ABSTRACT
Background:
Although multiple myeloma is still incurable, an abundance of novel treatments have become available for relapsed and or refractory multiple myeloma (RRMM). Direct head-to-head comparisons between the novel treatments are lacking. We performed a network meta-analysis to evaluate immediate effects such as response quality of current novel-drugs combined therapeutic regimens, with the aim to identify treatments that could be more effective than others in RRMM.
Methods:
We searched Cochrane Library, PubMed, Embase, and Web of Science for randomized controlled clinical trials receiving novel-drugs combined treatments as means of interventions. The primary endpoint was objective response rates (ORRs). We used the surface under the cumulative ranking curve (SUCRA) to sequence treatments. Totally, 22 randomized controlled trials were identified for final evaluation. With the aim to include all regimens within one network analysis, we divided the treatment schemes into 13 categories according to the use of novel drugs.
Results:
Carfilzomib-, daratumumab-, and isatuximab-based treatments had better ORRs than bortezomib combined dexamethasone and lenalidomide combined dexamethasone. Daratumumab- and isatuximab-based treatments had better ORRs than pomalidomide combined dexamethasone. According to the SUCRA, daratumumab- and isatuximab-based triple-drug regimens had higher probabilities of achieving better ORRs, followed by carfilzomib, elotuzumab, venetoclax, selinexor, ixazomib, vorinostat, pomalidomide, panobinostat, lenalidomide.
Conclusions:
Our network meta-analysis performed a complete review of the ORRs of all current available novel-drugs based regimens for RRMM. By using the clinical data all from randomized controlled studies, daratumumab- and isatuximab-based treatments were identified to be the best treatments receiving better response quality.
Introduction
With the advent of thalidomide and bortezomib ending the era in which melphalan combined with dexamethasone was thought to be the standard treatment, the survival outcomes of multiple myeloma (MM) have been improved considerably[Citation1]. However, MM is still incurable, almost all patients would inevitably have disease progress or relapse[Citation2]. Fortunately, since 2005, a large number of novel agents have emerged for patients with RRMM, gving them more options. Currently, besides thalidomide and bortezomib, another 11 novel drugs such as lenalidomide[Citation3, Citation4], pomalidomide[Citation5, Citation6], carfilzomib[Citation7, Citation8], ixazomib[Citation9, Citation10], vorinostat[Citation11], panobinostat[Citation12], elotuzumab[Citation13–15], daratumumab[Citation16–20], isatuximab[Citation21–23], selinexor[Citation24], and venetoclax[Citation25] have become available for RRMM.
Due to no direct head-to-head comparisons between the novel agents, it is not known which novel drug combination regimen is superior for RRMM. To resolve this problem, we overviewed and synthesized all information about current available novel-drugs for RRMM from randomized controlled trials (RCTs). And then we conducted a network meta-analysis to assess the objective response rate (ORR) of each treatment.
Materials and methods
Search strategy
We searched four databases including the Cochrane Library, PubMed, Embase, and Web of Science for identifying eligible clinical trials from inception date to 15 June 2022. We used a combination of mesh terms and free words when reviewing the electronic databases. Considering that the term ‘combined therapeutic regimens’ is a too broad concept, we defined the treatments as containing at least one of the following drugs: lenalidomide, pomalidomide, carfilzomib, ixazomib, vorinostat, panobinostat, elotuzumab, daratumumab, isatuximab, selinexor and venetoclax. Then, we conducted the search with the following mesh terms: (‘lenalidomide’ or ‘pomalidomide’ or ‘carfilzomib’ or ‘ixazomib’ or vorinostat’ or ‘panobinostat’ or ‘elotuzumab’ or ‘daratumumab’ or ‘isatuximab’ or ‘selinexor’ or ‘venetoclax’) and (‘multiple myeloma’) and (‘randomized controlled trial’). In addition, it should be noted that KEYNOTE-183 showed the benefit–risk profile of pembrolizumab plus pomalidomide and dexamethasone is unfavorable for patients with RRMM and the trial was called off due to too much toxic and side effects[Citation26], so we didn’t consider pembrolizumab-based treatments for analysis. This meta-analysis was in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline.
Inclusion criteria
Studies were screened for the following inclusion criteria: (1) The studies were described as RCTs among adult patients with RRMM. (2) The regimens of the RCTs had to contain at least one of the prespecified novel agents as mentioned above. (3) The trials had outcome indicators available.
Exclusion criteria
Studies were ruled out for the following exclusion criteria: (1) Studies published in non-English, review, non-RCT, and meta-analysis. (2) The intervention in RCTs did not include the prespecified novel regimens. (3) Patients in a RCT were neither newly diagnosed MM nor untreated. (4) The trials had no outcome indicators.
Outcome assessment
The efficacy of novel-drugs combined therapeutic regimens was evaluated by the treatment response rate, which was defined as overall ORR. The ORR comprised partial, very good partial, complete, and stringent complete responses in accordance with the International Myeloma Working Group (IMWG) criteria[Citation27].
Data extraction
We imported all identified articles into Endnote X9. Firstly, duplicated articles were removed. Secondly, these studies underwent preliminary screening through reading titles and abstracts by two reviewers independently. Studies which met the prespecified inclusion criteria were selected in the second screening. After that, the full-text articles were further reviewed according to the inclusion criteria. If there were disputes in the process of literature screening, the third senior investigator could be consulted to discuss together and make a final decision.
The detail information of all studies, such as author names, the date of publication, the number of RCTs, the intervention treatment, the control treatment, the prior lines of regimens, the age of patients, response rates, and survival outcomes were collected and recorded.
Risk of bias assessment
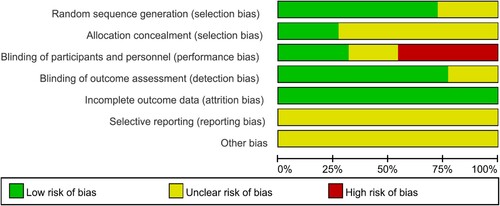
Two independent investigators performed a quality assessment for each RCT by software of the Review Manager 5.4 based on the Cochrane risk-of-bias assessment tool. Seven perspectives were considered as following: (1) random sequence generation, (2) allocation concealment, (3) blinding of participants and subjects, (4) incomplete results data, (5) selective report, (6) blinding of results assessment, and (7) other bias.
Data analysis
We conducted a network meta-analysis with Stata 15.1 software which under a frequency-based random-effects model. Data processing, network map, and network forest plots were conducted. Therapeutic strategies were ranked for each method by the SUCRA probabilities, and a lower SUCRA value indicates a lower chance of being the best treatment. We calculated the values of SUCRA to rank the treatments. We calculated odds ratios (OR) to compare the efficacy of one intervention versus another by the method of netleague. We also performed subgroup meta-analysis in patients with high-risk cytogenetic abnormalities using Hazard Ratios (HR) as the effect size. The I2 statistic was used as a criterion to assess the presence of heterogeneity between studies, and more than 50% was considered to have significant heterogeneity. A p-value less than 0.05 was considered statistically significant. We evaluated publication bias among researches contributing to primary conclusions by viewing the funnel diagram.
Results
Characteristics of the included studies
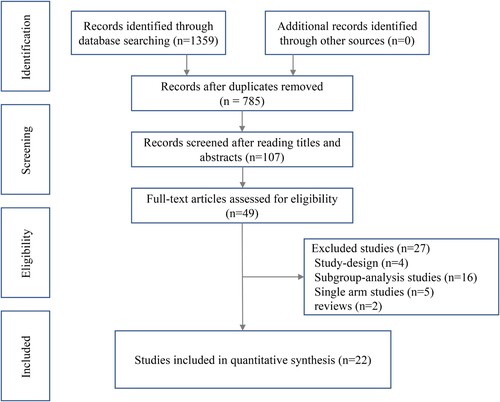
Totally, 1359 citations were retrieved from the databases. After removing duplicates, 785 citations were screened by reading titles and abstracts, and 687 were excluded from further analysis. In the second phase, 107 full texts were screened, of which 58 were excluded. A total of 49 citations were included for further full-text review. Ultimately, 22 citations were identified for final network meta-analysis. These citations contained 22 RCTs, including 11 kinds of novel-drugs combined therapeutic regimens. exhibits the PRISMA flowchart.
Among 22 RCTs, there were 13 kinds of therapeutic regimens according to the use of novel drugs: (1) dexamethasone (Dex), (2) bortezomib (Bor): bortezomib ± dexamethasone, (3) lenalidomide (Len): lenalidomide + dexamethasone, (4) pomalidomide (Pom): pomalidomide + dexamethasone, pomalidomide + bortezomib + dexamethasone, (5) carfilzomib (Car): carfilzomib + dexamethasone, carfilzomib + lenalidomide + dexamethasone, (6) ixazomib (Ixa): ixazomib + lenalidomide + dexamethasone, (7) vorinostat (Vorino): vorinostat + bortezomib, (8) panobinostat (Pano): panobinostat + bortezomib + dexamethasone, (9) elotuzumab (Elo): elotuzumab + lenalidomide + dexamethasone, elotuzumab + pomalidomide + dexamethasone, elotuzumab + bortezomib + dexamethasone, (10) daratumumab (Dara): daratumumab + bortezomib + dexamethasone, daratumumab + carfilzomib + dexamethasone, daratumumab + lenalidomide + dexamethasone, daratumumab + pomalidomide + dexamethasone, (11) isatuximab (Isa): isatuximab + carfilzomib + dexamethasone, isatuximab + pomalidomide + dexamethasone, (12) selinexor (Sel): selinexor + bortezomib + dexamethasone, (13) venetoclax (Ven): venetoclax + bortezomib + dexamethasone.
shows the summary features of the included RCTs. The median age ranged from 61 to 69 years, and patients received one to eleven prior lines of treatments. A total of 9932 patients were reported in these clinical trials.
Table 1. Characteristics of the included studies.
Four trials received immunomodulator-based treatment (Len or Pom), four received proteasome inhibitor–based treatment (Car or Ixa), ten received monoclonal antibody–based treatment (Elo, Dara, or Isa), two used histone deacetylase inhibitors–based treatments (Pano or Vorino), one received exportin-1 (XPO1) inhibitor-based treatments (Sel), and one received BCL-2 inhibitor-based treatment (Ven).
Network meta-analysis
Evidence network
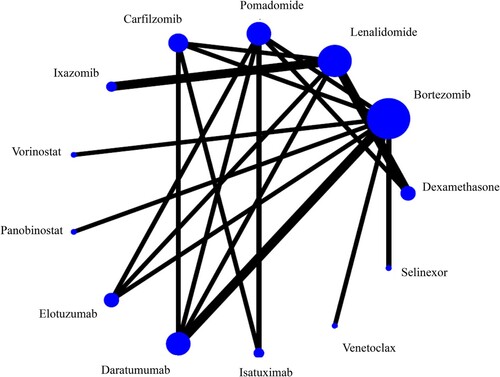
A total of 22 studies reported the overall ORRs, including 11 kinds of novel-drug combined treatments and 2 kinds of relative traditional treatments (Dex and Bor). The size of the blue dot represents the number of patients using the corresponding treatment regimen. The thickness of the black line between two dots indicates the number of clinical trials. There are eleven closed-loops. The network of the ORRs is showed in .
Publication bias
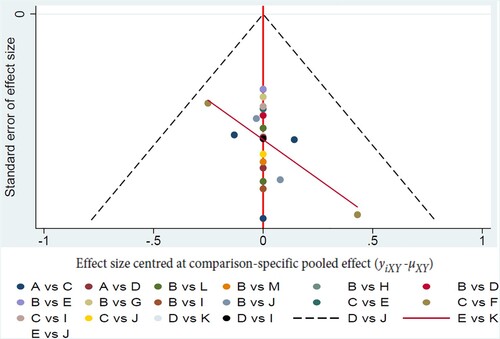
We performed funnel plots to assess the publication bias. The funnel plot of our study exhibited that most of the scatter points were concentrated in the middle line and no points fell outside the dotted line. The distribution of these points was basically symmetrical indicating very low degree of publication bias. The funnel plot of the ORRs of 13 kinds of treatments is presented in .
Network meta-analysis
A total of 22 pieces of literature reported ORRs involving 13 kinds of treatments, 11 of which were novel-drug combined treatments. The network comparison of ORRs was performed in 22 studies, yielding 79 pairwise comparisons, 20 of which were statistically significant. Compared with Dex, Bor (OR: 0.22, 95%CI: 0.09–0.56), len (OR: 0.22, 95%CI: 0.11–0.44), Pom (OR: 0.15, 95%CI: 0.07–0.23), Car (OR: 0.09, 95%CI: 0.04–0.23), Ixa (OR: 0.12, 95%CI: 0.04–0.34), Vorino (OR: 0.12, 95%CI: 0.03–0.47), Pano (OR: 0.17, 95%CI: 0.04–0.69), Elo (OR: 0.11, 95%CI: 0.04–0.27), Dara (OR: 0.06, 95%CI: 0.03–0.16), Isa (OR: 0.06, 95%CI: 0.02–0.19), Sel (OR: 0.11, 95%CI: 0.03–0.47), and Ven (OR: 0.11, 95%CI: 0.02–0.45) all had higher ORRs. Car (OR: 0.41, 95%CI: 0.21–0.82), Dara (OR: 0.29, 95%CI: 0.16–0.53), and Isa (OR: 0.27, 95%CI: 0.10–0.72) had better ORRs than Bor. Dara (OR: 0.29, 95%CI: 0.14–0.59), Isa (OR: 0.27, 95%CI: 0.10–0.76), and Car (OR: 0.41, 95%CI: 0.20–0.86) had better ORRs than Len. Dara (OR: 0.43, 95%CI: 0.22–0.85), and isa (OR: 0.40, 95%CI: 0.17–0.96) had better ORRs than Pom. The league table of comparisons between different treatments was showed in .
Table 2. Network meta-analysis comparisons for overall response rate.
Sucra probability ranking
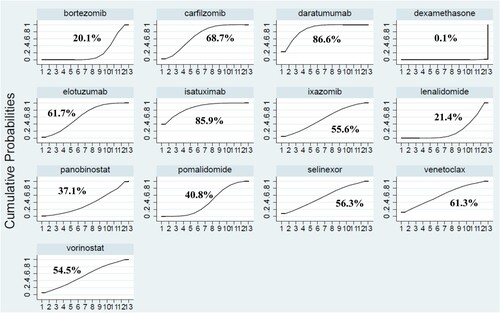
According to the area under the curve diagram of SUCRA, the ORRs of the 13 kinds of treatments were ranked from best to worst as follows: Dara (86.6%), Isa (85.9%), Car (68.7%), Elo (61.7%), Ven (61.3%), Sel (56.3%), Ixa (55.6%), Vorino (54.5%), Pom (40.8%), Pano (37.1%), Len (21.4%), Bor (20.1%), and Dex (0.1%). The results were showed in .
Comparing PFS HR results among patients with high-risk cytogenetic abnormalities
The ORR data for MM patients with high-risk cytogenetic abnormalities (HRCA) were missing from the majority of the included clinical studies. HRCA is defined as having t(4;14), t(4;16), or del(17p), and some clinical trials will also include those with 1q amplification as HRCA. However, 13 of these studies included HR values for PFS in patients with HRCA. Therefore, we did a comparison of PFS by HR in the clinical studies that contained HR values. According to the regimens of the control group, we did a 4-group meta-analysis. In all four groups of meta-analysis, the I2 statistic was <50% and the p-value was >0.05, indicating no significant heterogeneity. The risk of progression or death was reduced by 42%, 42% and 39% with CarDex/PanoBorDex/SelBorDex/DaraBorDex versus BorDex(HR, 0.58; 95% CI, 0.45–0.75; P<0.05), DaraCarDex/IsaCarDex versus CarDex (HR, 0.58; 95% CI, 0.37–0.93; P<0.05), CarLenDex/DareLenDex/EloLenDex versus LenDex (HR, 0.61; 95% CI, 0.42–0.87; P<0.05), respectively. And the HR for DaraPomDex/IsaPomDex/EloPomDex versus PomDex was 0.55 (95% CI, 0.28–1.1; P>0.05). The results were showed in .
Table 3. Subgroup meta-analysis based on HR for PFS between the intervention and control groups.
Discussion
Comprehensive evidence synthesis is increasingly demanded in RRMM, since a large number of novel agents-based combined regimens become available over the last 15 years. However, no final conclusion has been reached as to which novel drugs-based combination treatment has the best effect. Traditional meta-analysis has certain limitations due to only a two-by-two comparison, which could not provide valid methodological support for optimal selections from multiple interventions for the treatment of RRMM. In contrast, the network meta-analysis provides information for comparisons between multiple interventions. Therefore, our study is to compare the efficacy of different novel drugs-based combined treatments for RRMM, by using a network meta-analysis according to a frequency-based framework. The aim was to synthesize all current efficacy evidence from RCTs, enabling a comparison of all current treatments and providing more credible medical basis for the clinical treatment decisions of RRMM. We combined evidence from 22 phase II/III RCTs including 13 categories of treatments within one network analysis. Test of heterogeneity was not investigated due to the limited number of studies supporting each line in the network. Our study provides crucial information for choice of treatment decisions in patients with RRMM.
We used ORR as the primary end point, because it is simple to calculate included in almost all clinical trials and the fact that only better response has the opportunity to get longer progress free or overall survivals. According to the probability of ORR, the efficacy ranking sequence was Dara > Isa > Car > Elo > Ven > Sel > Ixa > Vorino > Pom > Pano > Len > Bor > Dex. The results indicated that daratumumab and isatuximab-based triple combination therapies ranked first regarding the effective response rate and also had the advantages of PFS in patients with HRCA. Under normal conditions, lymphocytes, myeloid cells, and other non-hematopoietic cells express relatively low levels of CD38, but almost all malignant plasma cells and myeloma cells express high levels of CD38[Citation28]. Both daratumumab and isatuximab are CD38 targeted monoclonal antibodies which have a unique dual mechanism of antitumor activity manifesting as immune-mediated actions, including antibody-dependent cell-mediated cytotoxicity, antibody-dependent cellular phagocytosis and complement-dependent cytotoxicity, and immunomodulatory functions which target and deplete CD38-positive regulator immune suppressor cells, leading to increase CD8-positive cytotoxic T cells and CD4-positive helper T cells in patients who have a response[Citation29, Citation30]. The regimen of CD38 targeted monoclonal antibody combined with proteasome inhibitors (bortezomib and carfilzomib) or immunomodulators (lenalidomide and pomalidomide) plus dexamethasone demonstrated very satisfactory clinical results in patients with RRMM[Citation29, Citation30]. In our study, carfilzomib ranked behind CD38 targeted monoclonal antibody, which is a new generation of proteasome inhibitors having more selective and robust ability to bind with proteasome subunits than bortezomib and ixazomib, resulting in potent, durable and precise anti-myeloma cell action and lower incidence rate of peripheral neuropathy[Citation31]. Carfilzomib in combination with dexamethasone plus lenalidomide or not also was a good option for patients with RRMM. In the meta-analysis, Venetoclax and selinexor had relatively high SUCRA scores of 61.3% and 56.3%, respectively. Venetoclax is an oral BCL-2 inhibitor, which has ability to induce apoptosis in myeloma cells. Venetoclax plus bortezomib and dexamethasone has exhibited impressive clinical efficacy with acceptable safety and tolerability in clinical trials. Studies have reported that venetoclax is more suitable for myeloma patients with t(11;14)[Citation32]. Selinexor is also an orally administered drug with selective and potent ability to inhibit XPO1 and suppress NF-κB signal and prevent the translation of oncoprotein mRNAs, resulting in anti-tumor cell effect[Citation33, Citation34]. Selinexor based regimens had shown encouraging clinical efficacy in heavily pretreated patients with RRMM. In the future, patients with multiple lines of relapsed myeloma may choose to be treated with a combination of drugs with different mechanisms. Then, we did a meta-analysis in patients with HRCA.
There were some limitations in our study as follows: Firstly, to connect all treatments options together into a single network model, we assumed that Bor and BorDex conferred the same efficacy as Professor Christy H. Y’s work [Citation35], and meanwhile we classified treatments based on the use of novel drugs. Secondly, we did not consider the impacts of drug dosage schemes and administration method on therapeutic efficacy. Thirdly, we only used the ORRs as markers for efficacy evaluation, as we mentioned before that the ORRs were included in all clinical trials and progress free survival or over survival were not always available in all trials. Fourthly, we did not consider the adverse-event profiles of novel agents’ combination therapies and patient's tolerance to drugs. Also, the quality of life in patients with RRMM was not taken into consideration. As similar to clinical trials, our study applied to the average patient with RRMM, treatments option still should be depended on individual patient characteristics and physical status. Fifthly, the BCMA-targeted immunotherapy, including antibody–drug conjugates, chimeric antigen receptor-T cells, and bispecific T cell engagers [Citation36], has achieved amazing clinical response in patients with RRMM. However, because there are not yet sufficient RCT data for this class of immunotherapy, it was not included in our network meta-analysis.
Despite the above limitations in our study, our results still could provide evidence-based decisions making for treating patients with RRMM. However, we underline that it is important to conduct phase III RCTs to obtain more accurate evidence of head-to-head comparisons among different treatments. Until such proof becomes available, clinicians could make better decisions for patients with RRMM according to the evidence provided in our results.
Acknowledgements
The authors would like to thank Dr. Liu for his guidance in writing our paper and statistical methods. Author contributions: Haimin Chen and Haiqi Chen contributed to the concept and design of the study. Yan Zhou, Weiwei Xu, Jingjing Yu and Yao Xu collected and assembled the data. Haimin Chen and Haiqi Chen performed the data analysis and interpretation. All authors participated in drafting the paper and approved the final version.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111(5):2516–2520. doi:10.1182/blood-2007-10-116129
- Joseph NS, Kaufman JL, Dhodapkar MV, et al. Long-Term follow-Up results of lenalidomide, bortezomib, and dexamethasone induction therapy and risk-adapted maintenance approach in newly diagnosed multiple myeloma. J Clin Oncol. 2020;38(17):1928–1937. doi:10.1200/JCO.19.02515
- Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357(21):2123–2132. doi:10.1056/NEJMoa070594
- Weber DM, Chen C, Niesvizky R, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357(21):2133–2142. doi:10.1056/NEJMoa070596
- Miguel JS, Weisel K, Moreau P, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(11):1055–1066. doi:10.1016/S1470-2045(13)70380-2
- Richardson PG, Oriol A, Beksac M, et al. Pomalidomide, bortezomib, and dexamethasone for patients with relapsed or refractory multiple myeloma previously treated with lenalidomide (OPTIMISMM): a randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(6):781–794. doi:10.1016/S1470-2045(19)30152-4
- Dimopoulos MA, Moreau P, Palumbo A, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17(1):27–38. doi:10.1016/S1470-2045(15)00464-7
- Stewart AK, Rajkumar SV, Dimopoulos MA, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372(2):142–152. doi:10.1056/NEJMoa1411321
- Moreau P, Masszi T, Grzasko N, et al. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;374(17):1621–1634. doi:10.1056/NEJMoa1516282
- Hou J, Jin J, Xu Y, et al. Randomized, double-blind, placebo-controlled phase III study of ixazomib plus lenalidomide-dexamethasone in patients with relapsed/refractory multiple myeloma: China continuation study. J Hematol Oncol. 2017;10(1):137. doi:10.1186/s13045-017-0501-4
- Dimopoulos M, Siegel DS, Lonial S, et al. Vorinostat or placebo in combination with bortezomib in patients with multiple myeloma (VANTAGE 088): a multicentre, randomised, double-blind study. Lancet Oncol. 2013;14(11):1129–1140. doi:10.1016/S1470-2045(13)70398-X
- San-Miguel JF, Hungria VT, Yoon SS, et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014;15(11):1195–1206. doi:10.1016/S1470-2045(14)70440-1
- Dimopoulos MA, Lonial S, White D, et al. Elotuzumab, lenalidomide, and dexamethasone in RRMM: final overall survival results from the phase 3 randomized ELOQUENT-2 study. Blood Cancer J. 2020 Sep 4;10(9):91.
- Jakubowiak A, Offidani M, Pegourie B, et al. Randomized phase 2 study: elotuzumab plus bortezomib/dexamethasone vs bortezomib/dexamethasone for relapsed/refractory MM. Blood. 2016;127(23):2833–2840. doi:10.1182/blood-2016-01-694604
- Dimopoulos MA, Dytfeld D, Grosicki S, et al. Elotuzumab plus pomalidomide and dexamethasone for multiple myeloma. N Engl J Med. 2018;379(19):1811–1822. doi:10.1056/NEJMoa1805762
- Spencer A, Lentzsch S, Weisel K, et al. Daratumumab plus bortezomib and dexamethasoneversusbortezomib and dexamethasone in relapsed or refractory multiple myeloma: updated analysis of CASTOR. Haematologica. 2018;103(12):2079–2087. doi:10.3324/haematol.2018.194118
- Bahlis NJ, Dimopoulos MA, White DJ, et al. Daratumumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: extended follow-up of POLLUX, a randomized, open-label, phase 3 study. Leukemia. 2020;34(7):1875–1884. doi:10.1038/s41375-020-0711-6
- Lu J, Fu W, Li W, et al. Daratumumab, bortezomib, and dexamethasone versus bortezomib and dexamethasone in Chinese patients with relapsed or refractory multiple myeloma: phase 3 LEPUS (MMY3009) study. Clin Lymphoma Myeloma Leuk. 2021;21(9):e699–e709. doi:10.1016/j.clml.2021.04.012
- Dimopoulos MA, Terpos E, Boccadoro M, et al. Daratumumab plus pomalidomide and dexamethasone versus pomalidomide and dexamethasone alone in previously treated multiple myeloma (APOLLO): an open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22(6):801–812. doi:10.1016/S1470-2045(21)00128-5
- Usmani SZ, Quach H, Mateos MV, et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): updated outcomes from a randomised, multicentre, open-label, phase 3 study. Lancet Oncol. 2022;23(1):65–76. doi:10.1016/S1470-2045(21)00579-9
- Attal M, Richardson PG, Rajkumar SV, et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): a randomised, multicentre, open-label, phase 3 study. Lancet. 2019;394(10214):2096–2107. doi:10.1016/S0140-6736(19)32556-5
- Moreau P, Dimopoulos MA, Mikhael J, et al. Isatuximab, carfilzomib, and dexamethasone in relapsed multiple myeloma (IKEMA): a multicentre, open-label, randomised phase 3 trial. Lancet. 2021;397(10292):2361–2371. doi:10.1016/S0140-6736(21)00592-4
- Richardson PG, Perrot A, San-Miguel J, et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): follow-up analysis of a randomised, phase 3 study. Lancet Oncol. 2022;23(3):416–427. doi:10.1016/S1470-2045(22)00019-5
- Grosicki S, Simonova M, Spicka I, et al. Once-per-week selinexor, bortezomib, and dexamethasone versus twice-per-week bortezomib and dexamethasone in patients with multiple myeloma (BOSTON): a randomised, open-label, phase 3 trial. Lancet. 2020;396(10262):1563–1573. doi:10.1016/S0140-6736(20)32292-3
- Kumar SK, Harrison SJ, Cavo M, et al. Venetoclax or placebo in combination with bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma (BELLINI): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2020;21(12):1630–1642. doi:10.1016/S1470-2045(20)30525-8
- Mateos MV, Blacklock H, Schjesvold F, et al. Pembrolizumab plus pomalidomide and dexamethasone for patients with relapsed or refractory multiple myeloma (KEYNOTE-183): a randomised, open-label, phase 3 trial. Lancet Haematol. 2019;6(9):e459–ee69. doi:10.1016/S2352-3026(19)30110-3
- Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International myeloma working group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–e548. doi:10.1016/S1470-2045(14)70442-5
- Lin P, Owens R, Tricot G, et al. Flow cytometric immunophenotypic analysis of 306 cases of multiple myeloma. Am J Clin Pathol. 2004 Apr;121(4):482–488. doi:10.1309/74R4TB90BUWH27JX
- Leleu X, Martin T, Weisel K, et al. Anti-CD38 antibody therapy for patients with relapsed/refractory multiple myeloma: differential mechanisms of action and recent clinical trial outcomes. Ann Hematol. 2022;101(10):2123–2137. doi:10.1007/s00277-022-04917-5
- Petrucci MT, Vozella F. The anti-CD38 antibody therapy in multiple myeloma. Cells. 2019 Dec 12;8(12):1629–1638. doi:10.3390/cells8121629.
- Stewart AK. Carfilzomib for the treatment of patients with relapsed and/or refractory multiple myeloma. Future Oncol. 2015;11(15):2121–2136. doi:10.2217/fon.15.123
- Kumar S, Kaufman JL, Gasparetto C, et al. Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma. Blood. 2017;130(22):900–909. doi:10.1182/blood-2017-01-763599
- Richard S, Richter J, Jagannath S. Selinexor: a first-in-class SINE compound for treatment of relapsed refractory multiple myeloma. Future Oncol. 2020;16(19):1331–1350. doi:10.2217/fon-2020-0054
- Podar K, Shah J, Chari A, et al. Selinexor for the treatment of multiple myeloma. Expert Opin Pharmacother. 2020;21(4):399–408. doi:10.1080/14656566.2019.1707184
- van Beurden-Tan CHY, Franken MG, Blommestein HM, et al. Systematic literature review and network meta-analysis of treatment outcomes in relapsed and/or refractory multiple myeloma. J Clin Oncol. 2017;35(12):1312–1319. doi:10.1200/JCO.2016.71.1663
- Yu B, Jiang T, Liu D. cGAS-STING, an important pathway in cancer immunotherapy. J Hematol Oncol. 2020;13(1):125. doi:10.1186/s13045-020-00916-z