ABSTRACT
In this study, we present a case of acute myeloid leukemia characterized by the t(11;12)(p15;q13) translocation, exhibiting clinical, immunophenotypical, and morphological features consistent with acute promyelocytic leukemia (APL). The RNA sequencing analysis of the patient's bone marrow samples revealed the presence of the NUP98-retinoic acid receptor gamma (RARG) (NUP98::RARG) gene resulting from the translocation. Furthermore, the presence of a mutation in the ARID1B gene in the patient under study indicates a potential association with resistance to all-trans retinoic acid (ATRA).
Introduction
Acute promyelocytic leukemia (APL) is a subtype of acute myeloid leukemia (AML) characterized by fatal coagulation abnormalities. More than 98% of patients with APL have a promyelocytic leukemia–retinoic acid receptor alpha (PML::RARA) fusion gene caused by the chromosomal translocation t (15; 17) (q22; q21), which is sensitive to therapy with ATRA and arsenic trioxide (ATO) therapy[Citation1,Citation2]. However, certain AML subtypes exhibit the APL phenotype and APL-like syndromes, with X::RARs fusion genes rather than the PML–RARA fusion gene [Citation2]. In the past few years, X::RARA has been well described, including PLZF::RARA, NPM1::RARA, and STAT5B::RARA. Furthermore, retinoic acid receptor beta(RARB) and retinoic acid receptor gamma(RARG) rearrangements have been discovered to contribute to APL-like syndromes [Citation3]. Notably, RARB and RARG share high sequence homology with RARA[Citation4], and unlike PML::RARA fusions, most novel X::RAR fusions are ATRA or ATO resistant. In this study, we report the case of a patient with AML with a NUP98::RARG rearrangement and review our current understanding of patients with AML harboring X::RAR fusion gene.
Case description
On 22 April 2021, a 53-year-old man was referred to our department with asthenia and fever. His blood test showed a white blood cell count (WBC) of 3.23 × 109/L with 87% promyelocytes, hemoglobin level (HGB) of 85 g/L, and platelet count (PLT) of 39 × 109/L. His prothrombin time, activated partial thromboplastin time, and fibrinogen levels were 13.7 s (reference, 9–13 s), 27.3 s (reference, 24.2–32.8 s), and 1.57 g/L (reference, 2–4 g/L), respectively. Fibrin degradation products and D-dimer levels were 157.57 μg/mL (reference, 0–5.0 μg/mL) and 76.30 μg/mL (reference, 0–0.55 μg/ml). Morphologic examination of bone marrow (BM) smears revealed infiltration by 97.6% of hypergranular promyelocytes without Auer bodies. The nuclei were round, quasi-circular, or irregularly shaped. The leukemia cells expressed myeloperoxidase (MPO), CD117, CD13, and CD33, and partially expressed HLA-DR and CD9, but lacked the expression of CD56, cKappa, cLambda, CD3, CD4, CD8, CD7, CD5, cCD3, cCD79a, CD16, CD15, CD123, CD10, CD64, CD2, CD36, CD14, CD19, CD34, CD38, CD11b, or CD11c. APL was highly suspected as the primary diagnosis based on the clinical features, the BM morphology, and the immunophenotype. He received ATRA 40 mg/day and ATO 10 mg/day subsequently. However, both the reverse transcription-polymerase chain reaction (RT–PCR) and fluorescence in situ hybridization (FISH) failed to detect the PML::RARA transcript in the BM (A). After treatment for 10 days, the coagulation abnormality did not improve, and differentiation syndromes did not occur. The WBC count was 1.87 × 109/L, the HGB level was 65 g/L, and there was a PLT count of 24 × 109/L; he developed gastrointestinal hemorrhage. We stopped the use of ATRA, and we planned to switch the treatment to a standard 3 + 7 chemotherapy schedule. However, the patient refused further treatment due to persistent gastrointestinal hemorrhage and severe pulmonary infection.
Figure 1. (A) Dual-color FISH analysis of the patient with LSI PML::RARA dual-color. The PML gene (15q24.1) was labeled as orange, and the RARA gene (17q21.1-21.2) was labeled as green. (B) Karyotype analysis showed 46, XY, t (11; 12) (p15; q13). (C) RNA sequencing using HiSeq X Ten exhibited a recurrent NUP98::RARG fusion.
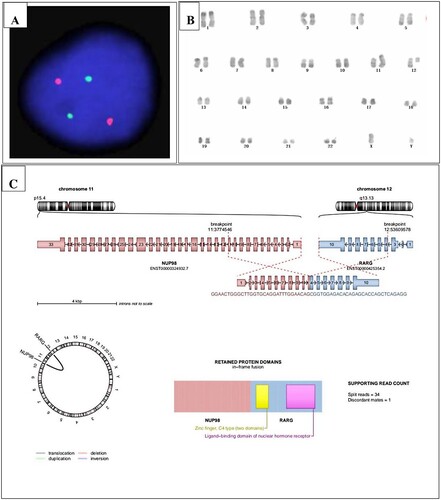
The karyotype analysis revealed 46, XY, t (11; 12) (p15; q13) [Citation5] (B). Meanwhile, we detected WT1 mutation by RT–PCR. To search for the potential fusion gene, we used the patient’s BM samples collected at the time of diagnosis to perform RNA sequencing using HiSeq X Ten and found a recurrent NUP98::RARG fusion (C). In addition, SPEN mutation and ARID1B mutation were identified.
Discussion
Compared to RARA rearrangement, RARG rearrangement is the second most common X::RARs. Thirty-four AML with RARG rearrangements were identified in a recent global cooperative study[Citation6]. In addition to NUP98::RARG, the partner genes of RARG include PML::RARG, CPSF6::RARG, NPM1::RARG::NPM1, HNRNPm::RARG, and HNRNPc::RARG. Thus far, 11 patients have been reported to have NUP98::RARG fusions from a case series of 34 cases.
From a recent literature review, we evaluated all reported patients with NUP98::RARG gene rearrangement, and the detailed information of the 11 patients, including the patient reported herein, are summarized in . The morphology of the patients’ bone marrow resembled that of APL, and the most common symptoms were bleeding and spontaneous ecchymosis (8 of 11 patients [72.7%]). Half of the patients presented with coagulopathy(low fibrinogen levels and high D-dimer levels), and cytogenetic abnormalities were found in 9(81.8%) of the 11 patients. The typical karyotype abnormality is t(11;12)(p15;q13).
Table 1. The clinical and genetic feature of APL with NUP98–RARG gene rearrangement.
All patients who underwent second-generation sequencing harbored a WT1 mutation that may play a role in treatment resistance. Owing to the small number of patients, the impact of the high frequency of WT1 mutations should be investigated in larger and more diverse cohorts in the future. In our patient, SPEN mutation and ARID1B mutation were first reported in this type of patient. ARID1B mutations have been associated with a poor prognosis. Prior studies have reported that silencing ARID1B in the APL cell line NB4 leads to impaired differentiation in response to ATRA. Furthermore, another previous study showed a higher incidence of ARID1B mutations in APL than in non-M3 AML[Citation7]. Therefore, more attention needs to be paid to gene mutations in AML with NUP98::RARG rearrangements, to determine whether the disease has a similar mutational spectrum to APL or non-M3 AML. SPEN gene mutation is common in mature B-cell lymphomas such as diffuse large B-cell lymphoma; however, whether the mutations are involved in the presence of phenotypical APL remains to be elucidated. All patients who received ATRA + ATO induction therapy (≥7 days) showed ATRA and ATO resistance, and subsequently received AML-like induction therapy.
A prior in vitro study identified ATRA resistance in primary leukemia cells from patients with NUP98::RARG rearrangements[Citation8]. However, an in vivo experiment found that primary murine cells transformed by the NUP98::RARG fusion were sensitive to ATRA[Citation9]. These contradictory ATRA sensitivities may be influenced by the different genetic backgrounds of various species or by the acquisition of additional mutations. Furthermore, responses to the standard 3 + 7 regimen included IA (idarubicin 10–12 mg/m2 for 3 days, cytarabine100 mg/m2 per day for 7 days) and DA (daunorubicin 45–60 mg/m2 for 3 days, cytarabine100 mg/m2 per day for 7 days), which also were different. Regarding patient outcomes, six patients achieved complete remission (CR), and the remaining four patients died within 35 days. After achieving CR, one patient underwent autologous hematopoietic stem cell transplant (HSCT), relapsed two years later, and died due to an infection complication after umbilical cord blood transplantation. Three patients underwent allogeneic HSCT (allo-HSCT), and no relapse or death occurred during follow-up. In general, this AML entity has a high risk of early death due to the hemorrhagic features shared with APL; however, the condition benefits from non-M3 AML treatment (intensive chemotherapy and allo-HSCT).
The PML::RARG rearrangement derived from t (12; 15) (q13; q22) was identified in a 64-year-old woman with morphologically hypergranular APL. The patient went into morphological and cytogenetic remission following treatment of IA plus ATRA; however, the lack of an early response after 18 days of ATRA treatment may indicate resistance to ATRA[Citation10].
CPSF6::RARG is the most common fusion and has been reported by several groups to present with different transcripts, including RARG::CPSF6[Citation11–14]. None of the patients showed sensitivity to ATRA or ATO. Similarly, both NPM1::RARG::NPM1 and HNRNPC::RARG have been reported in patients with APL-like disease and are resistant to ATRA and ATO[Citation15, Citation16]. Therefore, patients with X::RARG fusion AML should be treated with AML chemotherapy.
X::RARB rearrangements are rare, and TBL1XR1::RARB derived from t(3;3)(q26;q24) is the only RARB translocation that has been identified[Citation17–19]. Patients with AML with TBL1XR1::RARB show resistance to ATRA; Chemotherapy should be more effective.
X::RARA rearrangement is common and except PML::RARA another 16 types have been identified including PLZF::RARA, NPM1::RARA, NUMA::RARA, STAT5B::RARA, PRKAR1A::RARA, BCOR::RARA, FIP1L1::RARA, OBFC2A::RARA, TBLR1::RARA, GTF2I::RARA, IRF2BP2::RARA, FNDC3B::RARA, STAT3::RARA, TFG::RARA, NUP98::RARA and TNRC18::RARA. The sensitivities to ATRA and ATO of each type are different listed in [Citation2].
Table 2. ATRA/ATO therapy response for X::RARs fusions.
Conclusion
In conclusion, we confirmed a novel NUP98::RARG gene rearrangement in a patient with AML, and summarized the morphological, cytogenetic, molecular, and biological features of this disease. The condition presented clinical and morphological features similar to those of classic APL, and the typical karyotype abnormality was t(11;12)(p15;q13). Our literature review revealed that the disease is associated with a high risk of early death due to hemorrhagic features shared with APL but it shows resistance to ATRA and ATO treatment, in concordance with the poor outcomes. Patients benefit from non-M3 AML treatment (intensive chemotherapy and allo-HSCT). Therefore, when APL is present but without PML::RARA, this unusual form of AML should be considered. RNA sequencing can facilitate an early diagnosis and guide direct treatment strategies to avoid death. More attention needs to be paid to gene mutations because specific mutations can confer treatment resistance and guide targeted treatment. Whether the disease has a similar mutational spectrum to APL, non-M3 AML, or a specific one should be determined in the future, in view of the high frequency of WT1 mutations of this entity compared to APL.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Lo-Coco F, Avvisati G, Vignetti M, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369:111–121. doi:10.1056/NEJMoa1300874
- Geoffroy MC, de The H. Classic and variants APLs, as viewed from a therapy response. Cancers (Basel). 2020;12; doi:10.3390/cancers12040967
- Zhang X, Sun J, Yu W, et al. Current views on the genetic landscape and management of variant acute promyelocytic leukemia. Biomark Res. 2021;9:33. doi:10.1186/s40364-021-00284-x
- Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954.
- Luo H, Zhang S, Li K, et al. A novel entity of acute myeloid leukaemia with recurrent RARG-rearrangement resembling acute promyelocytic leukaemia. Leuk Res. 2019;77:14–16. doi:10.1016/j.leukres.2018.12.009
- Zhu H, Qin Y, Zhang ZL, et al. A global study for acute myeloid leukemia with RARG rearrangement. Blood Adv. 2023. doi:10.1182/bloodadvances.2022008364
- Madan V, Shyamsunder P, Han L, et al. Comprehensive mutational analysis of primary and relapse acute promyelocytic leukemia. Leukemia. 2016;30:1672–1681. doi:10.1038/leu.2016.69
- Such E, Cordon L, Sempere A, et al. In vitro all-trans retinoic acid sensitivity of acute myeloid leukemia blasts with NUP98/RARG fusion gene. Ann Hematol. 2014;93:1931–1933. doi:10.1007/s00277-014-2073-5
- Qiu JJ, Zeisig BB, Li S, et al. Critical role of retinoid/rexinoid signaling in mediating transformation and therapeutic response of NUP98-RARG leukemia. Leukemia. 2015;29:1153–1162. doi:10.1038/leu.2014.334
- Ha JS, Do YR, Ki CS, et al. Identification of a novel PML-RARG fusion in acute promyelocytic leukemia. Leukemia. 2017;31:1992–1995. doi:10.1038/leu.2017.167
- Han X, Jin C, Zheng G, et al. Acute myeloid leukemia with CPSF6-RARG fusion resembling acute promyelocytic leukemia with extramedullary infiltration. Ther Adv Hematol. 2021;12; doi:10.1177/2040620720976984
- Miller CA, Tricarico C, Skidmore ZL, et al. A case of acute myeloid leukemia with promyelocytic features characterized by expression of a novel RARG-CPSF6 fusion. Blood Adv. 2018;2:1295–1299. doi:10.1182/bloodadvances.2017014183
- Zhang Z, Jiang M, Borthakur G, et al. Acute myeloid leukemia with a novel CPSF6-RARG variant is sensitive to homoharringtonine and cytarabine chemotherapy. Am J Hematol. 2020;95:E48–E51. doi:10.1002/ajh.25689
- Liu T, Wen L, Yuan H, et al. Identification of novel recurrent CPSF6-RARG fusions in acute myeloid leukemia resembling acute promyelocytic leukemia. Blood. 2018;131:1870–1873. doi:10.1182/blood-2017-11-818716
- Chen X, Wang F, Zhang Y, et al. A novel NPM1-RARG-NPM1 chimeric fusion in acute myeloid leukaemia resembling acute promyelocytic leukaemia but resistant to all-trans retinoic acid and arsenic trioxide. Br J Cancer. 2019;120:1023–1025. doi:10.1038/s41416-019-0456-z
- Su Z, Liu X, Xu Y, et al. Novel reciprocal fusion genes involving HNRNPC and RARG in acute promyelocytic leukemia lacking RARA rearrangement. Haematologica. 2020;105:e376–e378. doi:10.3324/haematol.2019.244715
- Shiba N, Yoshida K, Hara Y, et al. Transcriptome analysis offers a comprehensive illustration of the genetic background of pediatric acute myeloid leukemia. Blood Adv. 2019;3:3157–3169. doi:10.1182/bloodadvances.2019000404
- Zhao J, Liang JW, Xue HL, et al. The genetics and clinical characteristics of children morphologically diagnosed as acute promyelocytic leukemia. Leukemia. 2019;33:1387–1399. doi:10.1038/s41375-018-0338-z
- Osumi T, Tsujimoto SI, Tamura M, et al. Recurrent RARB translocations in acute promyelocytic leukemia lacking RARA translocation. Cancer Res. 2018;78:4452–4458. doi:10.1158/0008-5472.CAN-18-0840
- Such E, Cervera J, Valencia A, et al. A novel NUP98/RARG gene fusion in acute myeloid leukemia resembling acute promyelocytic leukemia. Blood. 2011;117:242–245. doi:10.1182/blood-2010-06-291658
- Zhang X, Li F, Wang J, et al. RARgamma-rearrangements resemble acute promyelocytic leukemia and benefit from 3 + 7 regimen. Leuk Lymphoma. 2019;60:1831–1834. doi:10.1080/10428194.2018.1553302
- Wei W, Liu Q, Song F, et al. Alkaloid-based regimen is beneficial for acute myeloid leukemia resembling acute promyelocytic leukemia with NUP98/RARG fusion and RUNX1 mutation: A case report. Medicine (Baltimore. 2020;99:e22488. doi:10.1097/MD.0000000000022488
- Tao S, Song L, Deng Y, et al. Acute myeloid leukemia with NUP98-RARG gene fusion similar to acute promyelocytic leukemia: case report and literature review. Onco Targets Ther. 2020;13:10559–10566. doi:10.2147/OTT.S273172
- Wang M, Lin H, Chu X, et al. Identification of a recurrent fusion NUP98-RARG in acute myeloid leukaemia resembling acute promyelocytic leukaemia. Br J Haematol. 2022;197:e73–e78. doi:10.1111/bjh.18144
- Zhang J, Shen H, Song H, et al. A novel NUP98/RARG gene fusion in pediatric acute myeloid leukemia resembling acute promyelocytic leukemia. J Pediatr Hematol Oncol. 2022;44:e665–e671. doi:10.1097/MPH.0000000000002331
- Yuan J, Pei R, Lu Y. Successful haploidentical hematopoietic stem cell transplantation with azacitidine and venetoclax maintenance therapy for acute myeloid leukemia with NUP98-RARG gene fusion. Turk J Haematol. 2023;40:75–76. doi:10.4274/tjh.galenos.2022.2022.0475
- Jiang M, Zhou YR, Zhan Y, et al. Application of transcriptome sequencing and fusion genes analysis in the diagnosis of myeloid leukemia with normal karyotype. Zhonghua Yi Xue Za Zhi. 2021;101:939–944. doi:10.3760/cma.j.cn112137-20201103-03005
