3 inhibitors, with dose reduction and drug withdrawal, may ensure safety and efficacy.
Meanwhile, this profile of adverse events differs from those of bridging therapy to HCT, and attention should be paid to infections and organ damage rather than to myelosuppression. FLT3 inhibitor maintenance therapy seems to have little effect on the exacerbation of GVHD. It is important to note that three patients died due to transplant-related mortality in this study. One of the causes of death was sepsis during gilteritinib treatment. Although our patients suffered several different complications, particular attention should be paid to infection.
Surprisingly, despite the dose reduction of the FLT3 inhibitors, disease relapse was found in only one of our patients. Recently, it has been reported that gilteritinib enhances graft-versus-leukemia effects via IL-15, and has a therapeutic effect in post-transplant maintenance therapy without exacerbating GVHD [Citation22]. According to previous studies, post-transplant relapse rates ranged from 30 to 63% without maintenance therapy [Citation23–26]. Compared to these outcomes, despite the reduction or discontinuation of FLT3 inhibitors, the relapse rate in this study was relatively low. In our center, WT1 is used as a monitoring of measurable residual disease. The high remission rate with negative WT1 at HCT and negative WT1 at the start of post-transplant maintenance therapy may have therefore contributed to the low relapse rate. Between bridging therapy to post-transplant therapy, monitoring of measurable residual disease may be significant to prevent relapse in FLT3-ITD mutated AML [Citation27].
Our data showed promising outcomes for overall survival and disease-free survival rates among a high-risk cohort of relapse/refractory AML, although this study did lack an adequate number of patients and follow-up period. Furthermore, since allografted patients, even younger ones, or those with reduced conditioning regimens or who are on low doses of FLT3 inhibitors, have reduced immune and organ capacities, careful attention should be paid to patient safety when conducting maintenance therapy. Given the low recurrence rate and high transplant-related mortality, a reduced dose of FLT3 inhibitors is recommended from the start of maintenance therapy. If an adverse event occurs, promptly consider reducing reduction or discontinuation. In monitoring, it is important to focus on symptoms and perform blood tests and electrocardiograms as appropriate.
In conclusion, in cases of relapsed and refractory FLT3 mutation-positive AML, the use of FLT3 inhibitors can lead to high response rates and provide a safe bridge to HCT. In post-transplant maintenance therapy, if sufficient attention is devoted to safety, this therapy is expected to prevent disease relapse even in reduced doses.
Acknowledgements
The authors would like to acknowledge and thank all the physicians and medical staff of the institutions who treated, consulted, and retreated the patients in this cohort before and after their treatments.
Disclosure statement
TT reports honoraria from Pfizer, Otsuka, MSD, Chugai, and Astellas, outside the submitted work. HN reports honoraria from Novartis and Daiichi-Sankyo, scholarships from Daiichi-Sankyo, Cellgene, Chugai, Nihon-Shinyaku, Astellas, Asahikasei-pharma, Chugai, Takeda, Pfizer, and Eisai outside the submitted work.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Gale RE, Hills R, Kottaridis PD, et al. No evidence that FLT3 status should be considered as an indicator for transplantation in acute myeloid leukemia (AML): an analysis of 1135 patients, excluding acute promyelocytic leukemia, from the UK MRC AML10 and 12 trials. Blood. 2005;106(10):3658–3654.
- Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99(12):4326–4335.
- Fröhling S, Schlenk RF, Breitruck J, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100(13):4372–4380.
- Schmid C, Labopin M, Socié G, et al. Outcome of patients with distinct molecular genotypes and cytogenetically normal AML after allogeneic transplantation. Blood. 2015;126(17):2062–2069.
- Perl AE, Altman JK, Cortes JE, et al. Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: a multicentre, first-in-human, open-label, phase 1-2 study. Lancet Oncol. 2017;18(8):1061–1075.
- Perl AE, Martinelli G, Cortes JE, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. 2019;381(18):1728–1740.
- Cortes JE, Perl AE, Döhner H, et al. Quizartinib, an FLT3 inhibitor, as monotherapy in patients with relapsed or refractory acute myeloid leukaemia: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2018;19(7):889–903.
- Cortes JE, Khaled S, Martinelli G, et al. Quizartinib versus salvage chemotherapy in relapsed or refractory FLT3-ITD acute myeloid leukaemia (QuANTUM-R): a multicentre, randomised, controlled, open-label, phase 3 trial. The Lancet Oncol. 2019;20(7):984–997.
- Maziarz RT, Patnaik MM, Scott BL, et al. Radius: a Phase 2 randomized trial investigating standard of care ± midostaurin after allogeneic stem cell transplant in FLT3-ITD-mutated AML. Blood. 2018;132:662.
- Maziarz RT, Levis M, Patnaik MM, et al. Midostaurin after allogeneic stem cell transplant in patients with FLT3-internal tandem duplication-positive acute myeloid leukemia. Bone Marrow Transplant. 2021;56(5):1180–1189.
- Xuan L, Wang Y, Huang F, et al. Sorafenib maintenance in patients with FLT3-ITD acute myeloid leukaemia undergoing allogeneic haematopoietic stem-cell transplantation: an open-label, multicentre, randomised phase 3 trial. Lancet Oncol. 2020;21(9):1201–1212.
- Xuan L, Wang Y, Huang F, et al. Effect of sorafenib on the outcomes of patients with FLT3-ITD acute myeloid leukemia undergoing allogeneic hematopoietic stem cell transplantation. Cancer. 2018;124(9):1954–1963.
- Burchert A, Bug G, Fritz FV, et al. Sorafenib maintenance after allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia with FLT3-internal tandem duplication mutation (SORMAIN). J Clin Oncol. 2020;38(26):2993–3002.
- Ganguly S, Cortes JE, Krämer A, et al. Clinical outcomes in patients with FLT3-ITD-mutated relapsed/refractory acute myelogenous leukemia undergoing hematopoietic stem cell transplantation after quizartinib or salvage chemotherapy in the QuANTUM-R trial. Transplant Cell Ther. 2021;27(2):153–162.
- Perl AE, Larson RA, Podoltsev NA, et al. Outcomes in patients with FLT3-mutated relapsed/ refractory acute myelogenous leukemia who underwent transplantation in the Phase 3 ADMIRAL trial of Gilteritinib versus salvage chemotherapy. Transplant Cell Ther. 2023;29(4):265.e1–265.e.10.
- Schroeder T, Rautenberg C, Haas R, et al. Hypomethylating agents for treatment and prevention of relapse after allogeneic blood stem cell transplantation. Int J Hematol. 2018;107(2):138–150.
- Xuan L, Liu Q. Maintenance therapy in acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation. J Hematol Oncol. 2021;14(1):4.
- Burchet A. Maintenance therapy for FLT3-ITD-mutated acute myeloid leukemia. Haematologica. 2021;106(3):664–670.
- Blackmon A, Aldoss I, Ball BJ. FLT3 inhibitors as maintenance therapy after allogenic stem-cell transplantation. Blood Lymphat Cancer. 2022;12:137–147.
- Gagelmann N, Wolschke C, Klyuchnikov E, et al. TKI maintenance after stem-cell transplantation for FLT3-ITD positive acute myeloid leukemia: a systematic review and meta-analysis. Front Immunol. 2021;12:630429.
- Bewersdorf JP, Allen C, Mirza1 AS, et al. Hypomethylating agents and FLT3 inhibitors as maintenance treatment for acute myeloid leukemia and myelodysplastic syndrome after allogeneic hematopoietic stem cell transplantation – a systematic review and meta-analysis. Transplantation and Cellular Therapy. 2021;27:997.e1–997.e11.
- Zhang Z, Hasegawa Y, Hashimoto D, et al. Gilteritinib enhances graft-versus-leukemia effects against FLT3-ITD mutant leukemia after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2022;57(5):775–780.
- Brunet S, Labopin M, Esteve J, et al. Impact of FLT3 internal tandem duplication on the outcome of related and unrelated hematopoietic transplantation for adult acute myeloid leukemia in first remission: a retrospective analysis. J Clin Oncol. 2012;30(7):735–741.
- Schiller GJ, Tuttle P, Desai P. Allogeneic hematopoietic stem cell transplantation in FLT3-ITD-positive acute myelogenous leukemia: the role for FLT3 tyrosine kinase inhibitors post-transplantation. Biol Blood Marrow Transplant. 2016;22(6):982–990.
- Song Y, Magenau J, Li Y, et al. FLT3 mutational status is an independent risk factor for adverse outcomes after allogeneic transplantation in AML. Bone Marrow Transplant. 2016;51(4):511–520.
- Bazarbachi A, Bug G, Baron F, et al. Clinical practice recommendation on hematopoietic stem cell transplantation for acute myeloid leukemia patients with FLT3-internal tandem duplication: a position statement from the acute leukemia working party of the European society for blood and marrow transplantation. Haematologica. 2020;105(6):1507–1516.
- Liang EC, Chen C, Lu R, et al. Measurable residual disease status and FLT3 inhibitor therapy in patients with FLT3-ITD mutated AML following allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2021;56(12):3091–3093.