ABSTRACT
Objectives
Optimal post-remission treatment for individual favorable and intermediate risk acute myeloid leukemia (AML) patients has not yet been established. Human leukocyte antigen (HLA)-mismatched stem cell microtransplantation (MST), may improve outcomes and avoid graft-versus-host disease in patients with first complete remission of AML.
Methods
We retrospectively analyzed the efficacy, safety, and survival of 63 patients with favorable- or intermediate-risk AML who received MST, autologous stem cell transplantation (ASCT), or cytarabine single agent (CSA) as post-remission treatment from January 2014 to August 2021.
Results
The neutrophil recovery time was shorter in the MST group than in the CSA group. The 2-year cumulative incidences of relapse in the MST, ASCT, and CSA groups were 27.27%, 29.41%, and 41.67%, respectively. During follow-up, 21 patients (33.30%) died of relapse, including six (9.52%), five (7.94%), and 10 (15.84%) in the MST, ASCT, and CSA groups, respectively. The estimated 2-year overall survival (OS) and relapse-free survival (RFS) were 62.20% vs. 50.00% (P = 0.101) and 57.10% vs. 50.00% (P = 0.136), in the >60 years MST and CSA groups (P = 0.101). The estimated 2-year OS was 100%, 66.20%, and 69.10% in the MST, ASCT, and CSA groups (MST vs CSA, P = 0.044), meanwhile, the estimated 2-year RFS was 100%, 65.40%, and 59.80% in patients ≤60 years.
Conclusion
MST, ASCT, and CSA are acceptable post-remission treatments for patients with favorable- and intermediate-risk AML and may not only improve the prognosis of the elderly but also prolong the OS and RFS of favorable- or intermediate-risk patients ≤60 years.
Introduction
Acute myeloid leukemia (AML) is a heterogeneous hematological malignancy characterized by the proliferation and abnormal differentiation of myeloid blasts [Citation1,Citation2]. AML represented 1.2% of all new cancer cases in the United States in 2019 with <28.3% 5-year survival rate [Citation1]. Therefore, treatment options to prolong the AML long-term patient survival is key. Recent advances in new treatment regimens, including cytotoxic chemotherapy, novel targeted agents, and cellular therapies, have improved the prognosis of AML patients [Citation1].
For decades the ‘3 + 7’ regimen consisting of anthracycline and cytarabine has been the induction therapy for AML, while the post-remission treatment is stratified according to the risk status. Favorable-, intermediate-, and high-risk groups are identified using the National Comprehensive Cancer Network (NCCN) and European Leukemia Net (ELN) 2017 risk stratification. High-dose cytarabine chemotherapy, hematopoietic stem cell transplantation, immunotherapy, and hypomethylating drug maintenance therapy can be selected for AML patients in favorable- or intermediate-risk groups [Citation3]. In addition, human leukocyte antigen (HLA)-mismatched hematopoietic stem cell microtransplantation (MST), a novel protocol, was found to be capable of donor microchimerism, precluding graft-versus-host disease (GVHD), and improving survival [Citation4]. MST prolongs the leukemia-free survival (LFS) of elderly AML patients and the overall survival (OS) of intermediate-risk young AML patients [Citation4,Citation5].
However, definitive studies are lacking to confirm which treatment option is more effective for favorable- and intermediate-risk AML. Our study retrospectively analyzed the data of 63 patients to compare the differences in the efficacy and safety of MST and autologous stem cell transplantation (ASCT) or chemotherapy alone as post-remission treatments in favorable and intermediate-risk AML patients.
Patients and methods
Patient population
Sixty-three patients with AML were diagnosed and treated between January 2014 and August 2021 at Huan’ an No.1 People’s Hospital of Nanjing Medical University. The patient ages ranged from 20 to 80 years. This retrospective study was approved by the Institutional Review Committee of the Huai’an No.1 People’s Hospital. AML was diagnosed using the 2008 and 2016 WHO criteria [Citation6,Citation7]. Sixty-three patients received MST, ASCT, or cytarabine single agent (CSA) as consolidation therapy after complete remission (CR) with induction chemotherapy. Patients were divided into three groups: the CSA group did not receive any form of hematopoietic stem cell transplantation, the MST group could not tolerate ASCT or did not yield enough autologous peripheral blood stem cells, and the others received ASCT treatment.
Treatment regimens
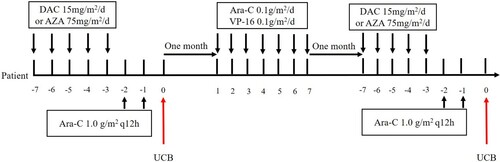
Patients who achieved CR were divided into three groups according to post-remission therapy. The peripheral blood MST (PBMST) regimen consisted of a median dose of cytarabine (0.5–2.5 g/m2 every 12 h intravenously on days 1–3) according to age, followed by infusion of HLA mismatched peripheral blood stem cell 48 h after each course of cytarabine chemotherapy, with three-month intervals between two cycles (). Patients without available donors received HLA mismatched umbilical cord blood stem cell MST (CBMST). The treatment consisted of decitabine (15 mg/m2 every day on days 1–5) or azacitidine (75 mg/m2 every day on days 1–5) and median cytarabine (1.0 g/m2 per 12 h intravenously on days 1–2) followed by infusion of HLA mismatched umbilical cord blood stem cell 24 h after chemotherapy [Citation8], with three-month intervals between two courses, EA regimen (cytarabine 0.1 g/m2 every day on days 1–7 and etoposide 0.1 g/m2 every day on days 1–7) was given in the interval as consolidation chemotherapy (). The preconditioning regimen for ASCT was BuCy (busulfan 0.8 mg/m2 per 6 h on days 1–4 and cyclophosphamide 60 mg/kg every day on days 1–2). Twenty-four patients received 4–6 cycles of medium or high doses of cytarabine (2.0–3.0 g/m2) for consolidation.
Figure 1. Treatment schedule of MST infusion of HLA mismatched peripheral blood stem cell. The donors were subcutaneously injected with 5–7 μg/kg granulocyte colony-stimulating factor, every 12 h for 5 days for mobilization. The patients received a median dose of cytarabine (0.5 g/m2–2.5 g/m2 per 12 h intravenously on days 1–3) according to age followed by infusion of HLA mismatched peripheral blood stem cells 48 h after each course of the cytarabine chemotherapy, with three-month intervals between two cycles.
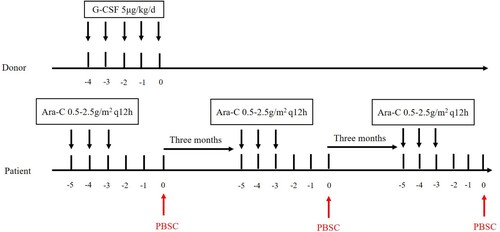
Figure 2. Treatment regimen of MST infusion of HLA mismatched umbilical cord blood stem cell. Umbilical cord blood stem cells were derived from the Cord Blood Bank of Shandong, China. The patients were administered decitabine (15 mg/m2 every day on days 1–5) or azacitidine (75 mg/m2 every day on days 1–5) and median cytarabine (1.0 g/m2 every 12 h intravenously on days 1–2) followed by infusion of HLA mismatched umbilical cord blood stem cell 24 h after chemotherapy, with three months intervals between two courses, EA regimen (cytarabine 0.1 g/m2 every day on days 17 and etoposide 0.1 g/m2 every day on days 1–7) was given in the interval as consolidation chemotherapy.
Mobilization and acquisition of donor peripheral stem cells and source of umbilical cord blood stem cells
HLA-mismatched donors were subcutaneously injected with 5–7 μg/kg granulocyte colony-stimulating factor (G-CSF) every 12 h for 5 days. The acquisition of donor cells was performed by a COM.TEC cell separator (Germany Fresenius Company), the fresh donor cells were used in the first cycle, and the remaining cells were stored at −80°C. The number of mononuclear cells per infusion was ≥3.0 × 108/kg. HLA-mismatched umbilical cord blood stem cells were derived from the Shandong Cord Blood Bank.
Response criteria and evaluation
Responses were evaluated according to standard criteria defined by the NCCN Guidelines for AML (www.nccn.org). CR was defined as the disappearance of clinical symptoms and recovery of normal hematopoiesis, with absolute neutrophil counts ≥1.0 × 109/L, platelets ≥100 × 109/L, bone marrow blasts ≤5%, and no evidence of extramedullary leukemia. Relapse was defined as the recurrence of leukemic blasts in the peripheral blood, ≥ 5% blasts in the bone marrow after CR excluding bone marrow regeneration after consolidation chemotherapy or other causes, or evidence of extramedullary leukemia. Flow cytometry and polymerase chain reaction were used to detect minimal residual disease (MRD). Relapse-free survival (RFS) was defined as the time from CR to relapse or death from any cause. Overall survival (OS) was measured as the time from initial diagnosis to death for any reason.
Statistical analysis
SPSS (version 22.0; SPSS Inc., Chicago, Illinois, USA) and GraphPad Prism 5.0 (Windows, GraphPad Software, La Jolla, California, USA) were used for all statistical analyses. Analysis of variance and Pearson chi-square tests were used to assess the probability of significant differences in age and sex. Survival data were analyzed using the log-rank test, Kaplan-Meier method, and multivariate regression analysis. Statistical significance was defined as P < 0.05.
Results
Patient characteristics
Patient characteristics are summarized in . Sixty-three patients were enrolled between 2014 and 2021 with a median follow-up time of 23.5 months, 21 months, and 11.5 months in the MST (22 patients), ASCT (17), and CSA (24) treatment groups, respectively. All eligible AML patients who received ‘3 + 7’ chemotherapy regimen consisting of cytarabine 100 mg/m2 daily for 7 days by intravenous infusion and idrubicin (10 mg/m2), daunorubicin (60 mg/m2), or CAG regimen ((aclarubicin hydrochloride 20 mg/d for 4 days, cytarabine 10 mg/m2 per 12 h intravenously for 14 days, G-CSF 200 μg/m2 daily subcutaneously for 14 days until the white blood cell counts were >20 × 109/L) and the patient achieved CR. The median ages were MST: 64 years (range 20–80), ASCT: 42 years (23–58), and CSA: 51 years (range 30–65) and most patients who received MST were older than 60 years. According to the French-American-British classification, M1, M2, M4, and M5 were included. According to ELN 2017 risk stratification for AML [Citation9], patients with t(8;21), t(16;16) or inv(16), RUNX1/RUNX1T1, CBFB/MYH11, mutated NPM1 without FLT3-ITD or with FLT3-ITDlow, or biallelic mutated CEBPA were defined as favorable risk, and patients with mutated NPM1 and FLT3-ITDhigh, wild type NPM1 without FLT3-ITD or with FLT3-ITDlow, t(9;11), MLLT3-KMT2A, cytogenetic abnormalities not classified as favorable or adverse were defined as intermediate risk [Citation9]. There were no significant differences among the three treatment groups in terms of the physical status score, blood counts, and bone marrow blast ratio.
Table 1. Patient characteristics, treatment regimens and outcomes according to three different consolidation treatments.
Treatment response and complications
In MST group, the median transfusion total nucleus cells dose and CD34 positive cell dose of grafts were 3.01 (range 0.28–5.40) × 108/kg and 0.80 (range 0.07–4.07) × 106/kg (). In the ASCT group, the median transfusion mononuclear cells dose and CD34+ cell dose of grafts were 4.11 (range 2.69–10.37) × 108/kg and 3.14 (range 1.74–5.65) × 106/kg. Two patients were positive for MRD after induction therapy in the MST group, while both the ASCT and CSA groups were negative. Neutrophil recovery time was shorter in the MST group than in the CSA group (median time to neutrophil recovery, 11 (9–15) days vs. 13 (11–18) days, P = 0.003) (). There were no significant differences in neutrophil or platelet recovery times between the MST and ASCT groups (P = 0.342 and P = 0.259, respectively). The most common complication was hematologic toxicity, including infection and bleeding, with no significant difference in incidence among the three groups. No GVHD was observed in the MST group ().
Table 2. Clinical outcomes after different post-remission treatments.
Table 3. The characteristics and clinical outcomes of 22 patients received MST.
Relapse and non-relapse related to mortality
The median follow-up times were MST: 23.5 months, ASCT: 21, and CSA: 11.5 (). The 2-year cumulative incidences of relapse were 27.27% and 29.41% in the MST and ASCT groups, respectively (P = 0.581) (). The 2-year cumulative incidence of relapse in the CSA group was 41.67% higher than those in the MST and ASCT groups; however, the difference was not significant (). At last follow-up, 21 patients (33.30%) had died of relapse, including 6 (9.52%), 5 (7.94%), and 10 (15.84) in the MST, ASCT, and CSA groups, respectively. There were no treatment-related or non-relapse-related deaths among the three groups ().
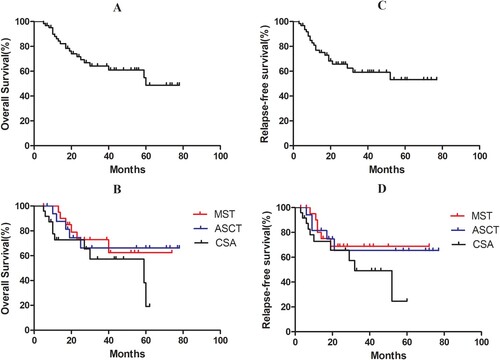
Figure 3. Survival analysis of all patients and different groups’ patients. (A) The median OS in 63 patients was 5 years; (B) The estimated 2-year OS was 72.90%, 66.20%, and 65.50% in the MST, ASCT, and CSA groups, respectively (χ2 = 3.079, P = 0.215); (C) The median for RFS time in all patients was undefined; (D) The estimated 2-year RFS was 68.70%, 59.90%, and 57.40% in the MST, ASCT, and CSA groups, respectively (χ2 = 2.159, P = 0.340).
Survival
The median OS was 5 years for all patients. The estimated 2-year OS was 72.90%, 66.20%, and 65.50% in the MST, ASCT, and CSA groups (χ2 = 3.079, P = 0.215) (A and B). The estimated 2-year RFS was 68.70%, 59.90%, and 57.40% in the MST, ASCT, and CSA groups, respectively (χ2 = 2.159, P = 0.340) (C and D). These results indicate that MST and ASCT did not improve the low- or intermediate-risk AML patients’ OS and RFS compared with CSA. For patients over 60 years of age, the estimated 2-year OS and RFS were 58.30% and 54.20%, respectively; and the estimated 2-year OS was 62.20% in the MST and 50.00% in the CSA group (P = 0.101), the estimated 2-year RFS was 57.10% and 50.00%, respectively (P = 0.136) (A–D). For patients under 60 years of age, the estimated 2-year OS and RFS were 74.60% and 67.70%, respectively; the estimated 2-year OS was 100%, 66.20%, and 69.10% in the MST, ASCT, and CSA groups, respectively (MST vs. CSA, HR = 0.030, P = 0.044); and the estimated 2-year RFS was 100%, 65.40%, and 59.80%, respectively (MST vs. CSA, HR = 0.029, P = 0.050) (E–H). The median RFS and OS in the PBMST group were longer than those in the ASCT and CSA groups (). Multivariate regression analysis showed that age, ELN risk stratification, and MRD status before myeloablative conditioning were prognostic factors affecting the patients’ OS and RFS with favorable- or intermediate-risk AML.
Figure 4. Survival analysis of patients over 60 years and under 60 years. For patients over 60 years of age, (A,B) the estimated 2-year OS and RFS was 58.30% and 54.20%, respectively; (C) the estimated 2-year OS was 62.20% and 50.00% in MST and CSA groups (P = 0.101); (D) the estimated 2-year RFS was 57.10% and 50.00% in MST and CSA groups (P = 0.136). For patients under 60 years of age, (E,F) the estimated 2-year OS and RFS was 74.60% and 67.70%; (G) the estimated 2-year OS 100%, 66.20%, and 69.10% in the MST, ASCT, and CSA groups, respectively (MST vs CSA, P = 0.044); (H) the estimated 2-year RFS was 100%, 65.40%, and 59.80% (MST vs CSA, P = 0.050).
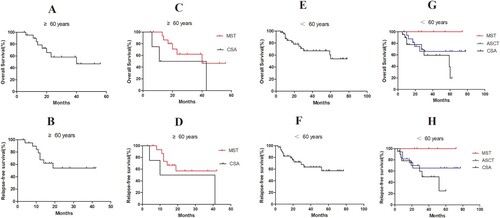
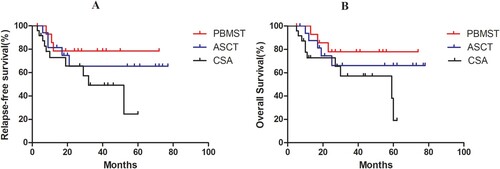
Figure 5. Survival analysis of PBMST and other patient groups. (A) The median for RFS time in the PBMST and ASCT groups were both undefined, and median survival in the CSA group was 59 months (χ2 = 3.948, P = 0.138); (B) The estimated 2-year RFS was 78.50%, 65.40%, and 65.60% in the PBMST, ASCT, and CSA groups, respectively (χ2 = 3.242, P = 0.197).
Discussion
In recent decades, advances in remission induction treatment strategies for specific AML subtypes have significantly improved CR and OS rates. However, almost all AML patients relapse during their first CR period without appropriate post-remission therapy [Citation10], even with favorable and intermediate risk according to the ELN 2017, risk stratification for AML. ELN recommends 2–4 cycles of intermediate-dose cytarabine for favorable-risk genetics in younger AML patients (18–60/65 years) eligible for intensive chemotherapy, allogeneic hematopoietic stem cell transplantation (allo-HSCT) from a matched-related or unrelated donor, 2–4 cycles of intermediate-dose cytarabine (IDAC), high-dose therapy, and ASCT for intermediate-risk genetics patients. For older patients (>60/65 years) eligible for intensive chemotherapy, 2–3 cycles of intermediate-dose cytarabine (HiDAC), allo-HSCT in patients with a low hematopoietic cell transplant comorbidity index, or investigational therapy could be chosen [Citation9]. In addition, previous studies have shown that MST as a post-remission therapy may improve outcomes and prevent GVHD in first CR (CR1) AML patients [Citation11]. Owing to the heterogeneity of AML and the paucity of randomized controlled trial data with large sample sizes, the optimal post-remission treatment for favorable and intermediate-risk genetic AML patients has not yet been established [Citation10]. This retrospective study aimed to compare the efficacy and safety of MST, ASCT, or CSA as post-remission treatments in patients with favorable- and intermediate-risk AML.
The majority of favorable-risk AML patients receive IDAC or HiDAC as consolidation therapy in CR1 according to ELN 2017 [Citation9]. However, the preferred dose of cytarabine and the optimal number of cycles necessary to achieve the best outcomes are unknown. It was recently reported that the 3-year risk of relapse was significantly higher in IDAC than in HiDAC, which is the preferred dose for single-agent cytarabine consolidation in younger patients with favorable-risk AML [Citation12]. A meta-analysis including nine studies also compared the efficacy of HiDAC to that of IDAC or low-dose cytarabine as a post-remission treatment for patients with AML. HiDAC improved RFS in the favorable risk group but it did not translate into an OS benefit [Citation13]. Moreover, CPX-351, hypomethylating agents, and targeted agents (i.e. gemtuzumab ozogamicin) were also used as post-remission treatments for patients with AML, but there was no material OS benefit from these regimens [Citation14–17]. Recent studies have shown that targeted therapies have important value in AML, and IDH1/IDH2 mutations in leukemogenesis and progress in targeted therapeutics for AML will be highlighted [Citation18]. In addition, venetoclax, an inhibitor of Bcl-2, combined with hypomethylating agents as highly effective frontline AML therapies for patients unfit for intensive chemotherapy showed a higher incidence of remission and longer OS [Citation19,Citation20]. However, there have been no randomized controlled studies on targeted therapy versus MST, and targeted therapy plus MST may be a novel therapeutic regimen. ASCT is feasible when a matched sibling donor is not available for patients with favorable- and intermediate-risk AML in CR1; however, the MRD status before HSCT is a key factor in determining suitability for ASCT and predicting the outcome after transplantation [Citation21–24]. Clinical studies have shown that MST can improve remission rates and OS with rapid hematopoietic recovery and without GVHD in patients with AML, particularly in elderly patients, although the precise mechanism of MST remains unclear [Citation5,Citation24,Citation25]. The majority of MST patients received HLA-mismatched peripheral blood stem cells from donors, but for AML patients without available donors, mismatched CBMST was also an optimal option [Citation8], both regimens implied a low-risk procedure with chemotherapy and no risk of GVHD [Citation5,Citation8]. In this study, seven patients were treated with CBMST and 15 were treated with PBMST; the median RFS and OS times were 19 and 40 months, respectively, in the CBMST group, and RFS and OS times were undefined in the PBMST group.
In this study, 63 patients were enrolled in the MST, ASCT, and CSA treatment groups, respectively. There were no significant differences in baseline characteristics among the three groups, except for age. The median age in the MST group was 64 years (range 20–80), which showed that MST could improve the outcomes of older AML patients with low or intermediate risk without increasing treatment-related toxicity, consistent with a previous report [Citation26]. Compared to CSA, neutrophil recovery time was shorter in the MST group, median time 11 (9–15) vs 13 (11–18) days, (P = 0.003), and rapid neutrophil recovery significantly reduced the incidence of infections in the granulocytosis phase. There were no significant differences among the three groups in terms of the most common complications: infection and bleeding, and there was no grade of GVHD in the MST group.
The 2-year cumulative incidence of relapse was 27.27% and 29.41% in the MST and ASCT groups, respectively (P = 0.581), which was 41.67% higher in the CSA than in the MST and ASCT groups. During the follow-up period, 21 patients (33.30%) died due to relapse. The estimated 2-year OS and RFS were 62.20% and 50.00% (P = 0.101) and 57.10% and 50.00% (P = 0.136), respectively, in the MST and CSA groups (P = 0.101) for patients aged >60 years. The estimated 2-year OS was 100%, 66.20%, and 69.10% in the MST, ASCT, and CSA groups, respectively (MST vs CSA, P = 0.044), the estimated 2-year RFS was 100%, 65.40%, and 59.80% (MST vs CSA, P = 0.050) in patients ≤60 years. These results suggest that MST could improve the outcomes of favorable and intermediate AML patients >60 years and prolong the OS and RFS for patients ≤60 years. However, prospective randomized controlled studies with a larger patient numbers are required for further investigation.
Our results showed that MST, ASCT, and CSA were acceptable options for patients with favorable- and intermediate-risk AML as a post-remission treatment. However, what is the optimal post-remission regimen for different individuals? Guo et al. [Citation27] first reported that MST increased the 2-year OS rate from 11.0% to 39.0% in older patients with AML, and a long-term follow-up study showed that the LFS and OS rates were 84.4% and 89.5%, respectively, in low-risk AML patients treated with MST for post-remission consolidation [Citation11]. In particular, in older patients with AML, MST achieved a high CR rate and 1-year OS [Citation24]. Moreover, in recent years, MST for refractory secondary AML and the immunomodulatory agent lenalidomide combined with MST for AML have been reported [Citation28,Citation29]. Compared to HLA-matched sibling donor (MSD) transplantation, the OS and LFS of MST were inferior to those of MSD transplantation for favorable and intermediate-risk AML patients in CR1 [Citation30]. Our results indicate that MST as a post-remission treatment may be suitable for elderly patients and younger patients without an available donor for allo-HSCT for favorable and intermediate-risk AML in CR1. The potential mechanisms of MST include direct cytotoxicity mediated by transferred alloreactive or tumor-specific donor cells, rejection of donor cells and concomitant cytokine release, and donor CD4 + cell-enhanced host cytotoxic T cell responses[Citation5,Citation24,Citation31–34]. However, the precise mechanism underlying MST remains unclear. A prospective randomized controlled design with large sample sizes and basic experiments on the mechanism of MST are needed. Recent studies have shown that ASCT is an acceptable option for the post-remission treatment of favorable- and intermediate-risk AML in CR1, especially in patients with favorable risk. Compared with ASCT, allo-HSCT is the preferred post-remission strategy for patients with intermediate-risk AML [Citation23,Citation35]. Furthermore, repeated courses of HiDAC and idarubicin with limited autologous CD34-positive peripheral blood stem cell support have been proven feasible and effective in non-high-risk AML patients [Citation36]. Therefore, ASCT should be used as a post-remission treatment for younger AML patients in CR1 whose MRD was negative, with favorable and intermediate risk in the absence of a donor. However, the limitations of our study were the small sample size and the lack of a prospective, randomized controlled design.
In conclusion, MST could not only improve the prognosis of the elderly but also prolong the OS and RFS of favorable- or intermediate-risk patients ≤60 years. MST, ASCT, and CSA are all acceptable options for patients with favorable- and intermediate-risk AML as a post-remission treatment. MST is more suitable for elderly and younger patients without an available donor with favorable and intermediate-risk AML in CR1, and ASCT is the preferred strategy for younger patients with favorable-risk AML in CR1 with MRD negativity. Further investigation is required to confirm this conclusion.
Ethical approval
This study was approved by the Institutional Review Committee of the Affiliated Huai’an No.1 People’s Hospital of Nanjing Medical University (KY-2022-031-01), and the need for individual consent for this retrospective analysis was waived.
Statement of human rights
All procedures were conducted in accordance with the Institutional Review Committee of the Affiliated Huai’an No.1 People’s Hospital of Nanjing Medical University, Jiangsu Province, China.
Statement of informed consent
Verbal informed consent was obtained from the patients for the publication of their anonymized information in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Acknowledgements
SDT, CL, and L Y conceptualized the original idea, designed the experiments, and analyzed the data. SD T wrote the paper. LX S, D Z, Y D, Y C, BH D, and ZM H collected patient information, treated patients with the regimens, and analyzed the data using statistical software. CLW and L Y revised the manuscript. All the authors have read and approved the final version of the manuscript.
Additional information
Funding
References
- Newell LF, Cook RJ. Advances in acute myeloid leukemia. Br Med J. 2021;375:n2026. doi:10.1136/bmj.n2026.
- Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–1152. doi:10.1056/NEJMra1406184.
- Pollyea DA, Bixby D, Perl A, et al. NCCN guidelines insights: acute myeloid leukemia, version 2.2021. J Natl Compr Canc Netw. 2021;19(1):16–27. doi:10.6004/jnccn.2021.0002
- Hu KX, Du X, Guo M, et al. Comparative study of micro-transplantation from HLA fully mismatched unrelated and partly matched related donors in acute myeloid leukemia. Am J Hematol. 2020;95(6):630–636. doi:10.1002/ajh.25780
- David KA, Cooper D, Strair R. Clinical studies in hematologic microtransplantation. Curr Hematol Malig Rep. 2017;12(1):51–60. doi:10.1007/s11899-017-0361-6
- Leonard JP, Martin P, Roboz GJ. Practical implications of the 2016 revision of the world health organization classification of lymphoid and myeloid neoplasms and acute leukemia. J Clin Oncol. 2017;35(23):2708–2715. doi:10.1200/JCO.2017.72.6745
- Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–951. doi:10.1182/blood-2009-03-209262
- Li X, Dong Y, Li Y, et al. Egfr and c-MET cooperate to enhance resistance to PARP inhibitors in hepatocellular carcinoma. BMC Cancer. 2019;79(1):819. doi:10.1158/0008-5472.CAN-18-1273
- Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447. doi:10.1182/blood-2016-08-733196
- Derman BA, Larson RA. Post-remission therapy in acute myeloid leukemia: are we ready for an individualized approach? Best Pract Res Clin Haematol. 2019;32(4):101102. doi:10.1016/j.beha.2019.101102
- Guo M, Hu KX, Liu GX, et al. HLA-mismatched stem-cell microtransplantation as postremission therapy for acute myeloid leukemia: long-term follow-up. J Clin Oncol. 2012;30(33):4084–4090. doi:10.1200/JCO.2012.42.0281
- Kolla BC, Halim NAA, Cao Q, et al. High risk of relapse with intermediate dose cytarabine for consolidation in young favourable-risk acute myeloid leukaemia patients following induction with 7+3: a retrospective multicentre analysis and critical review of the literature. Br J Haematol. 2021;194(1):140–144. doi:10.1111/bjh.17462
- Kolonen A, Sinisalo M, Huhtala H, et al. Efficacy of conventional-dose cytarabine, idarubicin and thioguanine versus intermediate-dose cytarabine and idarubicin in the induction treatment of acute myeloid leukemia: long-term results of the prospective randomized nationwide AML-2003 study by the Finnish Leukemia Group. Eur J Haematol. 2022;109(3):257–270. doi:10.1111/ejh.13805
- Blum W, Sanford BL, Klisovic R, et al. Maintenance therapy with decitabine in younger adults with acute myeloid leukemia in first remission: a phase 2 cancer and leukemia group B study (CALGB 10503). Leukemia. 2017;31(1):34–39. doi:10.1038/leu.2016.252
- Guo Y, Deng L, Qiao Y, et al. Clinical prognostic risk analysis and progression factor exploration of primary breast lymphoma. Hematology. 2022;27(1):1272–1281. doi:10.1080/16078454.2022.2150389
- Lancet JE, Uy GL, Cortes JE, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018;36(26):2684–2692. doi:10.1200/JCO.2017.77.6112
- Huls G, Chitu DA, Havelange V, et al. Azacitidine maintenance after intensive chemotherapy improves DFS in older AML patients. Blood. 2019;133(13):1457–1464. doi:10.1182/blood-2018-10-879866
- Cerchione C, Romano A, Daver N, et al. Mechanisms of action of the new antibodies in use in multiple myeloma. Front Oncol. 2021;11:639387. doi:10.3389/fonc.2021.684561
- Pollyea DA, Pratz K, Letai A, et al. Venetoclax with azacitidine or decitabine in patients with newly diagnosed acute myeloid leukemia: long term follow-up from a phase 1b study. Am J Hematol. 2021;96(2):208–217. doi:10.1002/ajh.26039
- DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617–629. doi:10.1056/NEJMoa2012971
- Chen J, Yang L, Fan Y, et al. Comparison of autologous stem cell transplantation versus haploidentical donor stem cell transplantation for favorable- and intermediate-risk acute myeloid leukemia patients in first complete remission. Biol Blood Marrow Transplant. 2018;24(4):779–788. doi:10.1016/j.bbmt.2017.12.796
- Zhao Y, Chen X, Feng S. Autologous stem cell transplantation for multiple myeloma: growth factor matters. Biol Blood Marrow Transplant. 2019;25(9):e293–e297. doi:10.1016/j.bbmt.2019.05.035
- Li Z, Liu Y, Wang Q, et al. Autologous stem cell transplantation is a viable postremission therapy for intermediate-risk acute myeloid leukemia in first complete remission in the absence of a matched identical sibling: a meta-analysis. Acta Haematol. 2019;141(3):164–175. doi:10.1159/000495206
- Guo M, Chao NJ, Li JY, et al. HLA-Mismatched microtransplant in older patients newly diagnosed with acute myeloid leukemia. JAMA Oncol. 2018;4(1):54–62. doi:10.1001/jamaoncol.2017.2656
- Pan B, Lazarus HM, Gale RP. Microtransplantation for acute myeloid leukemia. JAMA Oncol. 2020;6(10):1614–1620. doi:10.1001/jamaoncol.2020.1706
- Sung AD, Jauhari S, Siamakpour-Reihani S, et al. Microtransplantation in older patients with AML: a pilot study of safety, efficacy and immunologic effects. Am J Hematol. 2020;95(6):662–671. doi:10.1002/ajh.25781
- Guo M, Hu KX, Yu CL, et al. Infusion of HLA-mismatched peripheral blood stem cells improves the outcome of chemotherapy for acute myeloid leukemia in elderly patients. Blood. 2011;117(3):936–941. doi:10.1182/blood-2010-06-288506
- Fathi AT, Hobbs G, Dey BR, et al. Lenalidomide combined with mismatched microtransplantation for acute myeloid leukemia. Am J Hematol. 2018;93(10):E331–E333. doi:10.1002/ajh.25218
- Punwani N, Merin N, Mohrbacher A, et al. Unrelated HLA mismatched microtransplantation in a patient with refractory secondary acute myeloid leukemia. Leuk Res Rep. 2018;9:18–20. doi:10.1016/j.lrr.2018.02.002
- Liu L, Zhang X, Qiu H, et al. HLA-mismatched stem cell microtransplantation compared to matched-sibling donor transplantation for intermediate/high-risk acute myeloid leukemia. Ann Hematol. 2019;98(5):1249–1257. doi:10.1007/s00277-018-3583-3
- Rubio MT, Zhao G, Buchli J, et al. Role of indirect allo- and autoreactivity in anti-tumor responses induced by recipient leukocyte infusions (RLI) in mixed chimeras prepared with nonmyeloablative conditioning. Clin Immunol. 2006;120(1):33–44. doi:10.1016/j.clim.2006.03.004
- Symons HJ, Levy MY, Wang J, et al. The allogeneic effect revisited: exogenous help for endogenous, tumor-specific T cells. Biol Blood Marrow Transplant. 2008;14(5):499–509. doi:10.1016/j.bbmt.2008.02.013
- Colvin GA, Berz D, Ramanathan M, et al. Nonengraftment haploidentical cellular immunotherapy for refractory malignancies: tumor responses without chimerism. Biol Blood Marrow Transplant. 2009;15(4):421–431. doi:10.1016/j.bbmt.2008.12.503
- Krakow EF, Bergeron J, Lachance S, et al. Harnessing the power of alloreactivity without triggering graft-versus-host disease: how non-engrafting alloreactive cellular therapy might change the landscape of acute myeloid leukemia treatment. Blood Rev. 2014;28(6):249–261. doi:10.1016/j.blre.2014.08.002
- Rodríguez-Arbolí E, Martínez-Cuadrón D, Rodríguez-Veiga R, et al. Long-Term outcomes after autologous versus allogeneic stem cell transplantation in molecularly-stratified patients with intermediate cytogenetic risk acute myeloid leukemia: a PETHEMA study. Transplant Cell Ther. 2021;27(4):311.e1–311.e10. doi:10.1159/000495206
- Borlenghi E, Cattaneo C, Cerqui E, et al. Postremission therapy with repeated courses of high-dose cytarabine, idarubicin, and limited autologous stem cell support achieves a very good long-term outcome in European leukemia net favorable and intermediate-risk acute myeloid leukemia. Hematol Oncol. 2020;38(5):754–762. doi:10.1002/hon.2806
