ABSTRACT
Objective
Cell division cycle 37 (CDC37) modulates disease progression and bortezomib resistance in multiple myeloma by regulating X-box binding protein 1, nuclear factor-kappa-B, etc. This study aimed to explore the prognostic implication of CDC37 before and after bortezomib-based induction treatment in multiple myeloma patients.
Methods
CDC37 was detected from plasma cells of bone marrow by reverse transcription-quantitative polymerase chain reaction at baseline and after bortezomib-based induction treatment in 82 multiple myeloma patients, and in 20 disease controls and 20 healthy controls.
Results
CDC37 was increased in multiple myeloma patients versus disease controls and healthy controls (both P < 0.001). In multiple myeloma patients, CDC37 was related to increased serum creatinine (P = 0.017) and beta-2-microglobulin (P = 0.027), as well as unfavorable revised International Staging System stage (P = 0.041). Notably, CDC37 was reduced after bortezomib-based induction treatment versus that at baseline (P < 0.001). Furthermore, CDC37 at baseline was reduced in patients who achieved complete response versus those who did not achieve that (P = 0.023). Additionally, CDC37 after bortezomib-based induction treatment was also decreased in patients who achieved complete response (P < 0.001) and objective response (P = 0.001) versus those who did not reach them. Meanwhile, CDC37 at baseline only predicted worse progression-free survival (P = 0.033). Notably, CDC37 after bortezomib-based induction treatment estimated both shorter progression-free survival (P = 0.006) and overall survival (P = 0.005), which was confirmed by multivariate regression analysis.
Conclusion
CDC37 decreases after bortezomib-based induction treatment, while its higher expression reflects unsatisfactory induction treatment response and survival in multiple myeloma.
Introduction
Multiple myeloma is the second most common hematological malignancy, and nearly 588,000 people are diagnosed with this disease each year [Citation1–3]. According to the Global Cancer Statistics 2020, the incidence of multiple myeloma is slightly higher in males than in females [Citation4]. Generally, bortezomib-based induction treatment followed by autologous hematopoietic stem cell transplantation and maintenance treatment is recommended for transplant-eligible multiple myeloma patients [Citation5–7]. While for multiple myeloma patients who are not eligible for autologous hematopoietic stem cell transplantation, bortezomib-based induction treatment is also necessary to improve their prognosis [Citation5, Citation8, Citation9]. However, even after receiving bortezomib-based induction treatment, 5-year overall survival is still unsatisfactory (ranging from 57% to 80%) in multiple myeloma patients [Citation2, Citation10, Citation11]. Thus, investigating potential markers that predict bortezomib-based induction treatment response is necessary, which may assist in better stratifying multiple myeloma patients and ultimately extending their survival.
Cell division cycle 37 (CDC37) is a co-chaperone to heat shock protein 90, which plays an essential role in the pathology and progression of hematological malignancies [Citation12–14], including multiple myeloma [Citation15–17]. For instance, a previous study reports that CDC37 accelerates multiple myeloma cell proliferation through the nuclear factor kappa-B pathway [Citation18]. Additionally, a prior research finds out that CDC37 knockdown leads to plasma cell immaturation in multiple myeloma by modulating X-box binding protein 1 [Citation15]. Notably, one study discovers the involvement of CDC37 in bortezomib resistance, which indicates that CDC37 modulates bortezomib resistance through the regulation of autophagy activity in multiple myeloma cells [Citation16]. Although previous studies have disclosed the underlying mechanism of CDC37 in multiple myeloma progression and bortezomib resistance, the longitudinal change of CDC37 after bortezomib-based induction treatment and its prognostic implication in multiple myeloma patients remains unknown.
The present study aimed to evaluate the longitudinal change in CDC37 and its correlation with bortezomib-based induction treatment response and survival in multiple myeloma patients.
Methods
Subjects
This study consecutively enrolled 82 de novo primary multiple myeloma patients from January 2018 to December 2021. Enrollment criteria contained: (i) diagnosis of de novo multiple myeloma per the International Myeloma Working Group criteria [Citation19]; (ii) older than 18 years; (iii) received bortezomib-based induction treatment; (iv) had a willingness to participation; (v) voluntary for providing bone marrow specimen and follow-up. Exclusion criteria contained: (i) secondary or relapsed multiple myeloma; (ii) had other plasma-cell diseases or immunoglobulin-related diseases; (iii) had other malignancies; (iv) female during pregnancy or breastfeeding. Besides, 20 patients with non-malignant hematologic diseases that received bone marrow examinations were recruited as disease controls, as well as 20 healthy subjects that had bone marrow donations were recruited as healthy controls. This study had approval from Institutional Review Board. Subjects or their guardians signed the informed consent.
Collection and detection
Multiple myeloma patients’ characteristics were gained for study analysis. Bone marrow specimens were taken from multiple myeloma patients after recruitment and after bortezomib-based induction treatment (4-6 cycles), as well as from disease controls and healthy controls after bone marrow examinations. Then, CD138+ plasma cells in bone marrow specimens were isolated, and CDC37 expression in plasma cells was detected by reverse transcription-quantitative polymerase chain reaction (RT-qPCR), and the experiment was triplicated. Total RNA isolations were completed using PureZOL RNA isolation reagent (Bio-Rad, America). cDNA syncretization were performed using the ReverTra Ace® qPCR RT Kit (Toyobo, Japan). Next, qPCR was completed using KOD SYBR® qPCR Mix (Toyobo, Japan). Relative quantification calculation was based on the 2-ΔΔCT method. The primers were: CDC37, F: 5’-TGAAGACGAGACGCACC-3’, R: 5’-TCAGTTTCCTCTGGCACTCG-3’; GAPDH, F: 5’-GAAGGTGAAGGTCGGAGTC-3’, R: 5’-GAAGATGGTGATGGGATTTC-3’ [Citation18].
Treatment
Multiple myeloma patients received bortezomib-based therapy that was a combination of bortezomib, lenalidomide, and dexamethasone or a combination of bortezomib, thalidomide, and dexamethasone. Bortezomib was administered intravenously at a dose of 1.3 mg/m2 on days 1, 4, 8, and 11; dexamethasone was administered intravenously at a dose of 40 mg on days 1–4 (4 cycles), and days 9–12 (1-2 cycles); lenalidomide was administered orally at a dose of 25 mg on days 1–14, and thalidomide was administered orally at a dose of 100 mg per day [Citation20].
Assessment
After bortezomib-based induction treatment, the treatment response of multiple myeloma patients was assessed per the International Myeloma Working Group criteria [Citation21]. Objective response referred to the sum of patients who achieved complete response, very good partial response, and partial response; in detail, objective response = complete response + very good partial response + partial response. Minimal residual disease (MRD) status was assessed by flow cytometry, and patients with ≥20 abnormal phenotypic plasma cells in 1 x106 cells were defined as MRD positive [Citation22]. Besides, multiple myeloma patients were regularly followed up until November 2022. The median follow-up period was 28.1 months, and the range was 8.3-55.8 months. Then, progression-free survival and overall survival were estimated.
Statistical analysis
SPSS V22.0 was used for analysis, and GraphPad Prism V7.01 was used for graphing. The multi-group comparison was carried out using the Kruskal-Willis H rank sum test with post hoc comparison using the Bonferroni test. The two-group comparison was carried out using the Mann–Whitney U test. Correlations were evaluated using Spearman's rank correlation test. Comparison of CDC37 expression before and after treatment was assessed using Wilcoxon signed-rank test. The correlation of CDC37 expression with progression-free survival and overall survival was evaluated using Kaplan-Meier curves and log-rank tests. Prognostic factors for progression-free survival and overall survival were assessed using Cox’s proportional hazards regression analysis. P < 0.05 was considered significant.
Results
Clinical features in multiple myeloma patients
The mean age of enrolled multiple myeloma patients was 67.0 ± 9.2 years. There were 31 (37.8%) females and 51 (62.2%) males. Besides, 33 (40.2%) patients had renal impairment, and the left 49 (59.8%) patients did not have that. Additionally, the median (interquartile range) values of serum creatinine and beta-2-microglobulin were 1.8 (1.2–2.4) mg/dL and 5.5 (3.4–8.9) mg/L, respectively. In terms of prognostic stratification, 7 (8.5%), 34 (41.5%), and 41 (50.0%) patients were classified at International Staging System stages I, II, and III, respectively. Based on the revised International Staging System stage, 4 (4.9%), 55 (67.1%), and 23 (28.0%) patients were classified at stages I, II, and III, respectively. Regarding treatment information, 21 (25.6%) patients received the combination of bortezomib, thalidomide, and dexamethasone, 61 (74.4%) patients received the combination of bortezomib, lenalidomide, and dexamethasone. In addition, 23 (28.0%) patients received autologous hematopoietic stem cell transplantation, while 59 (72.0%) patients did not receive that. The specific information is exhibited in .
Table 1. Clinical characteristics.
CDC37 expression in multiple myeloma patients, disease controls, and healthy controls
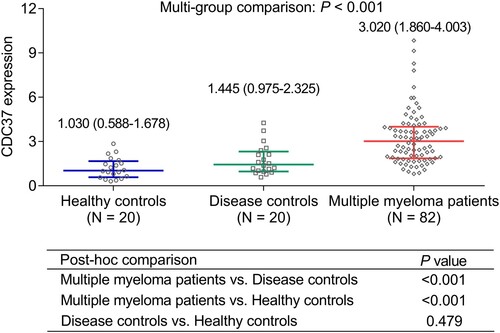
Multi-group comparison displayed that CDC37 was highest in multiple myeloma patients [median (interquartile range): 3.020 (1.860–4.003)], followed by disease controls [median (interquartile range): 1.445 (0.975–2.325)], and lowest in healthy controls [median (interquartile range): 1.030 (0.588–1.678)] (P < 0.001). Further, post-hoc comparison revealed that CDC37 was increased in multiple myeloma patients compared to disease controls (P < 0.001) and healthy controls (P < 0.001). However, CDC37 remained unchanged between disease controls and healthy controls (P = 0.479) ().
Correlation of CDC37 with clinical features in multiple myeloma patients
CDC37 was positively related to serum creatinine (r = 0.263, P = 0.017) and beta-2-microglobulin (r = 0.244, P = 0.027) in multiple myeloma patients. In addition, CDC37 was also related to the higher revised International Staging System stage (P = 0.041). Whereas CDC37 was not related to other clinical characteristics, such as age, gender, immunoglobulin subtype, bone lesion, etc., in multiple myeloma patients (all P > 0.05) ().
Table 2. Correlation of CDC37 expression with clinical characteristics in multiple myeloma patients.
Correlation of CDC37 at baseline with treatment response and survival in multiple myeloma patients
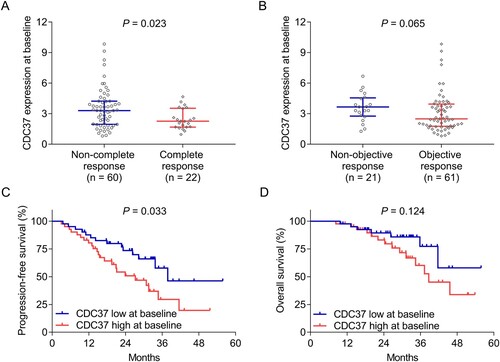
CDC37 at baseline was reduced in patients who achieved complete response compared with those who did not achieve that (P = 0.023) ((a)). While CDC37 at baseline was not different between patients who achieved an objective response and those who did not achieve that (P = 0.065) ((b)). CDC37 at baseline was divided into high and low expression based on median values. It was found that CDC37 high at baseline was related to shorter progression-free survival (P = 0.033) ((c)), whereas CDC37 at baseline was not linked to overall survival in multiple myeloma patients (P = 0.124) ((d)). Notably, it was also found that CDC37 at baseline was increased in patients with minimal residual disease-positive compared to those with minimal residual disease-negative (P = 0.004) (Supplementary Table 1).
Longitudinal change of CDC37 after induction treatment in multiple myeloma patients
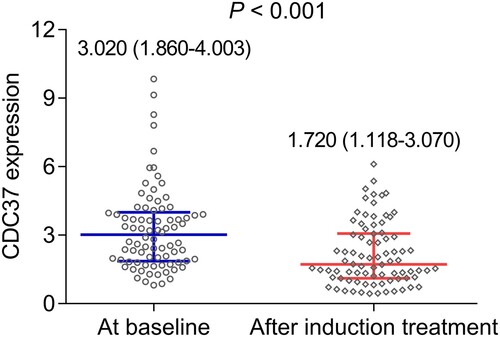
This study further explored the change in CDC37 after bortezomib-based induction treatment. It turned out that CDC37 was decreased after induction treatment [median (interquartile range): 1.720 (1.118–3.070)] compared to its expression at baseline [median (interquartile range): 3.020 (1.860–4.003)] in multiple myeloma patients (P < 0.001) ().
Relationship of CDC37 after induction treatment with treatment response and survival in multiple myeloma patients
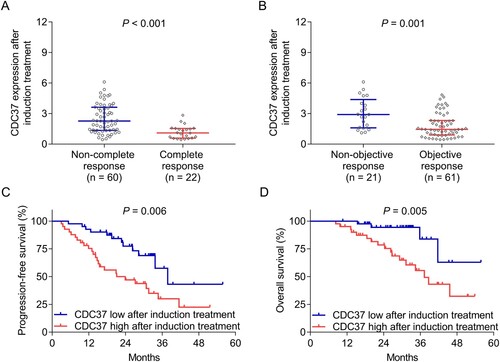
CDC37 after induction treatment was dramatically decreased in patients who achieved complete response (P < 0.001) ((a)) and objective response (P = 0.001) ((b)) compared to those who did not reach them. CDC37 after induction treatment was divided into high and low expression based on its median values. It was discovered that CDC37 high after induction treatment was noticeably associated with both unsatisfactory progression-free survival (P = 0.006) ((c)) and overall survival (P = 0.005) ((d)) in multiple myeloma patients. In addition, CDC37 after induction treatment was also elevated in patients with minimal residual disease-positive compared to those with minimal residual disease-negative (P < 0.001) (Supplementary Table 1).
Figure 4. CDC37 after induction treatment reflected poor treatment response and survival in multiple myeloma patients. Correlation of CDC37 after induction treatment with complete response (A), objective response (B), progression-free survival (C), and overall survival (D) in multiple myeloma patients.
Independent factors for progression-free survival and overall survival in multiple myeloma patients
Univariate regression analysis displayed that CDC37 at baseline (high vs. low) (hazard ratio = 2.038, P = 0.037) and after induction treatment (high vs. low) (hazard ratio = 2.567, P = 0.008) estimated poor progression-free survival in multiple myeloma patients. In addition, albumin (ALB) (abnormal vs. normal) (hazard ratio = 2.542, P = 0.020), beta-2-microglobulin (abnormal vs. normal) (hazard ratio = 2.012, P = 0.040), higher International Staging System stage (hazard ratio = 2.051, P = 0.015), and higher revised International Staging System stage (hazard ratio = 2.474, P = 0.003) were related to shorter progression-free survival in multiple myeloma patients. However, autologous hematopoietic stem cell transplantation (yes vs. no) was correlated with more prolonged progression-free survival (hazard ratio = 0.389, P = 0.026). Notably, treatment (bortezomib, thalidomide, and dexamethasone vs. bortezomib, lenalidomide, and dexamethasone) (hazard ratio = 1.073, P = 0.847) and cytogenetics (t (4; 14), t (14; 16), or Del (17p) vs. no) (hazard ratio = 1.392, P = 0.359) were not related to progression-free survival. Further forward-multivariate regression analysis revealed that only CDC37 after induction treatment (high vs. low) (hazard ratio = 2.257, P = 0.023) independently predicted shorter progression-free survival; however, CDC37 at baseline could not. Meanwhile, a higher revised International Staging System stage (hazard ratio = 2.212, P = 0.009) was also independently correlated with undesirable progression-free survival in multiple myeloma patients ().
Table 3. Cox’s proportional hazards regression analysis for progression-free survival.
In terms of overall survival, univariate regression analysis suggested that only CDC37 after induction treatment (high vs. low) (hazard ratio = 4.165, P = 0.011) was related to worse overall survival; however, CDC37 at baseline was not linked to that (hazard ratio = 2.013, P = 0.132). In addition, a higher revised International Staging System stage (hazard ratio = 2.424, P = 0.030) was also linked to shorter overall survival in multiple myeloma patients. Whereas autologous hematopoietic stem cell transplantation (yes vs. no) was related to prolonged overall survival (hazard ratio = 0.097, P = 0.023). It should be noticed that treatment (bortezomib, thalidomide, and dexamethasone vs. bortezomib, lenalidomide, and dexamethasone) (hazard ratio = 1.007, P = 0.988) and cytogenetics (t (4; 14), t (14; 16), or Del (17p) vs. no) (hazard ratio = 1.762, P = 0.210) were not correlated with overall survival. Further forward-multivariate regression analysis revealed that CDC37 after induction treatment (high vs. low) (hazard ratio = 3.580, P = 0.022) independently predicted worse overall survival in multiple myeloma patients; nevertheless, CDC37 at baseline could not independently estimate that. Besides, autologous hematopoietic stem cell transplantation (yes vs. no) independently predicted longer overall survival in multiple myeloma patients (hazard ratio = 0.113, P = 0.034) ().
Table 4. Cox’s proportional hazards regression analysis for overall survival.
Discussion
The dysregulation of CDC37 in hematological malignancies has been revealed by some studies [Citation18, Citation23, Citation24]. In terms of multiple myeloma, one study recently discovers that CDC37 is increased in multiple myeloma patients compared to healthy controls [Citation18]. Partly in line with this study, the current study observed that CDC37 was increased in multiple myeloma patients compared to disease controls and healthy controls. The potential reasons behind this might be that: (1) CDC37 might boost multiple myeloma cell proliferation through activating nuclear factor kappa-B [Citation18]. (2) CDC37 might also regulate plasma cell maturation to accelerate the pathology of multiple myeloma [Citation15]. Therefore, CDC37 could represent elevated multiple myeloma risk.
The correlation of CDC37 with clinical features in multiple myeloma patients has not been studied yet; the current study explored this field and discovered that CDC37 was positively related to serum creatinine, beta-2-microglobulin, and revised International Staging System stage in multiple myeloma patients. The potential reasons would be that: (1) CDC37 might regulate the plasma cell maturation to facilitate the production of immunoglobulin, and the accumulation of immunoglobulin would further lead to kidney injury [Citation15, Citation25], which was reflected by increased serum creatinine in multiple myeloma patients. Notably, from the results of this study, CDC37 was not related to the immunoglobulin subtype, and we speculate that it might be due to the small sample size. Thus, the explanation should be validated. (2) CDC37 could form a complex with heat shock protein 90 to regulate plasma cell differentiation, which further increased the secretion of beta-2-microglobulin [Citation15]; therefore, CDC37 reflected increased beta-2-microglobulin in multiple myeloma patients. (3) as discussed above, CDC37 could reflect increased beta-2-microglobulin; meanwhile, CDC37 might have a certain correlation with the occurrence of high-risk cytogenetics (however, this hypothesis was required to be further validated), and the latter was a component of unfavorable revised International Staging System stage [Citation26, Citation27]; taken together, CDC37 was related to higher revised International Staging System stage.
The current study further investigated the longitudinal change of CDC37 and its association with bortezomib-based induction treatment response and survival in multiple myeloma patients. It was found that CDC37 was reduced after induction treatment compared to baseline. A potential argument would be that CDC37 was highly expressed in malignant/active plasma cells, and the proportion of malignant/active plasma cells would be reduced after bortezomib-based induction treatment [Citation15]. Thus, CDC37 was decreased after bortezomib-based induction treatment compared to that at baseline. However, this speculation should be validated by subsequent experiments. Regarding treatment response, CDC37 after bortezomib-based induction treatment was related to non-complete response and non-objective response in multiple myeloma patients. An interpretation might be that CDC37 could modulate bortezomib resistance by regulating X-box binding protein 1 in multiple myeloma cells [Citation15]; therefore, it could reflect bortezomib-based induction treatment response. Regarding the correlation between CDC37 and survival, one previous study claims that CDC37 is linked to decreased survival in acute myeloid leukemia patients [Citation28]. The present study discovered that CDC37 after bortezomib-based induction treatment, was associated with shorter progression-free survival and overall survival in multiple myeloma patients. It could be hypothesized that CDC37 could accelerate the progression of multiple myeloma [Citation15, Citation18, Citation29], reflect a poor bortezomib-based induction treatment response [Citation15], and indicate a higher revised International Staging System stage [Citation30, Citation31]. As a result, CDC37 could predict worse survival.
It should be mentioned that there are two studies that explore the underlying mechanism of CDC37 in bortezomib resistance and multiple myeloma progression [Citation15, Citation16], and they find that CDC37 inhibition facilitates bortezomib resistance and multiple myeloma progression, which seems to contradict with our results. However, one of these two studies has the same finding as us, they find that CDC37 is increased in newly diagnosed multiple myeloma patients compared to healthy individuals (they do not show this data in the results section, but mention this in the discussion section), which conflicts with their results of cell experiment [Citation15]. The authors explain that the reason would be due to some subclones might already coexist within the tumor bulk at diagnosis. Bortezomib could serve as a selective pressure to eradicate the major subclones that are sensitive to bortezomib. While some minor subclones that are initially dormant and resistant to bortezomib would be survived. As a result, the key genes associated with bortezomib resistance are those diluted or quiescent ones [Citation15]. Overall, CDC37 might be increased in the major subclones at diagnosis, and its lower expression is associated with bortezomib resistance. In our opinion, the two studies report that CDC37 suppression in the multiple myeloma cell line leads to bortezomib resistance via Xbp1s and autophagy [Citation15, Citation16], while this procedure would be affected by the endogeneity of cells. Thus, the results of previous studies may lack the ability to reflect the role of CDC37 in vivo, and further experiments were required.
Several limitations existed in the current study: (1) the sample size of multiple myeloma patients was not large enough, and further studies could consider expanding that to draw a clearer conclusion; (2) this was a single-center study, which might result in selection bias; (3) the sample size of disease controls and healthy controls was relatively small; besides, the numbers of disease controls and healthy controls were unmatched with the number of multiple myeloma patients. Thus, the results might be affected.
In conclusion, CDC37 is reduced after induction treatment; notably, its high expression is associated with poor response to bortezomib-based induction treatment and survival in multiple myeloma patients. Clinically, the detection of CDC37 after bortezomib-based induction treatment is meaningful for estimating treatment response and survival in multiple myeloma patients, which may further improve the management of these patients.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- van de Donk N, Pawlyn C, Yong KL. Multiple myeloma. Lancet. 2021;397(10272):410–427. doi:10.1016/S0140-6736(21)00135-5
- Cowan AJ, Green DJ, Kwok M, et al. Diagnosis and management of multiple myeloma: a review. JAMA. 2022;327(5):464–477. doi:10.1001/jama.2022.0003
- Silberstein J, Tuchman S, Grant SJ. What is multiple myeloma? JAMA. 2022;327(5):497. doi:10.1001/jama.2021.25306
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
- Rajkumar SV. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. 2022;97(8):1086–1107. doi:10.1002/ajh.26590
- Swan D, Hayden PJ, Eikema DJ, et al. Trends in autologous stem cell transplantation for newly diagnosed multiple myeloma: Changing demographics and outcomes in European Society for Blood and Marrow Transplantation centres from 1995 to 2019. Br J Haematol. 2022;197(1):82–96. doi:10.1111/bjh.18025
- Rafae A, Ehsan H, Wahab A, et al. Evidence-based recommendations for induction and maintenance treatment of newly diagnosed transplant-ineligible multiple myeloma patients. Crit Rev Oncol Hematol. 2022;176:103744. doi:10.1016/j.critrevonc.2022.103744
- Facon T, San-Miguel J, Dimopoulos MA, et al. Treatment regimens for transplant-ineligible patients With newly diagnosed multiple myeloma: a systematic literature review and network meta-analysis. Adv Ther. 2022;39(5):1976–1992. doi:10.1007/s12325-022-02083-8
- Sandecka V, Pour L, Spicka I, et al. Bortezomib-based therapy for newly diagnosed multiple myeloma patients ineligible for autologous stem cell transplantation: Czech Registry Data. Eur J Haematol. 2021;107(4):466–474. doi:10.1111/ejh.13683
- Lonial S, Anderson KC. Association of response endpoints with survival outcomes in multiple myeloma. Leukemia. 2014;28(2):258–268. doi:10.1038/leu.2013.220
- Joseph NS, Kaufman JL, Dhodapkar MV, et al. Long-term follow-up results of lenalidomide, bortezomib, and dexamethasone induction therapy and risk-adapted maintenance approach in newly diagnosed multiple myeloma. J Clin Oncol. 2020;38(17):1928–1937. doi:10.1200/JCO.19.02515
- Ikebe E, Kawaguchi A, Tezuka K, et al. Oral administration of an HSP90 inhibitor, 17-DMAG, intervenes tumor-cell infiltration into multiple organs and improves survival period for ATL model mice. Blood Cancer J. 2013;3(8):e132. doi:10.1038/bcj.2013.30
- Kuravi S, Parrott E, Mudduluru G, et al. CDC37 as a novel target for the treatment of NPM1-ALK expressing anaplastic large cell lymphomas. Blood Cancer J. 2019;9(2):14. doi:10.1038/s41408-019-0171-2
- Casas S, Ollila J, Aventin A, et al. Changes in apoptosis-related pathways in acute myelocytic leukemia. Cancer Genet Cytogenet. 2003;146(2):89–101. doi:10.1016/S0165-4608(03)00102-X
- Zang M, Guo J, Liu L, et al. Cdc37 suppression induces plasma cell immaturation and bortezomib resistance in multiple myeloma via Xbp1s. Oncogenesis. 2020;9(3):31. doi:10.1038/s41389-020-0216-1
- Liu LT, Deng SH, Zang MR, et al. Cdc37 Contributes to bortezomib resistance in multiple myeloma via autophagy. Zhonghua Xue Ye Xue Za Zhi. 2020;41(7):583–588.
- Prince TL, Lang BJ, Okusha Y, et al. Cdc37 as a Co-chaperone to Hsp90. Subcell Biochem. 2023;101:141–158. doi:10.1007/978-3-031-14740-1_5
- Zang MR, Liu LT, Deng SH, et al. Cdc37 expression in multiple myeloma and its role in cell proliferation. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2021;29(5):1522–1527.
- Ludwig H, Miguel JS, Dimopoulos MA, et al. International Myeloma Working Group recommendations for global myeloma care. Leukemia. 2014;28(5):981–992. doi:10.1038/leu.2013.293
- Laurent V, Fronteau C, Antier C, et al. Autologous stem-cell collection following VTD or VRD induction therapy in multiple myeloma: a single-center experience. Bone Marrow Transplant. 2021;56(2):395–399. doi:10.1038/s41409-020-01033-8
- Rajkumar SV, Harousseau JL, Durie B, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117(18):4691–4695. doi:10.1182/blood-2010-10-299487
- Owen RG. Minimal residual disease (mrd) in multiple myeloma: prognostic and therapeutic implications (including imaging). Hemasphere. 2019;3(Suppl).
- Santon A, Garcia-Cosio M, Cristobal E, et al. Expression of heat shock proteins in classical Hodgkin lymphoma: correlation with apoptotic pathways and prognostic significance. Histopathology. 2011;58(7):1072–1080. doi:10.1111/j.1365-2559.2011.03803.x
- Thompson MA, Stumph J, Henrickson SE, et al. Differential gene expression in anaplastic lymphoma kinase-positive and anaplastic lymphoma kinase-negative anaplastic large cell lymphomas. Hum Pathol. 2005;36(5):494–504. doi:10.1016/j.humpath.2005.03.004
- Ying WZ, Li X, Rangarajan S, et al. Immunoglobulin light chains generate proinflammatory and profibrotic kidney injury. J Clin Invest. 2019;129(7):2792–2806. doi:10.1172/JCI125517
- Mellors PW, Binder M, Ketterling RP, et al. Metaphase cytogenetics and plasma cell proliferation index for risk stratification in newly diagnosed multiple myeloma. Blood Adv. 2020;4(10):2236–2244. doi:10.1182/bloodadvances.2019001275
- Abdallah NH, Binder M, Rajkumar SV, et al. A simple additive staging system for newly diagnosed multiple myeloma. Blood Cancer J. 2022;12(1):21. doi:10.1038/s41408-022-00611-x
- Sun Y, Wang R, Xie S, et al. A novel identified necroptosis-related risk signature for prognosis prediction and immune infiltration indication in acute myeloid leukemia patients. Genes (Basel). 2022;13(10).
- Zhao M, Ma J, Zhu HY, et al. Apigenin inhibits proliferation and induces apoptosis in human multiple myeloma cells through targeting the Trinity of CK2, Cdc37 and Hsp90. Mol Cancer. 2011;10:104. doi:10.1186/1476-4598-10-104
- D'Agostino M, Cairns DA, Lahuerta JJ, et al. Second revision of the international staging system (R2-ISS) for overall survival in multiple myeloma: a European myeloma network (EMN) report within the HARMONY project. J Clin Oncol. 2022;40(29):3406–3418. doi:10.1200/JCO.21.02614
- Cho HJ, Jung SH, Jo JC, et al. Development of a new risk stratification system for patients with newly diagnosed multiple myeloma using R-ISS and 18F-FDG PET/CT. Blood Cancer J. 2021;11(12):190. doi:10.1038/s41408-021-00577-2