ABSTRACT
Objectives
Since MPL mutation is a rare driver gene mutation found in a small number of essential thrombocythemia (ET) patients, the clinical characteristics of patients with MPL mutations and their association with thrombotic events have not yet been elucidated in Japan.
Methods
We enrolled 579 Japanese ET patients based on the diagnostic criteria of the WHO classification 2017 and compared clinical characteristics of MPL-mutated patients (n = 22; 3.8%) to JAK2V617F-mutated (n = 299; 51.6%), CALR-mutated (n = 144; 24.9%), and triple-negative (TN) (n = 114; 19.7%) patients.
Results
Thrombosis during follow up was observed in 4 out of 22 (18.2%) in the MPL-mutated group, which was the highest among all driver gene mutation groups (JAK2V617F-mutated, 8.7%; CALR-mutated, 3.5%; TN,1.8%). The MPL- and JAK2V617F-mutated groups had worse thrombosis-free survival (TFS) than the CALR-mutated (p = 0.043) and TN groups (p = 0.006). Univariable analysis revealed that a history of thrombosis was a possible risk factor for thrombosis among MPL-mutated patients (hazard ratio: 9.572, p = 0.032).
Conclusions
MPL-mutated ET patients should require more intensive management to prevent recurrence of thrombosis.
Introduction
Essential thrombocythemia (ET) is a subtype of Philadelphia chromosome-negative myeloproliferative neoplasms (MPNs). It is characterized by thrombocytosis with the presence of megakaryocytic hyperplasia in the bone marrow. As driver gene mutations of ET, Janus kinase 2 (JAK2)V617F mutation, frameshift mutations at calreticulin (CALR) exon 9, and non-synonymous mutations on Myeloproliferative leukemia virus oncogene (MPL) are frequently detected in ET patients. The frequency of JAK2V617F, CALR, and MPL mutations are about 60%, 20–25%, and 3–5%, respectively. The remaining 15% are negative for all three genes; thus, they are defined as triple-negative (TN)[Citation1].
MPL encodes thrombopoietin (TPO) receptor and TPO-MPL signaling regulates not only hematopoietic stem cell quiescence at early stage but also megakaryocytic proliferation and differentiation and subsequent platelet production at late stage[Citation2–5]. Mutated MPL molecules constitutively activate JAK2 tyrosine kinase and its downstream pathway, resulting in increased number of megakaryocytes and platelet overproduction in MPNs[Citation6]. Since the frequency of MPL mutations is much lower than that of other driver gene mutations in ET, clinical characteristics of MPL-mutated ET patients remain to be elucidated, especially in a Japanese cohort.
Herein, we aimed to analyze a large cohort of Japanese ET patients retrospectively to elucidate the clinical characteristics of patients with MPL mutations and their association with clinical events.
Materials and methods
Patients
We analyzed data obtained from 579 ET patients who met the diagnostic criteria of the WHO classification 2016[Citation7, Citation8]. Pathological diagnosis was performed by a pathologist at each institution. We excluded patients with familial ET or harboring more than one driver gene mutation. Data were retrospectively collected at Juntendo University Hospital for the period from 17 June 1993, to 2 December 2020. The following clinical parameters and events were included in the analysis: date of diagnosis, age, sex, driver gene mutations, white blood cell (WBC) count, red blood cell (RBC) count, hemoglobin (Hb), hematocrit (Hct), mean corpuscular volume (MCV), platelet (Plt) count, ferritin (FER), erythropoietin (EPO), the presence of cardiovascular risk (CVR) factors (hypertension [HT], diabetes mellitus [DM], hyper-LDL cholesterolemia, hypertriglyceridemia, smoking), a history of thrombosis, thrombotic events after diagnosis, transformation to myelofibrosis (MF) or acute myeloid leukemia (AML) after diagnosis, and secondary malignancies after diagnosis. The driver gene mutation analysis was performed as previously reported[Citation9, Citation10]. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Juntendo University (IRB #M12-0895).
Definition
The risk classification of prognosis was estimated according to the International Prognostic Score for Essential Thrombocythemia (IPSET)-survival[Citation11]. The conventional[Citation12], IPSET-thrombosis[Citation13], and revised IPSET-thrombosis[Citation14, Citation15] risk scoring models were used for estimating the risk of thrombosis. Thrombotic events in the present study included cerebral infarction, transient ischemic attack, myocardial infarction, angina pectoris, peripheral arterial occlusion, pulmonary embolism, deep vein thrombosis, and other events requiring treatment. Overall survival (OS) and thrombosis-free survival (TFS) were calculated as the term from the day at diagnosis to the end of observation and from the day at diagnosis to the onset of arterial and venous thrombosis, respectively. Discontinuation of patient visit and death were considered censored events.
Statistical analysis
Mann–Whitney U-test was used for comparing continuous variables (age, WBC count, RBC count, Hb, Hct, MCV, Plt count, FER, and EPO) and Fisher’s exact test was used to test independence between categorical variables (sex, presence of CVR factors, a history of thrombosis and events after diagnosis [thrombosis, death, transformation to MF or AML, secondary malignancies]) of MPL-mutated patients compared to JAK2V617F-mutated, CALR-mutated, and TN patients. The Kaplan-Meier method and Log-rank test were used to analyze OS and TFS. To identify risk factors for thrombotic events, univariate analysis using Cox proportional hazards regression was performed for clinically important variables based on previous studies. Two-tailed tests were used for all statistical analyses of valid variables, with p < 0.05 being considered significant. EZR version 1.55 (Jichi Medical University, Saitama Medical Center, Japan) was used for statistical analysis[Citation16]. EZR is a graphical user interface for R (The R Foundation for Statical Computing, Vienna, Austria).
Results
Patient characteristics
We compared clinical characteristics of patients harboring MPL mutations (n = 22, 3.8%) to those of patients harboring JAK2V617F (n = 299, 51.6%), CALR mutations (n = 144, 24.9%), and TN (n = 114, 19.7%) (). The median follow-up was 3.6 years (range, 0–28.3 years). WBC count, RBC count, Hb, and Hct were significantly lower in the patients harboring MPL mutation than those harboring JAK2V617F, whereas EPO and FER tended to be higher. There were no significant differences in Plt count between the MPL-mutated group and the other groups. The prevalence of one or more CVR factors and a history of thrombosis at diagnosis in the patients harboring MPL mutation were 71.4% and 9.5%, respectively, and these rates were the same levels as those of other mutations (50.8–61.4% and 5.4–19.4%, respectively).
Table 1. Clinical features of ET patients by gene mutation (n = 579).
MPL-mutated ET patients classified into the high-risk group for thrombosis have a high probability of developing thrombosis
We characterized MPL-mutated patients according to the three prognostic scoring systems of thrombosis: conventional, IPSET-thrombosis, and revised IPSET-thrombosis. MPL-mutated patients had the highest rate of thrombosis among the driver gene mutation groups (). MPL-mutated patients in the high-risk group stratified by IPSET-thrombosis and revised IPSET-thrombosis had the highest rate of thrombosis (100% and 100%, respectively), whereas less CALR-mutated patients developed thrombosis even among those classified into the high-risk group (IPSET-thrombosis: 7.7%; revised IPSET-thrombosis: 5.9%) and no patients in the TN high-risk group developed IPSET-thrombosis and revised IPSET-thrombosis criteria (Supplemental Table 1). Among the four MPL-mutated patients who developed thrombosis, two were positive for MPLW515L mutation (cerebral infarction and transient ischemia, respectively), one for MPLW515 K mutation (pulmonary embolism), and one for MPLW515A (transient ischemia) (Supplemental Table 2). The MPL-mutated group and the JAK2V617F-mutated group had low TFS rates; however, there was no significant difference (p = 0.419, A). On the other hand, the MPL-mutated group had significantly lower TFS rates than the CALR-mutated and TN groups (p = 0.043 [B] and p = 0.006 [C], respectively).
Figure 1. Kaplan-Meier curves comparing the thrombosis-free survival of patients with MPL mutation (black) versus JAK2V617F mutation (red) (A); MPL mutation versus CALR mutation (green) (B); and MPL mutation versus triple-negative (blue) (C). p < 0.05 was defined as significant.
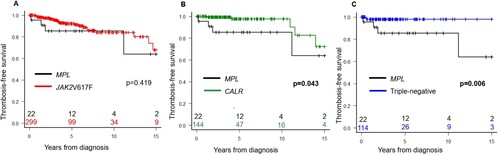
However, there was no significant difference in OS between the MPL-mutated group and the other groups (Supplemental Figures 1A–C). Among the MPL-mutated patients, one patient (4.5%) who had MPLW515L mutation progressed to MF. Transformation to AML during follow-up was not observed in the MPL-mutated group and rare regardless of the driver gene mutation (n = 8, 1.4%). Secondary malignancies occurred in two patients, both of whom exhibited MPLW515L mutations.
A history of thrombosis is a possible risk factor for thrombosis among MPL-mutated ET patients
As shown in , MPL-mutated patients with a history of thrombosis are more likely to have recurrent thrombosis after diagnosis, compared to the other gene mutation groups. To further evaluate this, we performed a univariate analysis against TFS as an explanatory variable among MPL-mutated patients. A history of thrombosis was identified as a possible risk factor for thrombosis (hazard ratio: 9.572, p = 0.032, ). Furthermore, MPL-mutated patients harboring a history of thrombosis showed worse TFS rates than those without a history of thrombosis (p = 0.013, data not shown). These results indicate that a history of thrombosis is a possible risk factor of recurrent thrombosis in MPL-mutated patients.
Table 2. Relationship between a history of thrombosis and thrombosis after diagnosis.
Table 3. Univariable analysis of predictors of thrombosis-free survival in MPL-mutated patients.
Discussion
To date, several previous studies with a small number of patients have summarized the clinical characteristics of ET harboring MPL mutations (Supplemental Table 3)[Citation17–26]. These studies reported a frequency of MPL mutations ranging from 1.4–6.7%, a median age in the 60s, and a median Plt count that exceeds 800 × 109/L. The incidence of thrombosis tends to be high in MPL-mutated patients with ET[Citation18, Citation27], and a high rate of progression to MF has been also reported[Citation26, Citation28]. However, these findings have been mainly identified in Western countries, and have not yet been elucidated in Asian cohorts.
In the present study, we analyzed the clinical characteristics and events developed during the follow up of MPL-mutated patients with ET and found that MPL mutation is one of risk factors for thrombosis. The presence of JAK2V617F mutation is a well-recognized risk factor for thrombosis[Citation29]; however, consistent with our results, a recent meta-analysis of thrombosis reported that MPL mutation is a higher risk factor for thrombosis compared to JAK2V617F mutation[Citation27]. Focusing on reports from Japan, Okabe et al. reported on 10 MPL-mutated patients and found no cases of thrombosis after diagnosis, although the information about thrombosis risk category and history of thrombosis were not available[Citation22]. Sugimoto et al. reported on two of six cases with MPL mutation (33.3%)[Citation25] and found that MPL-mutated patients had a high rate of thrombosis, which supports our findings. Therefore, MPL mutations as well as JAK2V617F should be considered risk factors for thrombosis in Japanese ET patients. In vitro analysis using MPN patients’ samples found that stimulated neutrophils from MPL-mutated patients induced a significant increase in neutrophil extracellular trap formation, which has been linked to thrombosis[Citation30]. Since MPL mutations are acquired in more primitive progenitors than JAK2V617F[Citation31], the thrombosis in MPNs may be affected by the broad expression of mutant MPL among blood cell lineages, although this hypothesis is speculative at present.
The median and mean Plt counts at the onset of thrombosis after diagnosis in MPL-mutated patients were 696 × 109/L and 58.6 × 109/L, respectively (Supplemental Table 2), which did not meet the European LeukemiaNet response criteria for ET [Citation32]. This suggests that in MPL-mutated patients, especially in those with a history of thrombosis, Plt count should be strictly controlled by cytoreductive therapy.
We further analyzed OS for patients with ET and found that the mortality rate of patients harboring MPL mutations was higher in intermediate-risk patients stratified by IPSET-survival, whereas those of patients with non-MPL mutations tended to be high with increasing IPSET-survival. Therefore, we cannot conclude from our study that patients with MPL mutation can be clearly stratified by IPSET-survival. Furthermore, the frequency of secondary malignancies of the MPL-mutated patients was the highest in our cohort (MPL-mutated vs. the others; 9.1% vs. 2.1-4.2%). Therefore, secondary malignancy may be a factor causing death in MPL-mutated ET patients. An analysis of patients in Western countries reported that MPL mutation is a high risk for MF in the long term (≥ 10 years)[Citation15, Citation26]. In the present study, there was only one case that progressed to MF, presumably due to the small number of patients analyzed and short observation period. Further investigation is necessary.
The limitations of this study are that it was a retrospective study, and that statistical validity was unobtainable due to the small number of MPL-mutated cases. Therefore, the possibility could not be excluded that thrombosis-relevant factors such as age have influenced our results showing a higher frequency of thrombosis in MPL-mutated ET patients. In fact, patients with MPL mutations were significantly older than those with triple negative ET (). However, it is noteworthy that our results are consistent with published data from Europe and the United States. Another limitation is that pathological diagnosis was performed by a pathologist at each institution. Since, in general, histological criteria of prefibrotic primary myelofibrosis have a limited diagnostic accuracy due to low sensitivity[Citation33], some patients with prefibrotic primary myelofibrosis may have been included as ET patients in our cohort. In conclusion, ET patients harboring MPL mutation, especially those with a history of thrombosis, exhibited a high incidence of thrombosis. Therefore, MPL mutation, although less frequent, should be considered as a thrombosis risk factor equivalent to JAK2V617F mutation in ET.
Author contribution
Chiho Furuya, Yoshinori Hashimoto and Norio Komatsu designed the study, performed research, analyzed and interpreted data, and wrote the manuscript; Soji Morishita, Tadaaki Inano, Tomonori Ochiai, Shuichi Shirane, Yoko Edahiro, Marito Araki and Miki Ando interpreted data and revised the manuscript. All authors approved final version of the manuscript for submission.
Ethics approval statement
This study was approved by the Ethics Committee of the School of Medicine, Juntendo University (IRB#M12-0895) and was conducted in accordance with the 1975 Helsinki Declaration.
Patient consent statement
Written informed consent was obtained prior to the use of patient samples and collection of clinical records.
Supplemental Material
Download MS Word (28.9 KB)Supplemental Material
Download MS Word (18.6 KB)Supplemental Material
Download JPEG Image (284.2 KB)Acknowledgements
We thank all members of Department of Hematology, Department of Advanced Hematology, and Laboratory for the Development of Therapies against MPN at Juntendo University Graduate School of Medicine for sample collection and technical assistance. We sincerely thank all the institutions that participated into this study: Juntendo University Shizuoka Hospital; Juntendo University Urayasu Hospital; Sapporo Hokuyu Hospital; Juntendo University Nerima Hospital; Saiseikai Yokohama-shi Nanbu Hospital; Japanese Red Cross Narita Hospital; Chiba Aoba Municipal Hospital; Kansai Medical University Hospital; Nihon University Itabashi Hospital; Toyama City Hospital; Toyama Red Cross Hospital; Tokai University Hachioji Hospital; Aichi Medical University Hospital; Japanese Red Cross Society Nagano Hospital; Eiju General Hospital; Iwate Prefectural Central Hospital; Meirikai Chuo General Hospital; National Hospital Organization Disaster Medical Center; Tokyo Metropolitan Tama Medical Center; Teine Keijinkai Hospital; Tenshi Hospital; Toyama University Hospital; Jichi Medical University Saitama Medical Center; Atami Tokoro Memorial Hospital; Wakayama Medical University Hospital; Fukui Prefectural Hospital; Kochi Medical School Hospital; Toyama Prefectural Central Hospital; Chofu Touzan Hospital; Kawasaki Saiwai Clinic; Saga University Hospital; Kameda Medical Center; Japanese Red Cross Kyoto Daiichi Hospital; Japanese Red Cross Medical Center; Shinmatsudo Central General Hospital; Aizu Medical Center; Fukui-ken Saiseikai Hospital; Fukushima Medical University Hospital; Jichi Medical University Hospital; Kuwana City Medical Center; Matsuyama Red Cross Hospital; Saiseikai Fukushima General Hospital; and Sapporo Medical University Hospital.
Disclosure statement
Araki is an employee of Meiji Seika Pharma, and Komatsu has received a salary from PharmaEssentia Japan where he is a board member. All other authors declare no conflict of interest.
Data availability statement
Data available on request from the corresponding author.
Additional information
Funding
References
- Tefferi A, Pardanani A. Essential thrombocythemia. N Engl J Med. 2019;381(22):2135–2144. doi:10.1056/NEJMcp1816082
- Kimura S, Roberts AW, Metcalf D, et al. Hematopoietic stem cell deficiencies in mice lacking c-Mpl, the receptor for thrombopoietin. Proc Natl Acad Sci U S A. 1998;95(3):1195–1200. doi:10.1073/pnas.95.3.1195
- Solar GP, Kerr WG, Zeigler FC, et al. Role of c-mpl in early hematopoiesis. Blood. 1998;92(1):4–10. doi:10.1182/blood.V92.1.4.413k38_4_10
- Buza-Vidas N, Antonchuk J, Qian H, et al. Cytokines regulate postnatal hematopoietic stem cell expansion: opposing roles of thrombopoietin and LNK. Genes Dev. 2006;20(15):2018–2023. doi:10.1101/gad.385606
- Kaushansky K. Thrombopoietin: the primary regulator of megakaryocyte and platelet production. Thromb Haemost. 1995;74(1):521–525. doi:10.1055/s-0038-1642732
- Pikman Y, Lee BH, Mercher T, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3(7):e270), doi:10.1371/journal.pmed.0030270
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi:10.1182/blood-2016-03-643544
- Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. rev. 4th ed. International Agency for Research on Cancer; 2017.
- Morishita S, Komatsu N, Kirito K, et al. Alternately binding probe competitive PCR as a simple, cost-effective, and accurate quantification method for JAK2V617F allele burden in myeloproliferative neoplasms. Leukemia Res. 2011;35(12):1632–1636. doi:10.1016/j.leukres.2011.06.016
- Misawa K, Yasuda H, Araki M, et al. Mutational subtypes of JAK2 and CALR correlate with different clinical features in Japanese patients with myeloproliferative neoplasms. Int J Hematol. 2018;107(6):673–680. doi:10.1007/s12185-018-2421-7
- Passamonti F, Thiele J, Girodon F, et al. A prognostic model to predict survival in 867 World Health Organization-defined essential thrombocythemia at diagnosis: a study by the international working group on myelofibrosis research and treatment. Blood. 2012;120(6):1197–1201. doi:10.1182/blood-2012-01-403279
- Barbui T, Tefferi A, Vannucchi AM, et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia. 2018;32(5):1057–1069. doi:10.1038/s41375-018-0077-1
- Barbui T, Finazzi G, Carobbio A, et al. Development and validation of an international prognostic score of thrombosis in World Health Organization-essential thrombocythemia (IPSET-thrombosis). Blood. 2012;120(26):5128–5133. doi:10.1182/blood-2012-07-444067
- Barbui T, Vannucchi AM, Buxhofer-Ausch V, et al. Practice-relevant revision of IPSET-thrombosis based on 1019 patients with WHO-defined essential thrombocythemia. Blood Cancer J. 2015;5:e369. doi:10.1038/bcj.2015.94
- Haider M, Gangat N, Lasho T, et al. Validation of the revised international prognostic score of thrombosis for essential thrombocythemia (IPSET-thrombosis) in 585 Mayo clinic patients. Am J Hematol. 2016;91(4):390–394. doi:10.1002/ajh.24293
- Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013;48(3):452–458. doi:10.1038/bmt.2012.244
- Beer PA, Campbell PJ, Scott LM, et al. MPL mutations in myeloproliferative disorders: analysis of the PT-1 cohort. Blood. 2008;112(1):141–149. doi:10.1182/blood-2008-01-131664
- Vannucchi AM, Antonioli E, Guglielmelli P, et al. Characteristics and clinical correlates of MPL 515W > L/K mutation in essential thrombocythemia. Blood. 2008;112(3):844–847. doi:10.1182/blood-2008-01-135897
- Schnittger S, Bacher U, Haferlach C, et al. Characterization of 35 new cases with four different MPLW515 mutations and essential thrombocytosis or primary myelofibrosis. Haematologica. 2009;94(1):141–144. doi:10.3324/haematol.13224
- Pietra D, Brisci A, Rumi E, et al. Deep sequencing reveals double mutations in cis of MPL exon 10 in myeloproliferative neoplasms. Haematologica. 2011;96(4):607–611. doi:10.3324/haematol.2010.034793
- Fu R, Xuan M, Zhou Y, et al. Analysis of calreticulin mutations in Chinese patients with essential thrombocythemia: clinical implications in diagnosis, prognosis and treatment. Leukemia. 2014;28(9):1912–1914. doi:10.1038/leu.2014.138
- Okabe M, Yamaguchi H, Usuki K, et al. Clinical features of Japanese polycythemia vera and essential thrombocythemia patients harboring CALR, JAK2V617F, JAK2Ex12del, and MPLW515L/K mutations. Leukemia Res. 2016;40:68–76. doi:10.1016/j.leukres.2015.11.002
- Prejzner W, Mital A, Bieniaszewska M, et al. Clinical characteristics of essential thrombocythemia patients depend on the mutation status. Acta Haematol Pol. 2020;51(4):230–235. doi:10.2478/ahp-2020-0040
- Alvarez-Larran A, Angona A, Andrade-Campos M, et al. -Cytoreductive treatment in patients with CALR-mutated essential thrombocythaemia: a study comparing indications and efficacy among genotypes from the Spanish registry of essential thrombocythaemia. Br J Haematol. 2021;192(6):988–996. doi:10.1111/bjh.16988
- Sugimoto Y, Nagaharu K, Ohishi K, et al. MPL exon 10 mutations other than canonical MPL W515L/K mutations identified by in-house MPL exon 10 direct sequencing in essential thrombocythemia. Int J Hematol. 2021;113(5):618–621. doi:10.1007/s12185-021-03134-6
- Loscocco GG, Guglielmelli P, Gangat N, et al. Clinical and molecular predictors of fibrotic progression in essential thrombocythemia: A multicenter study involving 1607 patients. Am J Hematol. 2021;96(11):1472–1480. doi:10.1002/ajh.26305
- Yang E, Wang M, Wang Z, et al. Comparison of the effects between MPL and JAK2V617F on thrombosis and peripheral blood cell counts in patients with essential thrombocythemia: a meta-analysis. Ann Hematol. 2021;100(11):2699–2706. doi:10.1007/s00277-021-04617-6
- Haider M, Elala YC, Gangat N, et al. MPL mutations and palpable splenomegaly are independent risk factors for fibrotic progression in essential thrombocythemia. Blood Cancer J. 2016;6(10):e487. doi:10.1038/bcj.2016.98
- Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779–1790. doi:10.1056/NEJMoa051113
- Wolach O, Sellar RS, Martinod K, et al. Increased neutrophil extracellular trap formation promotes thrombosis in myeloproliferative neoplasms. Sci Transl Med. 2018;10(436). doi:10.1126/scitranslmed.aan829
- Vainchenker W, Plo I, Marty C, et al. The role of the thrombopoietin receptor MPL in myeloproliferative neoplasms: recent findings and potential therapeutic applications. Expert Rev Hematol. 2019;12(6):437–448. doi:10.1080/17474086.2019.1617129
- Barosi G, Mesa R, Finazzi G, et al. Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG-MRT consensus project. Blood. 2013;121(23):4778–4781. doi:10.1182/blood-2013-01-478891
- Alvarez-Larrán A, Ancochea A, García M, et al. WHO-histological criteria for myeloproliferative neoplasms: reproducibility, diagnostic accuracy and correlation with gene mutations and clinical outcomes. Br J Haematol. 2014;166(6):911–919. doi:10.1111/bjh.12990
