ABSTRACT
Objective
Analyze the reasons for the mismatch between forward and reverse typing of ABO blood types and the mismatch between cross matching.
Methods
When the forward and reverse typing do not match, use physiological saline method, polyamine method, anti human globulin method, and anti screening positive samples are used for antibody identification.
Results
The factors contributing to discrepancies in blood typing between forward and reverse typing include weakened serum typing, condensation, monoclonal immunoglobulin influence, bone marrow transplantation, and blood type subtypes. The causes of cross matching incompatibility include homologous antibody, warm Autoantibody, cold Autoantibody and daretozumab.
Conclusion
Regular red blood cell homologous antibody screening should be conducted based on disease type, blood transfusion history, and medication history. Antigen matched red blood cells should be selected for cross matching, and different experimental methods should be used for testing to ensure the safety of clinical blood transfusion.
1. Introduction
Transfusing blood is a crucial therapeutic and life-saving procedure in the medical field. However, it is crucial to confirm the blood group of the recipient before a transfusion is given. Typically, crossmatching of blood is conducted in cases where time is not of the essence. Discrepancies in forward and reverse blood group typing and/or crossmatch incompatibility are common clinical occurrences [Citation1]. In such a scenario, the blood transfusion (blood bank) staff must investigate the root causes, run numerous tests, rule out potential interferences, double-check the accuracy of blood group results, and select compatible blood for transfusion. In this study, we conducted a retrospective analysis of 23 cases of forward and reverse blood group typing discrepancy and 57 cases of crossmatch incompatibility, to determine the causes and treatments that facilitate accurate blood group typing and crossmatching, and thus, ensure timely and safe clinical blood transfusion.
2. Data and methods
2.1 Specimen source
Between January 2015 and December 2021, we analyzed 23 instances of forward and reverse blood group typing discrepancies at the blood transfusion department of our hospital and 57 instances of crossmatch incompatibility tested at Jiaxing Central Blood Station. This study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of our hospital. Written informed consent was obtained from all participants.
2.2 Instruments
Centrifuge KA-2200, Japan KUBOTA; Electric thermostatic water bath S.H.W 21.600S, Shanghai Yuejin Medical Device Co., Ltd.
2.3 Reagents
Monoclonal anti-A/B positive setting reagent 20210608, ABO negative setting reagent 20215349, irregular antibody screening reagent 20217056, spectrum cell 20211129, acid release, anti-spheroid human protein reagent, 2-Me, and anti-A1 were all purchased from Shanghai Blood Biomedicine; polybrene reagent A1210803 was purchased from Zhuhai Beso; imported spectrum cells 8000459055 were purchased from Sanquin.
2.4 Methods
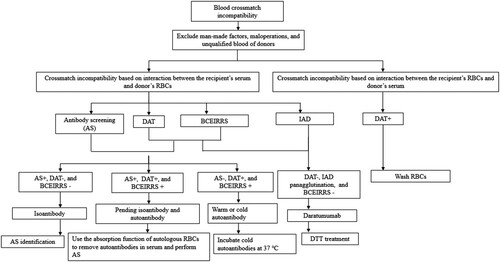
We performed positive and negative blood group typing, antibody screening, and the selection of ABO and Rh homologous blood for cross matching prior to the transfusions (Flowchart 1). Forward and reverse blood group typing and Rh(D) blood group identification method: Microlab STAR IVD Automatic Blood Analyzer. Irregular antibody screening was carried out using the normal saline method, coacylamine method, and anti-human globulin method. We followed the manufacturer’s instructions provided in the package inserts and National Clinical Laboratory Procedures [Citation2] for the other tests, including antiglobulin test, absorption test, and diffusion test. Saline tube test, microcolumn agglutination, and polybrene were adopted for crossmatch tests, and we followed the manufacturer’s instructions provided in the package inserts. Antibody subtypes were identified with a heat release test, while isoantibodies and autoantibodies were detected with an acid release test. The flowchart about blood crossmatch incompatibility test was shown in .
Figure 1. The flowchart about blood crossmatch incompatibility test. DAT: direct antiglobulin test; IAT: indirect antiglobulin test; BCEIRRS: blood crossmatch by examining the interaction between the recipient’s RBCs and serum.
The procedures of the diffusion test are:
Cool all reagents to 4°C for testing.
Washing before diffusion: wash the red blood cells in the whole blood to remove impurities.
Add 1 unit of hematocrit to 1 unit of citric acid diffusion solution and place in a 13 mm × 100 mm test tube.
Seal and shake the tube upside down for 90 s.
Unseal and centrifuge for 45 s.
Remove the supernatant into a clean test tube and add the neutralizing solution into the diffusion solution until the pH is adjusted to about 7.0.
Re-centrifuge to remove impurities and collect the upper layer of the diffusion liquid.
Diffusion test analysis:
Antigen-positive cells were subjected to a reaction with the solution from step (7) and the supernatant solution from the final cell wash prior to diffusion.
The diffusion test was successful and the diffusion solution could be used if the reaction with the diffusion solution was positive and with the supernatant of the final cell wash was negative.
There was either no antibody in the diffusion solution or it was a drug-dependent antibody if the reaction of both, the diffusion solution and the supernatant of the final cell wash, were negative.
The diffusion test failed, and the cells need to be washed and diffused again if the reaction of both, the diffusion solution and the supernatant of the last cell wash were positive.
Criteria: ABO positive setting /RhD agglutination degree should meet 4+, and anti-setting agglutination degree should not be less than 2+ (except subtype). At the same time, the agglutination degree of the Oc tube and self-tending should be 0. The standard of cross-matching blood is that there is no agglutination and hemolysis on the primary and secondary sides. The results of antibody identification were interpreted against the matching 'Red Blood Group Antibody Identification Cell Response Pattern Table.'
Steps of the polybrene test:
Add the serum into the labeled test tube.
Add +/- control serum to the quality control tube of the +/- control, respectively.
Add 3% concentration Rh(D) positive erythrocyte suspension to the quality control tube and mix the test tubes.
Add 500∼1000 μL of low ion solution and mix again.
Incubate at room temperature for 1 min (or longer).
Add 2∼4 drops of polycoagulant reagent to each test tube.
Centrifuge for 1 min (3100 r/min).
Discard the supernatant, shake the test tube gently, and observe whether there is strong agglutination after the red blood cells are re-suspended.
Add 1 drop of the re-suspension.
Gently shake the test tube and observe and record the results within 1 min.
Result analysis:
1. Step (8) above: Observe whether all test tubes have strong agglutination.
If there is strong agglutination, it can be used for the next test.
If there is low or medium mass agglutination, the next test can also be carried out, but the test results can only be negative or positive, not strong or weak.
If there is no agglutination, the test failed; need to be tested again.
2. Step (10) above: Gently shake the test tube to fully mix the suspension with the clot. Observe the results for 1 min; do not shake at this time.
Observe the quality control tube – negative and positive control. The weak positive control should have small clots that do not disperse within 1 min, while all clots should disperse within 1 min in the negative control.
Observe the results of all test tubes for 1 min, and determine the agglutination intensity according to the size of the clot.
3. Step (10) above: Directly record the results and end the test.
3. Results
3.1 Cause analysis for discrepant forward and reverse blood group typing
Different factors contribute to discrepant forward and reverse blood group typing, the most common of which is a serotyping weak response or no response. 11 cases in total, including 6 instances of cold agglutinin, 2 instances of monoclonal immunoglobulin, 2 instances of bone marrow transplant, and 2 instances of blood group subtype (). Conditions that cause weakened serotype included multiple myeloma (MM), pancytopenia, and leukemia chemotherapy. There was 1 case of cold autoantibody in a patient with hemolytic anemia; 1 case of acute hepatitis E among the 6 cases of cold agglutinin (high potency cold agglutinin); 1 case of abnormal monoclonal immunoglobulin secretion due to MM; and 1 case of bone marrow transplant in a patient with acute leukemia ().
Table 1. The forward and reverse blood group typing in our study.
Table 2. Cause analysis and treatment of forward and reverse blood group typing discrepancy.
3.2 Cause of crossmatch incompatibility
Among the 57 cases of crossmatch incompatibility, there were 23 cases with a combination of cold autoantibody and warm autoantibody, 18 cases with a combination of alloantibody and autoantibody, 6 cases of alloantibody, 6 cases of warm autoantibody, 3 cases of cold autoantibody, and 1 case with a combination of daratumumab therapy combined with autoantibody. Among these causes, combinations of cold autoantibodies and warm autoantibodies accounted for the largest share (40.35%), while combinations of alloantibodies and autoantibodies accounted for the second-largest share (31.58%) ().
Table 3. Cause analysis of 57 cases of crossmatch incompatibility.
3.3 Specificity test results of blood transfusion recipients
The most common cause of crossmatch incompatibility among the 24 blood transfusion recipients was an irregular antibody of the Rh blood group system, with 6 cases of anti-E (25.00%), 5 cases of anti-c, E (20.83%), 2 cases of anti-C, e (8.33%), 1 case of anti-c (4.17%). The MNS blood group system accounted for the second biggest proportion, with 7 cases of anti-M (29.17%) and 1 case of anti-S (4.17%). The Lewis blood group accounted for the third highest proportion, with 1 case of Lea (29.17%) ().
Table 4. Specificity of irregular antibodies in anti-ethmoid positive patients with cross-matched blood.
3.4 Blood transfusion recipient disease type
Types of diseases among the 57 blood transfusion recipients: there were 11 cases of immune disorders, including immune hemolytic anemia, rheumatoid arthritis, and immune thrombocytopenic purpura; 32 cases of hematologic diseases, including MM, myelodysplastic syndrome (MDS), diffuse large B-cell tumor, B-cell lymphoma, plasma cell lymphoma, acute myelogenous leukemia, and aplastic anemia; 4 cases of tumors, including pancreatic cancer, prostate cancer, and others; and 10 cases of other diseases, such as cirrhosis, hepatic failure, tuberculous pleurisy, and others.
4 Discussion
Safe blood transfusions depend on consistent positive and negative blood group typing and cross-matching of blood. Blood group typing and agglutination, as measured by major and (or) minor crossmatch, can occur in clinical practice. Diseases, individual patient circumstances, and medication administration are just some of the causes.
Among all other causes of discrepant forward and reverse ABO blood group typing, serotyping weak response or no response accounted for the largest proportion (6 cases of MM, 1 case of whole blood cell decrease, 1 case of tumor, 2 cases of leukemia chemotherapy, and 1 case of ectopic pregnancy) with reverse blood group typing agglutination < 2 + in all cases. Herein we provide four treatment options for the exclusion: enhancing the serum volume for the reverse blood group typing in the tube test (serum: red blood cell = 3:1); centrifuging the specimen after it is stored in a refrigerator at 4°C [Citation4];incubating the specimen for 30–60 min at 4°C and submitting it for absorption and diffusion test; and enhancing antigen–antibody response by adding a low ionic solution [Citation5] to the specimen to lower its red blood cell surface potential.
MM, a malignant plasma cell dyscrasia, produces a considerable quantity of aberrant immunoglobulins, the M-protein. Significant amounts of M-protein can invert the ratio of plasma albumin to globulin [Citation6]. An excessive increase in plasma globulin and plasma globulin-coated red blood cells decreases negative charges on the surface of red blood cells and repulsion between blood cells, ultimately leading to aberrant rouleaux agglutination of red blood cells [Citation7]. Herein we provide two treatment options. Option 1: Isotonic saline dilution. Add 1 drop of saline onto a glass slide; the abnormal rouleaux agglutination of red blood cells disappears instantly [Citation8]. Option 2: Saline replacement. Replace the clear plasma supernatant liquid for reverse blood group typing with 100 μl normal saline and resuspend it. The agglutination disappears instantly [Citation9].
Blood groups of bone marrow transplant patients more closely resemble those of donors as a result of hematopoietic reconstitution of hematopoietic stem cells in recipients [Citation1]. Considering that both donor and recipient red blood cells can be found in the peripheral blood, forward blood group typing is a hybrid technique. Variations in reverse blood group typing are to be expected after a transplant. Using forward and reverse blood group typing, we found that a patient with blood group B could successfully receive a bone marrow transplant from a donor who was typed as blood group O and B in forward and reverse blood group typing, respectively. Our study also included a patient with blood group AB who received a bone marrow transplant from a donor with blood group B. This donor was typed as B and AB in forward and reverse group typing, respectively. Within 4 months of their non-homotypic hematopoietic stem cell transplants, both patients underwent a transformation that rendered ABO blood group typing difficult. Patients with forward and reverse blood group typing discrepancies should have their medical records reviewed to learn more about their diseases, and both the patient and donor involved in the bone marrow transplant should be questioned about their blood types. With this information, it is possible to confidently determine the blood group of the patients and provide them with the correct blood transfusion.
ABO subtype, also known as ABO variant, is associated with the following blood group test results: (1) forward and reverse ABO blood group typing discrepancy; (2) consistent forward and reverse ABO blood group typing with forward blood group typing response intensity ≤ 3 + or reverse blood group typing response intensity ≤ 2, as seen in the two cases of subtype AB in this study. Anti-H agglutination intensity is the major criterion for defining ABO subtypes. From strong to weak, the H antigen of each blood group is O>A2>B>A1>A2B>A1B. The antigen identification method was determined based on the results of the absorption and diffusion test in which the absorptive capacity of subtypes was lower than that of normal red blood cells but the diffusion capacity was higher [Citation10]. The absorption and diffusion tests are typically used to detect ABO subtypes when there are discrepancies between forward and reverse ABO blood group typing, or when forward blood group typing is O. This method, however, has a few drawbacks. An additional genetic test based on molecular biology is recommended if possible.
Serum cold agglutinins are typically IgM, which can agglutinate an individual’s red blood cells or isotype red blood cells at temperatures below 32 °C, and reversibly disperse agglutinated red blood cells at 37°C [Citation11]. Cold agglutinins are most effective at a temperature of 4°C, where their agglutination abilities are at their highest. In addition, cold antibodies are active at temperatures lower than 30°C, suggesting that temperature plays a significant role. However, nonspecific agglutination, the reaction product of red blood cell and cold agglutinins, can interfere with forward and reverse ABO blood group typing due to its role in mycoplasma pneumoniae infection, autoimmune hemolytic anemia, and other diseases. Treatments: Forward and reverse blood group typing should be performed at 37°C. For forward blood group typing, the red blood cells of the patient can be repeatedly washed with normal saline at 37°C, and the serum of the patient can be absorbed and reused multiple times for reverse blood group typing. Forward blood group typing should be performed after specimens have been incubated for 15 min in a water bath at 37°C and the red blood cells have been treated with 2-Mercaptoethanol (2-ME) to prevent red cell suspension preparation failure caused by high potency cold autoantibodies [Citation1]. The capacity of cold agglutinin to agglutinate corresponds with its potency. Patients with low potency requiring a blood transfusion should have a crossmatch conducted in a 37°C water bath. Crossmatching with absorbed serum and diffused red blood cells should be performed on patients with high potency requiring a transfusion. Patients with high potency should get a gradual blood transfusion using an infusion warmer while their vital signs are closely monitored [Citation12].
Most warm autoantibodies are IgG, while IgM is uncommon. Warm autoantibodies can obfuscate clinically relevant alloantibodies and impede blood transfusion assays. Life-threatening complications can arise from transfusing the wrong type of blood.
Treatments: The presence of alloantibodies in serum can be determined by performing a diffusion test on red blood cells with positive direct antiglobulin, which exposes the antibody binding site, absorbs autoantibodies in the serum, and prevents autoantibodies from disturbing the red blood cells, allowing for accurate blood group typing and crossmatching [Citation13]. (1) In the event of a transfusion emergency, it is best to use blood from a donor whose ABO and Rh types are consistent with those of the recipient's, whose blood has fewer reactive direct antiglobulins than the recipient's blood, and whose blood agglutinates only slightly with the recipient's serum. (2) For blood transfusions under normal circumstances, it is best to use blood from a donor whose ABO and Rh types are consistent with those of the recipient’s, and with isotypic MN, Ss, and kidd.
MM has high levels of CD38 expression. When it comes to treating relapsed, refractory multiple myeloma, daratumumab (DARA), a monoclonal antibody that targets CD38, is both safe and effective [Citation14,Citation15]. In this study, we found one case of daratumumab therapy combined with autoantibodies. CD38 is widely expressed in human cell membranes and is a type II transmembrane protein. Indirect antiglobulin tests (IATs) including those used for screening and identifying abnormal antibodies, red blood cell antigen phenotyping analyses, and crossmatch tests rely on the ability of DARA to agglutinate with the reagent or donor red blood cells [Citation16,Citation17]. However, DARA has no effect on crossmatching between ABO blood groups, RhD blood groups, or saline medium. Consequently, polybrene can be used for blood specimens, antibody screening tests, and crossmatches for patients with CD38 monoclonal antibodies. Despite its limited capacity to detect Kell antibody, polybrene crossmatch remains a relatively safe test in China and Southeast Asia as the Kell antibody is quite rate in these populations [Citation18]. Dithiothreitol (DTT), a thiol-reducing agent, may inhibit anti-CD38 antibodies from binding to donor red blood cells by denatured CD38 on the surface of the cells by breaking disulfide bonds in the extracellular region. Anti-globulin cards are used to identify red blood cells; it has been observed that treating them immediately with 0.04 mol/L DTT for 15 min at 37°C totally eliminates the action of the CD38 monoclonal antibody. At the same time, the detection abilities of K antigen, LW antigen, JMH antigen, Lub antigen, Dia antigen, Jka antigen and Rh blood group system antigen are still present and functional [Citation19]. Red blood cell antibodies that are neither anti-A nor anti-B are referred to as irregular antibodies. Extreme hemolytic transfusion responses and failed red blood cell transfusion may occur if this antibody is present, making a compatible crossmatch more difficult [Citation20]. The Rh blood group system accounts for the vast majority of the reported instances of significant irregular antibodies, although the MNS blood group system and the Lewis blood group system also play a role. This result is consistent with prior findings [Citation21].
Treatments: Antibody identification should be performed on positive antibody specimens to determine the type and clinical significance of the antibody.
5 Conclusion
Forward and reverse ABO blood group type, as well as crossmatch incompatibility, may occur for a variety of reasons. The study of blood types and crossmatch incompatibility is of utmost importance, and those working in the field of blood transfusions must have a comprehensive awareness of all test techniques. The diagnostic process should not, however, prevent or postpone an emergency blood transfusion. If a blood transfusion facility does not have the necessary equipment, it should send blood samples to the regional blood center so that they may be properly typed. This allows for a more precise crossmatch, and hence a safer and more effective blood transfusion.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Zheng ZL, Liu J, Cheng XM. Cause analysis and solution of ABO positive and negative stereotypes inconsistency in 51 cases. Chin Mod Med. 2021;28(29):170–173. doi:10.3969/j.issn.1674-4721.2021.29.049.
- Shang H, Wang YS, Shen ZY. National operating procedures for clinical examination. 4th ed. Beijing: People's Medical Publishing House. 2015; p. 118–143.
- Yudin J, Heddle NM. A 13-question approach to resolving serological discrepancies in the transfusion medicine laboratory. Lab Med. 2014 Summer;45(3):193–206. doi:10.1309/LMEWVSNT2F3O5JDN
- Sun Y, Wang XN, Liu B. Clinical analysis of positive and negative typification of blood group in 152 cases. Chinese J Labor Diag. 2020;24(7):1218.
- Ma HL, Yun ZQ, Yang B, et al. Application of"three step analysis"of complicated ABO blood group. J Clin Hemat. 2011;24(2):75–77. doi:10.3969/j.issn.1004-2806-B.2011.01.005.
- Liu ZY, Zhang GQ, Yu WZ, et al. Experimental study of hypercoagulability in patients with multiple myeloma. J Exp Hemat. 2015;23(1):142–145. doi:10.7534/j.issn.1009-2137.2015.01.027.
- Park J, Jekarl DW, Park SY, et al. Combined group I and III ABO discrepancies in multiple myeloma with IgG-lambda type: A case report. Med Princ Pract. 2017;26(1):90–92. doi:10.1159/000450579
- Cui Y, Du J, Yang SM, et al. The influencing factors and treatment of blood grouping and cross matching in patients with multiple myeloma. Chin J Cell Mol Immunol. 2017;33(10):1419–1421. doi:10.13423/j.cnki.cjcmi.008465.
- Cheng J, Jiang M, Lin CY, et al. Cause analysis and treatment of ABO blood type abnormality in patients with multiple myeloma. Chin J Clin Lab Sci. 2020;38(6):440–442. doi:10.13602/j.cnki.jcls.2020.06.13.
- Cao HR, Meng XJ, Wang ZJ, et al. Analysis and report of ABO subtype identification results: one case report of B(A)04 subtype. Journal of Clinical Hematology. 2017;30(2):152–154. doi:10.13201/j.issn.1004-2806-b.2017.02.024.
- Meny GM. Recognizing and resolving ABO discrepancies. Immunohematol. 2017;33(2):76–81. doi:10.21307/immunohematology-2019-012
- Xie HY, Zhou ZX, Gu HH, et al. Blood type incompatibility caused by cold agglutination: a study of 16 cases. Chinese J Blood Trans. 2021;34(1):40–43. doi:10.13303/j.cjbt.issn.1004-549x.2021.01.013.
- Yang R, Luo YP, Xin H. Autoantibody combined with anti-Wra in a patient with autoimmune hemolytic anemia. Chinese J Blood Trans. 2019;32(07):718–720. doi:10.13303/j.cjbt.issn.1004-549x.2019.07.031.
- Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(8):754–766. doi:10.1056/NEJMoa1606038
- Chari A, Suvannasankha A, Fay JW, et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood. 2017;130(8):974–981. doi:10.1182/blood-2017-05-785246
- Chapuy CI, Nicholson RT, Aguad MD, et al. Resolving the daratumumab interference with blood comp atibility testing. Transfusion. 2015;55(6pt2):1545–1554. doi:10.1111/trf.13069
- Oostendorp M, Lammerts van Bueren JJ, Doshi P, et al. When blood transfusion medicine becomes complicated due to interference by monoclonal antibody therapy. Transfusion. 2015;55(6 Pt 2):1555–1562. doi:10.1111/trf.13150
- Liu F, Liu J. Possible insensitivity of the polybrene antibody screen to detect anti-Jka. Ann Clin Lab Sci. 2006;36(1):101–102.
- Song J, Kong YK, Wang SY, et al. To remove monoclonal anti-CD38 interference from compatibility testing:A simple method. Chinese J Blood Trans. 2021;34(9):974–977. doi:10.13303/j.cjbt.issn.1004-549x.2021.09.010.
- Noiret L, Slater A, Higgins JM. Determinants of red blood cell alloantibody detection duration: analysis of multiply alloimmunized patients supports peritransfusion factors. Transfusion. 2017;57(8):1930–1937. doi:10.1111/trf.14157
- Hou RQ, Yang HY, Cui YP, et al. Analysis of the results of irregular antibody identification in patients and its clinical significance. J Exp Hemat. 2020;28(3):961–966. doi:10.19746/j.cnki.issn1009-2137.2020.03.040.
