ABSTRACT
Objectives
Multiple myeloma (MM) is a malignant disease characterized by a single clonal proliferation of B cell-derived plasma cells in the bone marrow. It is the second most common haematologic malignancy in adults. The objective of this retrospective study is to evaluate the diagnostic and prognostic value of haematologic parameters in MM.
Methods
The difference of NLR/ALB ratio (NAR) and NLR/HDL-C ratio (NHR) between the 151 newly diagnosed MM patients and 153 healthy controls was compared. According to NAR and NHR cutoff values obtained from the ROC curve, MM patients were divided into low group and high group. The differences in hematological parameters and survival time between the two groups were compared. Independent prognostic analysis was performed using Cox proportional hazard regression model.
Results
The NAR and NHR values in MM group were significantly higher than those in control group (P < 0.001). Higher NAR levels were significantly associated with lower albumin (ALB), higher β2 microglobulin(β2-MG), higher creatinine (Crea), and highe ISS stage (All P<0.05). High NHR group was significantly correlated with age , β2-MG and ISS stage (All P<0.05). In high NAR or NHR groups, OS and DFS was significantly shortened and the prognosis was poor (P < 0.05). Multivariate analysis showed that PLT, ISS stage and NAR were independent prognostic indicators of OS in MM patients, while ALB, PLT and NAR were independent prognostic factors of DFS.
Conclusion
NAR and NHR are inexpensive, readily available diagnostic indicators for MM, and NAR is an independent prognostic factor for MM.
Introduction
Multiple myeloma (MM) is a rare cancer, with approximately 114,000 new cases worldwide each year. It is the second most common haematological malignancy in adults, and it accounts for about 1% of all malignancies and about 10% of haematological malignancies. It most commonly occurs in middle-aged and elderly people, and is more common in men [Citation1]. It is characterized by the accumulation of malignant plasma cells in the bone marrow, leading to anaemia, bone pain, renal impairment, hypercalcemia, and infection[Citation2].
The proliferation and survival of malignant plasma cells are largely dependent on signals from the microenvironment. Cytokines and growth factors produced by stromal cells in the microenvironment (such as IL-6, vascular endothelial growth factor, insulin-like growth factor 1, tumour necrosis factor, transforming growth factor β1, and IL-10) can regulate the growth, survival, and migration of myeloma cells[Citation3]. These alterations of inflammatory components in the tumour microenvironment could be reflected by peripheral whole blood cell count (WBCC) in a certain extent. The International Staging System (ISS) has demonstrated the prognostic value of β2-MG and ALB levels in patients with MM [Citation4]. In addition, the deletion of 17p13 (the locus for the tumour-suppressor gene, p53) leads to the loss of heterozygosity of TP53, which is considered to be a high-risk feature in MM [Citation5]. Other high-risk chromosomal abnormalities in MM are characterized by structural alterations, and several studies have confirmed that patients with t (4;14) and t (14;16) have a poor prognosis [Citation6]. However, MM patients with the same ISS stage also have different prognosis, and fluorescence in situ hybridization (FISH) is very expensive to evaluate the prognosis of MM patients. Therefore, we need to explore some prognostic indicators that are convenient and easily obtain. A range of inflammation-related indexes derived from WBCC are considered to be a kind of potential biomarkers, such as neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), lymphocyte-to-monocyte ratio (LMR) or monocyte-to-lymphocyte ratio (MLR), which are easily available and inexpensive.
Abnormal lipid metabolism is an important feature of tumour cells and a potential target for tumour therapy [Citation7, Citation8]. High-density lipoprotein cholesterol (HDL-C) has antioxidant and anti-inflammatory properties, which may protect against cancer. Furthermore, HDL-C can inhibit the proliferation of myeloma by reducing cytokine levels in granulocytes, monocytes, progenitors, and bone marrow cells [Citation9].
At present, many literatures have demonstrated the relationship between NLR, MLR, PLR and the prognosis of myeloma patients. However, the relationship between the ratio of NLR/ALB or the ratio of NLR/HDL and the prognosis of MM is relatively limited. The objective of this study was to investigate the predictive effect of NLR/ALB ratio and NLR/HDL ratio on survival and prognosis of patients with MM.
Materials and methods
Subjects
In this study, 151 patients with MM newly diagnosed in the Department of Haematology in the Second Hospital of Shandong University from 2010 to 2018 were retrospectively analysed. Among them, 58 were females aged 11–78 years and 93 were males aged 15–84 years. All patients were diagnosed according to the 2016 World Health Organization diagnostic criteria for multiple myeloma [Citation10]. Excluding other diseases of the blood system, autoimmune diseases, inflammation-related diseases, malignant tumours and other diseases such as hypertension, diabetes, and cardiovascular disease, all patients did not receive radiotherapy and chemotherapy therapy. A total of 153 age and sex-matched healthy volunteers were collected as the control group. All subjects signed informed consent. We obtained permission from the hospital's research Ethics Committee.
Methods
All patients underwent complete blood count (CBC) and biochemical examination (including Ca, ALB, β2-MG, Crea, HDL-C) when they were diagnosed with MM. ISS was used as staging criteria. The CBC was detected using a haematology analyzer XE-2100(Sysmex Corporation, Kobe, Japan), and the neutrophil count to lymphocyte count ratio (NLR) was calculated. The biochemical indexes of ALB, HDL-C, Ca, β2-MG and Crea were detected by an automatic biochemical analyzer Roche c702(Roche Diagnostics GmbH, Mannheim, Germany). The ratio of NLR to ALB (NAR thinsp;= NLR/ALB*100) and the ratio of NLR to HDL-C (NHR = NLR/HDL-C*100) were calculated. Overall survival (OS) was defined as the time from diagnosis to death for any cause. Disease-free survival (DFS) was defined as the number of days from diagnosis until local or regional recurrence or distant metastasis.
Statistical analysis
Statistical analysis of the data was performed using the social science statistical software package (SPSS 16.0, IBM Corp, Armonk NY, USA). The differences between the two groups were analysed by Mann–Whitney U test or Wilcoxon signed rank test for continuous variables, and chi-square test for categorical variables. The area under curve (AUC) of NAR and NHR was obtained by receiver operating characteristic (ROC) curve analysis, and the optimal cutoff values of NAR and NHR were determined. The patients with MM were divided into a high group and a low group according to cutoff values. OS and DFS were analysed using Kaplan-Meier method. Statistical differences between survival curves were assessed using a two-tailed log-rank test. Cox proportional hazard regression models were used to assess independent prognostic factors for OS and DFS in univariate and multivariate analyses. P < 0.05 was considered to indicate a statistically significant difference.
Results
Comparison of characteristics between the MM patients and the controls
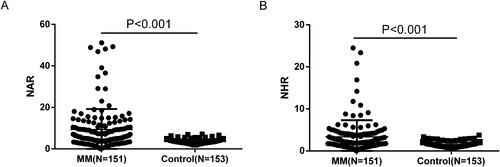
The general characteristics of the patients and the controls are shown in . when comparing MM patients with the controls, no statistical difference was found in age, sex, WBC and neutrophil (all P values >0.05). The mean values of RBC, HGB and PLT in the patients with MM were significantly lower those in the controls (P < 0.001, P < 0.001 and P = 0.034, respectively). Meanwhile, the median values of lymphocyte, ALB and HDL-C in the patients with MM were significantly lower than those in the controls (all P values <0.001). The median value of NLR in MM group was significantly.
Table 1. Characteristics of the patients with MM and the controls.
higher than that in control group, and the difference was statistically significant (P < 0.001).
Compare the difference of NAR and NHR between the MM group and the healthy control group
In the MM group, the median of NAR was 5.98 (4.02-10.49) and the median of NHR was 2.05 (1.29-4.05). While in the control group, the median of NAR was 3.37 (2.80-4.13) and the median of NHR was 1.42 (1.02-1.79). The NAR and NHR values in MM group were significantly higher than those in control group. The differences were statistically significant (both P values <0.001) ().
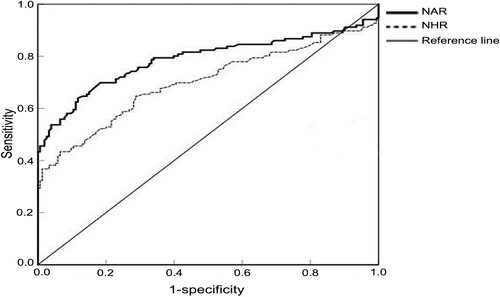
The diagnostic value of NAR and NHR for MM was determined by ROC curve
The area under curve (AUC) of NAR and NHR were 0.787 (95%CI = 0.730-0.845, P < 0.001) and 0.700(95%CI = 0.636-0.763, P < 0.001), respectively. According to ROC curve analysis, the optimal cutoff values of NAR and NHR were determined to be 4.87 (sensitivity is 64.0% and specificity is 88.2%) and 2.51 (sensitivity is 43.4% and specificity is 93.5%), respectively. MM patients were divided into high NAR group and low NAR group, high NHR group and low NHR group according to the optimal cutoff values of NAR and NHR ().
Relationship between preoperative NAR, NHR and clinicopathologic data in patients with MM
A total of 151 MM patients were divided into a high group and a low group according to the optimal cutoff values of NAR and NHR, and the clinicopathologic data of the two groups were compared (). It was found that higher NAR levels were significantly associated with lower albumin (P = 0.043), higher β2-MG(P = 0.018), higher Crea (P = 0.017), and higher ISS stage (P = 0.042). There was no significant difference in age, sex, Hb, Ca and PLT between the high NAR group and the low NAR group. A comparison of high and low NHR groups showed that high NHR group was significantly correlated with age (P = 0.042), β2-MG(P = 0.023) and ISS stage (P = 0.021), but not with other pathological parameters (All P > 0.05).
Table 2. Comparison of clinicopathological characteristics of 151 patients with MM between high and low groups in terms of NAR and NHR.
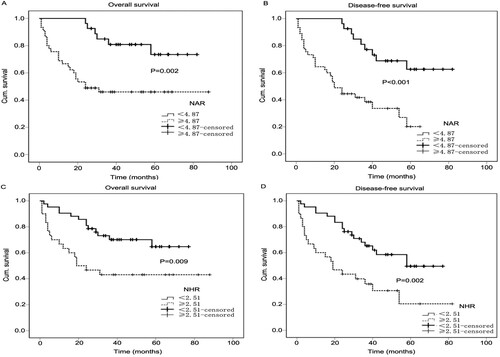
The survival analysis for patients with MM according to NAR and NHR
Kaplan-Meier survival curves of 151 patients with MM were shown in . Median follow-up time for MM patients was 32 months (range 1–88 months). The median OS time was 48 months in the low NAR group and 24 months in the high NAR group. In addition, the median DFS time was 49 months in the low NAR group and 20 months in the high NAR group. Compared with the low NAR group, the high NAR group exhibited unfavorable OS (P = 0.002) and shorter DFS (P < 0.001) ((A) and 3(B)). In terms of NHR, the median OS and DFS time was 37 and 35 months in the low NHR group respectively, and 21.5 and 19 months in the high NLR group respectively. The higher NHR value was significantly correlated with the shortening time of OS (P = 0.009) and DFS (P = 0.002) ((C) and 3(D)).
Univariate and multivariate analysis of patients with MM
To verify whether NAR and NHR were independent prognostic factors, univariate and multivariate survival analyses were performed for OS and DFS. Univariate analysis for OS showed that Hb (P = 0.008), ALB (P = 0.010), PLT (P = 0.001), ISS stage (P = 0.003), NAR (P = 0.005) and NHR (P = 0.012) were significantly correlated with OS (). Univariate analysis for DFS found that Hb (P = 0.013), ALB (P = 0.015), PLT (P = 0.002), ISS (P = 0.003), NAR (P < 0.001) and NHR (P = 0.003) were significantly correlated with DFS (). All significant prognostic factors obtained by univariate analysis were included in multivariate analysis to further obtain independent prognostic indicators. The results showed that PLT (P = 0.020), ISS stage (P = 0.018) and NAR (P = 0.045) were independent prognostic indicators for OS. ALB (P = 0.031), PLT (P = 0.005) and NAR (P = 0.001) were independent prognostic factors for DFS (see and ).
Table 3. Univariate and multivariate survival analysis for OS in 151 patients with MM.
Table 4. Univariate and multivariate survival analysis for DFS in 151 patients with MM.
Discussion
The importance of the tumour microenvironment in cancer progression has been swiftly recognized. Tumour cells and the cells in the tumour microenvironment communicate with each other through hormones and cytokines. Serum exosomal miRNAs can be employed as new markers for MM and may be implicated in the progress of subjects with monoclonal gammopathies. The prognostic relevance of circulating exosomal miRNAs was demonstrated. When exosomes obtained from uniformly treated myeloma patients were analysed, let-7b, let-7e, miR-106a, miR-106b, miR-155, miR-16, miR-17, miR-18a, and miR-20a were found to be significant risk factors for PFS. Among these, two miRNAs, let-7b and miR-18a, were significantly associated with both PFS and OS, even after adjustment for the ISS and adverse cytogenetics, supporting the use of circulating exosomal miRNAs to improve the identification of poor outcome of myeloma patients [Citation11].
With the deepening understanding of the tumour inflammatory microenvironment, we find that inflammation plays an important role in the occurrence, growth and development of tumours [Citation12, Citation13]. Previous studies have shown that inflammatory markers are associated with the prognosis of various tumours, including non-small cell lung cancer, gallbladder cancer, and diffuse large B-cell lymphoma [Citation14–16]. The role of inflammatory markers in MM has also received increasing attention, and it is believed that they can indirectly reflect the condition of bone marrow microenvironment, thus affecting the growth, survival and migration of myeloma cell, and even the regulatory process of drug resistance [Citation17]. Currently, we know that some inflammatory cells, including macrophages and dendritic cells, are involved in the coordination of the MM microenvironment [Citation18]. Therefore, systemic inflammatory markers (NLR, PLR and LMR/MLR) derived from WBCC have recently received close attention in MM. A meta-analysis showed that elevated NLR, decreased LMR, elevated MLR could predict poor OS/PFS in patients with multiple myeloma, but there was no significant correlation between PLR and prognosis of MM patients [Citation19]. Onec B et al. showed that elevated NLR in newly diagnosed MM patients suggests poor prognosis and is an independent risk factor for prognosis [Citation20]. Wongrakpanich et al. found a significant relationship between high NLR and OS in the patients with MM (NLR cutoff point 2.59) [Citation21]. In another study, Kelkitli et al. found that MM patients with a NLR <2.0 survived longer compared with NLR >2.0 [Citation22]. Romano et al. found NLR to be a predictor of PFS and OS in MM patients treated upfront with novel agent. They showed that the 5–year PFS and OS estimates were 18.2% and 36.4% for patients with NLR >2.0 versus 25.5 and 66.6% in patients with NLR <2.0. The prognostic relevance of NLR was even more prominent in the young patients and treated with autologous stem cell transplantation upfront [Citation23]. In the study by Shi et al., it was shown that elevated NLR and MLR and decreased PLR were associated with unfavorable outcomes in newly diagnosed MM patients. The cutoff points for NLR, PLR, and MLR were 4, 100, and 0.3, respectively, in their study [Citation24].
HDL is the most abundant lipoprotein in most species, which means that this particle has important functions in health and disease [Citation25]. There is increasing evidence that HDL regulates innate and adaptive immune responses and may have antioxidant, anti-apoptotic, and anti-inflammatory properties [Citation25–27]. Through dysfunction of some of these characteristics, low HDL may play a role in the development of cancer [Citation26]. On one hand, studies have shown that changes in HDL-C levels are associated with the risk of many cancers, such as breast cancer, prostate cancer, endometrial cancer and colorectal cancer [Citation28–31]. The possible mechanism is that HDL can regulate and activate the immune system to enter a more beneficial anti-cancer state. On the other hand, HDL-C and apolipoprotein A1 (ApoA1) have been shown to inhibit proliferation of haematopoietic stem cells and progenitor cells [Citation32]. Low HDL-C and ApoA1 are associated with an increased risk of haematological cancers [Citation33]. HDL may be essential for the strict control of proliferation and homeostasis of the haematopoietic system and may impede malignant transformation.
At present, the prognostic indicators of MM patients which have been proved include Hb, serum Ca, ALB, β2-MG, cytogenetics and tumour staging. It is still necessary to continuously find and improve the new prognostic indicators of MM patients, so as to provide the basis for advance intervention and targeted therapy, and to prolong the survival of MM patients. ALB is a protein with a molecular weight of 66,000 synthesized by the liver. ALB reduction is seen in liver damage, malnutrition, kidney disease, chronic consumption, and malignancy. ALB level is related to inflammatory response, nutritional status and tumour load [Citation34].
Multiple myeloma is still an incurable disease, and the clinical course is so highly variable. Therefore, we need to accurately and effectively evaluate the potential indicators which may be used to predict the prognosis of patients with multiple myeloma. WWBC is a necessary test for every patient, and it is very easy to obtain, so it is very meaningful for patients to use blood routine parameters to obtain valuable indicators for the diagnosis and prognosis of MM. In this study, NAR and NHR were calculated by WWBC and biochemical parameters, and the inflammatory state and nutritional state were comprehensively stabilized, so as to evaluate the prognosis of MM patients more comprehensively.
Our results showed that the NAR and NHR levels in MM patients were significantly higher than those in healthy controls (both P values <0.001). Meanwhile, by the area under ROC curve, it was found that NAR and NHR had good diagnostic efficiency in MM. By analysing the relationship between NAR, NHR and the age, sex, Hb, ALB, β2-MG, Crea and ISS stage of MM patients, it was found that, higher NAR levels were significantly associated with lower albumin (P = 0.043), higher β2-MG(P = 0.018), higher Crea(P = 0.017), and higher ISS stage (P = 0.042). The high NHR group was significantly correlated with age (P = 0.042), β2-MG(P = 0.023) and ISS stage (P = 0.021). These results suggested that NAR and NHR were associated with poor prognostic outcomes in MM patients. In this study, the difference of OS and DFS between MM patients and healthy controls according to NAR and NHR were further analysed. It was found that patients with high NAR and NHR group had significantly shorter OS and DFS than those with low value, and the prognosis was poor (P < 0.05). Univariate analysis showed that Hb, ALB, PLT, ISS stage, NAR and NHR were significantly correlated with OS (all P < 0.05). Hb, ALB, PLT, ISS stage, NAR and NHR were significantly correlated with DFS (all P < 0.05). Multivariate analysis showed that PLT, ISS stage and NAR were independent prognostic indicators of OS in MM patients, while ALB, PLT and NAR were independent prognostic factors of DFS (all P < 0.05). Although high NHR lost its independent prognostic significance for OS and DFS in multivariate analysis, it still provided considerable information for the clinical outcome of MM patients.
There are still some shortcomings in this study. On the one hand, the data in this study are all from the same institution, and the number of patients is limited. We need to expand the sample size and extend the follow-up time to further confirm the evidence. On the other hand, this study is a retrospective study, so data bias is inevitable.
Conclusion
NAR and NHR are simple, inexpensive, and readily available biomarkers related to inflammatory response. NAR and NHR have good diagnostic efficacy and can distinguish MM from healthy controls. In addition, NAR and NHR can be used as important prognostic indicators of MM patients, and NAR is an independent prognostic factor of MM patients. However, it must be noted that the measurement of a single biomarker is far from sensitive and specific enough for a certain disease, so the combination of NAR and NHR to evaluate the prognosis of MM patients will be of greater value in the clinic.
List of abbreviations
MM, multiple myeloma; NLR, neutrophil-to-lymphocyte ratio; ALB, albumin; HDL-C, high-density lipoprotein cholesterol; NAR, ratio of NLR to ALB; NHR, ratio of NLR to HDL-C; Hb, hemoglobin; β2-MG, β2 microglobulin; Crea, creatinine; ISS, International Staging System; MLR, monocyte-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; OS, overall survival; DFS, disease-free survival; AUC, the area under curve; ROC, receiver operating characteristic; ApoA1, apolipoprotein A1.
Acknowledgements
The authors wish to thank the staff of Pathology department, The Second Hospital of Shandong University for their help in data collection.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Fuyan Han
Fuyan Han is PhD. Candidates of clinical laboratory diagnostics. Her research focuses on the diagnosis and prognosis of hematologic tumors and solid tumors.
Nan Sheng
Nan Sheng is engaged in a clinical examination and test diagnosis.
Chenchen Sheng
Chenchen Sheng is PhD. Candidates of Obstetrics and Gynecology in Qilu Hospital.
Jing Meng
Jing Meng is a PhD of clinical laboratory diagnostics and a senior technologist of the Second Hospital of Shandong University. She is an expert in hematology and immunology.
References
- Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International myeloma working group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–e548. doi:10.1016/S1470-2045(14)70442-5
- Padala SA, Barsouk A, Barsouk A, et al. Epidemiology, staging, and management of multiple myeloma. Med Sci (Basel). 2021;9:3. doi:10.3390/medsci9010003.
- Allegra A, Innao V, Allegra AG, et al. Lymphocyte subsets and inflammatory cytokines of monoclonal gammopathy of undetermined significance and multiple myeloma. Int J Mol Sci. 2019;20:2822. doi:10.3390/ijms20112822.
- Ege H, Gertz MA, Markovic SN, et al. Prediction of survival using absolute lymphocyte count for newly diagnosed patients with multiple myeloma: a retrospective study. Br J Haematol. 2008;141:792–798. doi:10.1111/j.1365-2141.2008.07123.x
- Jovanović KK, Escure G, Demonchy J, et al. Deregulation and targeting of TP53 pathway in multiple myeloma. Front Oncol. 2018;8:665. doi:10.3389/fonc.2018.00665.
- Garrido D, Slavutsky I, Riva E. Survival analysis of transplant-eligible newly-diagnosed multiple myeloma patients harboring t(4;14), t(14;16), and/or del(17p) in the real-world setting. Curr Probl Cancer. 2023;47:100916). doi:101016/j.
- Koundouros N, Poulogiannis G. Reprogramming of fatty acid metabolism in cancer. Br J Cancer. 2020;122:4–22. doi:10.1038/s41416-019-0650-z
- Liu Q, Luo Q, Halim A, et al. Acetyl-CoA carboxylase rewires cancer metabolism to allow cancer cells to survive inhibition of the warburg effect by cetuximab. Cancer Lett. 2017;384:39–49. doi:10.1016/j.canlet.2016.09.020
- Wang H, Chen B, Shao R, et al. A new prediction model integrated serum lipid profile for patients with multiple myeloma. J Cancer. 2022;13:1796–1807. doi:10.7150/jca.69321
- Rajkumar SV. Updated diagnostic criteria and staging system for multiple myeloma. Am Soc Clin Oncol Educ Book. 2016;35:e418–e423. doi:10.1200/EDBK_159009.
- Manier S, Liu CJ, Avet-Loiseau H, et al. Prognostic role of circulating exosomal miRNAs in multiple myeloma. Blood. 2017;129:2429–2436. doi:10.1182/blood-2016-09-742296
- Dolan RD, Laird B, Horgan PG, et al. The prognostic value of the systemic inflammatory response in randomised clinical trials in cancer: A systematic review. Crit Rev Oncol Hematol. 2018;132:130–137. doi:10.1016/j.critrevonc.2018.09.016
- Podar K, Chauhan D, Anderson KC. Bone marrow microenvironment and the identification of new targets for myeloma therapy. Leukemia. 2009;23:10–24. doi:10.1038/leu.2008.259
- Zhao QT, Yuan Z, Zhang H, et al. Prognostic role of platelet to lymphocyte ratio in non-small cell lung cancers: A meta-analysis including 3,720 patients. Int J Cancer. 2016;139:164–170. doi:10.1002/ijc.30060
- Xu B, Chen Z, Zhang J, et al. Prognostic value of peripheral whole blood cell counts derived indexes in gallbladder carcinoma: A systematic review and meta-analysis. Front Oncol. 2021;11:707742), doi:10.3389/fonc.2021.707742
- Mu S, Ai L, Fan F, et al. Prognostic role of neutrophil-to-lymphocyte ratio in diffuse large B cell lymphoma patients: an updated dose-response meta-analysis. Cancer Cell Int. 2018;18:119), doi:10.1186/s12935-018-0609-9
- Podar K, Chauhan D, Anderson KC. Bone marrow microenvironment and the identification of new targets for myeloma therapy. Leukemia. 2009;23:10–24. doi:10.1038/leu.2008.259
- Chauhan D, Singh AV, Brahmandam M, et al. Functional interaction of plasmacytoid dendritic cells with multiple myeloma cells: a therapeutic target. Cancer Cell. 2009;16:309–323. doi:10.1016/j.ccr.2009.08.019
- Zhang X, Duan J, Wen Z, et al. Are the derived indexes of peripheral whole blood cell counts (NLR, PLR, LMR/MLR) clinically significant prognostic biomarkers in multiple myeloma? A Systematic Review And Meta-Analysis. Front Oncol. 2021;11:766672. doi:10.3389/fonc.2021.766672.
- Onec B, Okutan H, Albayrak M, et al. The predictive role of the neutrophil/lymphocyte ratio in survival with multiple myeloma: A single center experience. J Clin Lab Anal. 2017;31:e22032. doi:10.1002/jcla.22032.
- Wongrakpanich S, George G, Chaiwatcharayut W, et al. The prognostic significance of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in patients With multiple myeloma. J Clin Lab Anal. 2016;30:1208–1213. doi:10.1002/jcla.22004
- Kelkitli E, Atay H, Cilingir F, et al. Predicting survival for multiple myeloma patients using baseline neutrophil/lymphocyte ratio. Ann Hematol. 2014;93:841–846. doi:10.1007/s00277-013-1978-8
- Romano A, Parrinello NL, Consoli ML, et al. Neutrophil to lymphocyte ratio (NLR) improves the risk assessment of ISS staging in newly diagnosed MM patients treated upfront with novel agents. Ann Hematol. 2015;94:1875–1883. doi:10.1007/s00277-015-2462-4
- Shi L, Qin X, Wang H, et al. Elevated neutrophil-to-lymphocyte ratio and monocyte-to-lymphocyte ratio and decreased platelet-to-lymphocyte ratio are associated with poor prognosis in multiple myeloma. Oncotarget. 2017;8:18792–18801. doi:10.18632/oncotarget.13320
- Catapano AL, Pirillo A, Bonacina F, et al. HDL in innate and adaptive immunity. Cardiovasc Res. 2014;103:372–383. doi:10.1093/cvr/cvu150
- Pirro M, Ricciuti B, Rader DJ, et al. High density lipoprotein cholesterol and cancer: marker or causative? Prog Lipid Res. 2018;71:54–69. doi:10.1016/j.plipres.2018.06.001
- Yvan-Charvet L, Pagler T, Gautier EL, et al. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–1693. doi:10.1126/science.1189731
- Nowak C, Ärnlöv J. A Mendelian randomization study of the effects of blood lipids on breast cancer risk. Nat Commun. 2018;9:3957), doi:10.1038/s41467-018-06467-9
- Bull CJ, Bonilla C, Holly JM, et al. Blood lipids and prostate cancer: a Mendelian randomization analysis. Cancer Med. 2016;5:1125–1136. doi:10.1002/cam4.695
- Seth D, Garmo H, Wigertz A, et al. Lipid profiles and the risk of endometrial cancer in the Swedish AMORIS study. Int J Mol Epidemiol Genet. 2012;3:122–133. http://www.ijmeg.org/ISSN1989-1975/IJMEG1203002.
- Rodriguez-Broadbent H, Law PJ, Sud A, et al. Mendelian randomisation implicates hyperlipidaemia as a risk factor for colorectal cancer. Int J Cancer. 2017;140:2701–2708. doi:10.1002/ijc.30709
- Gao M, Zhao D, Schouteden S, et al. Abstract 8: effect of intensive lipid therapy on plaque lipid depletion as assessed by magnetic resonance imaging. Arterioscler Thromb Vasc Biol. 2014;34:1900–1909. doi:10.1161/atvb.34.suppl_1.8
- Feng Y, Schouteden S, Geenens R, et al. Hematopoietic stem/progenitor cell proliferation and differentiation is differentially regulated by high-density and low-density lipoproteins in mice. Plos One. 2012;7:e47286), doi:10.1371/journal.pone.0047286
- Ding D, Feng Y, Song B, et al. Effects of preoperative and postoperative enteral nutrition on postoperative nutritional status and immune function of gastric cancer patients. Turk J Gastroenterol. 2015;26:181–185. doi:10.5152/tjg.2015.3993