ABSTRACT
Objectives
The clinical characteristics, risk factors and survival prognosis of pericardial effusion (PE) after haematopoietic stem cell transplantation (HSCT) in children were investigated.
Methods
Clinical data of children who underwent HSCT at the Children's Hospital Affiliated with Chongqing Medical University from January 2016 to December 2022 were analysed retrospectively. Cox proportional hazards regression and the Kaplan-Meier method were used to analyse the risk factors for post-HSCT PE and its impact on outcomes, respectively.
Results
We enrolled 452 patients with HSCT: 307 males and 145 females, with a median age of 3.4 (1.8 to 6.5) years at transplantation. Forty-five patients (10%) had PE within a median time of 25 (10.5 to 44) days, 42 (93%) within 100 days. Three patients with large PE were treated with pericardiocentesis and drainage, while the others were treated conservatively. Of the 45 patients with PE, 24 survived, and their PE disappeared after treatment. Graft-versus-host disease (GVHD) grade, abnormal pre-HSCT electrocardiogram, hepatic veno-occlusive disease (HVOD), pulmonary infection and Epstein–Barr virus (EBV) infection were risk factors for PE. The overall survival (OS) rates at 1, 3, and 5 years were 86.0%, 84.2%, and 82.3%, respectively. PE had a significant negative effect on OS after HSCT (P < 0.0001). Particularly, one patient with large PE died of pericardial tamponade.
Conclusions
Post-HSCT PE usually occurred within 100 days. GVHD grade, abnormal pre-HSCT electrocardiogram, HVOD, pulmonary infection and EBV infection were closely related to PE. PE had a significant negative effect on OS rate.
Introduction
With the rapid development of haematopoietic stem cell transplantation (HSCT), more and more children with malignant and non-malignant diseases can be cured [Citation1]. Pericardial effusion (PE) is a post-HSCT cardiovascular complication, whose incidence in children is significantly higher than that in adults [Citation2]. The overall survival (OS) rates of children with post-HSCT PE is poor [Citation3–6]. Therefore, it is important to have a comprehensive understanding of the risks and outcomes of PE.
The amount of fluid in the pericardial space usually determines the severity of PE related symptoms. A slow-progressing PE is usually asymptomatic, while a rapid accumulation of PE can lead to a sudden increase in pericardial pressure and dramatic symptoms, and even life-threatening pericardial tamponade [Citation7]. In children, the occurrence of post-HSCT PE may be related to age, sex, disease type, disease status, donor type, stem cell source, human leukocyte antigen (HLA) mismatch, graft-versus-host disease (GVHD), thrombotic microangiopathy (TMA), heart failure, arrhythmia, systemic hypertension, total body irradiation, hepatic veno-occlusive disease (HVOD), delayed neutrophil engraftment, etc. [Citation2,Citation3,Citation5,Citation8–12]. In adults, age, multiple transplant procedures, GVHD, delayed neutrophil engraftment, TMA, and disease recurrence may be risk factors for PE [Citation13–16]. However, to the best of our knowledge, the risk factors, aetiology, and treatment of post-HSCT PE remain unclear.
The primary objective of our study is to analyse the clinical characteristics and to identify risk factors and survival outcomes of children with post-HSCT PE. We hope that this study will help in dealing with PE and reduce the risk of its occurrence.
Patients and methods
Patients
We conducted a retrospective single-centre study of 452 consecutive paediatric patients undergoing HSCT between January 2016 and December 2022. Patients with a clear diagnosis and no PE within one month prior to HSCT were included. Patients with non-first HSCT, incomplete clinical data, and loss to follow-up were excluded. This study was approved by the Ethics Committee of the Children's Hospital Affiliated with Chongqing Medical University.
Data collection
Case data were collected from the medical record browsing system and included sex, age, type of primary disease, donor-recipient relationship, HLA status, stem cell types, GVHD occurrence, pre-HSCT heart-related examination results [electrocardiogram (ECG), myocardial markers, echocardiography], post-HSCT complications [HVOD, pulmonary infection, cytomegalovirus infection, Epstein–Barr virus (EBV) infection, septicaemia], the diagnosis and treatment process of PE, and the follow-up survival status.
Transplantation details and conditioning regimens
High-resolution HLA-typing tests for 10 alleles (HLA-A, -B, -C, -DR and -DQ) were used to select donors for HSCT [Citation17]. HLA 10/10 was defined as HLA-typing matched, and the rest were defined as HLA-typing mismatched. Donor types included matched related and unrelated donors, mismatched related and unrelated donors, and autologous peripheral blood stem cells.
Our conditioning regimens were based on busulfan (BU)+cyclophosphamide (CY) or BU + fludarabine (FLU) and were adjusted according to the patient's disease characteristics. Most patients with thalassemia major were given thiotepa, and some patients with leukaemia were given cytarabine (Ara-C), etoposide (VP16) and Melphalan. A few autologous transplant patients received carboplatin according to their disease conditions. No patients received TBI.
Diagnosis and prophylaxis of GVHD
Acute graft-versus-host disease (aGVHD) occurred within 100 days after HSCT and was characterized by inflammatory reactions mainly in the skin, liver and gut. AGVHD included grades I, II, III, and IV, and the specific grading standards referred to the improved aGVHD Glucksberg grading standard. The diagnosis of chronic GVHD conformed to the NIH grading criteria [Citation18].
GVHD prophylaxis was mainly based on cyclosporine (CsA), which was combined with mycophenolate mofetil (MMF) and/or short-term methotrexate (MTX). Cord blood transplantation recipients received CsA and methylprednisolone, and some patients who underwent haploidentical HSCT received tacrolimus and MMF.
Prevention of HSCT-related complications
Alprostadil and ursodeoxycholic acid were used to prevent HVOD. Before HSCT, acyclovir or ganciclovir, voriconazole, levofloxacin, and imipenem were used to prevent viral, fungal, and bacterial infections.
Diagnosis of PE [Citation19]
PE was defined based on its size and location of accumulation around the heart. A normal PE was <5 mm. A small PE of 5 to 9 mm was observed along the length of the posterior wall of the heart but not anteriorly. A moderate PE was 10 to 19 mm during diastole and distributed circumferentially around the heart. A large PE was ≥20 mm and found circumferentially around the heart. Pericardial tamponade referred to the occurrence of right atrial collapse and limited cardiac diastolic function, regardless of whether PE was >20 mm. According to the occurrence of PE, the patients were divided into a PE group and a non-PE group.
Statistical analysis
Count data and measurement data with a normal distribution or nonnormal distribution are expressed as number and percentage, mean and standard deviation, or median (quartile 1, quartile 3). Univariate and multivariate analyses of potential risk factors were performed using a Cox proportional hazards model, and the results are expressed as hazard ratios (HRs) and their 95% confidential intervals (95% CIs). OS was defined as the length of time from HSCT to death from any cause. The Kaplan-Meier method was used for survival analysis, and the log-rank method was used to compare OS rates.
The primary endpoint of this study was the risk factors affecting the occurrence of PE, and the secondary endpoint was a statistically significant difference in OS. We analysed the potential risk factors affecting the occurrence of PE: sex, age, type of primary disease, donor-recipient relationship, HLA status, stem cell types, the occurrence and degree of GVHD, pre-HSCT heart-related examination results (ECG, myocardial markers, echocardiography), post-HSCT complications (HVOD, pulmonary infection, cytomegalovirus infection, EBV infection, septicaemia). Variables with P < 0.05 were included in the multivariate analysis. P < 0.05 indicated statistical significance.
Calculations of the Kaplan-Meier method and the log-rank method were performed using the R programming language, and other calculations were performed using SPSS 25.0 software.
Results
Patient characteristics
A total of 452 children with HSCT were enrolled, namely, 307 males (68%) and 145 females (32%). The median age at transplantation was 3.4 (1.8-6.5) years. There were 253 patients (56%) with haematopoietic system diseases (including leukaemia, aplastic anaemia, severe thalassemia, and myelodysplastic syndrome), 180 patients (40%) with primary immunodeficiency diseases, 14 patients (3%) with lymphoid system diseases (including various lymphoid tumors, haemophagocytic syndrome, and lymphoproliferative diseases), and 5 patients (1%) with solid tumors (including neuroblastoma and pinealoblastoma). There were 444 patients (98%) who underwent allogeneic HSCT and 8 patients (2%) who underwent autologous HSCT. There were 216 patients (48%) with matched HLAs and 236 patients (52%) with mismatched HLAs. Regarding the stem cell source, 427 patients (94.4%) received peripheral blood, 3 (0.7%) received bone marrow, 15 (3.3%) received cord blood, 3 (0.7%) received peripheral blood and cord blood, and 4 (0.9%) received bone marrow and cord blood. The characteristics of the study patients are summarized in .
Table 1. Characteristics of the study patients.
Clinical characteristics and treatment of post-HSCT PE
There were 407 patients (90%) with non-PE, 33 patients (7%) with small PE, 8 patients (2%) with moderate PE, and 4 patients (1%) with large PE, and the incidence of PE was 10% (45/452). The median time for the occurrence of PE was 25 (10.5-44) days. Of the 45, 42 patients (93%) had PE within 100 days, and the other 3 patients had PE within 101 days, 6 and 14 months. The main symptoms and signs of PE were anhelation, chest tightness, palpitation and dizziness, sinus tachycardia, facial or systemic oedema and hepatomegaly. PE was found by echocardiography in 39 patients, chest CT in 5 patients, and chest X-ray in 1 patient, and PE was confirmed by echocardiography in all patients. Serum albumin decreased to varying degrees when PE occurred in all patients, and the lowest level was 28.0 ± 4.2 g/L.
The causes of PE (n = 45) were as follows: infection (n = 23, 51%), hypoalbuminaemia (n = 16, 36%), capillary leakage syndrome (CLS) (n = 31, 69%), and post-transplant lymphoproliferative disorder (PTLD) (n = 2, 4%). Regarding treatment, all patients with PE were given albumin and diuresis, 31 were given methylprednisolone, and 11 were infused with hydroxyethyl starch; 3 patients with cardiac insufficiency were treated with phentolamine and dobutamine (2 patients) or milrinone (1 patient). Three patients with large PE were treated with pericardial puncture and drainage; 2 of these patients had negative pericardial fluid aetiology, and 1 patient died of aggravated pericardial tamponade and did not undergo pericardial fluid aetiological examination. Among the 45 patients, PE disappeared in 24 patients after treatment, and the outcomes of PE could not be confirmed in 21 patients because of death without echocardiography. The clinical data of patients with moderate to large PE and small PE are summarized in and Supplemental Table 1.
Table 2. Clinical characteristics of the 12 patients with moderate to large pericardial effusion.
Risk factors for PE
Univariate analysis showed that HLA typing (HR, 0.536; 95% CI, 0.289 to 0.997; P < 0.05), GVHD grade (HR, 0.368; 95% CI, 0.203 to 0.664; P < 0.05), pre-HSCT ECG (HR, 0.434; 95% CI, 0.235 to 0.798; P < 0.05), HVOD (HR, 0.185; 95% CI, 0.066 to 0.517; P < 0.05), pulmonary infection (HR, 0.261; 95% CI, 0.093 to 0.728; P < 0.05) and EBV infection (HR, 1.983; 95% CI, 1.092 to 3.600; P < 0.05) were associated with PE. The clinical factors with statistical significance in univariate analysis were input into multivariate analysis. The results showed that GVHD grade (HR, 0.428; 95% CI, 0.227 to 0.810; P < 0.05), abnormal pre-HSCT ECG (HR, 0.472; 95% CI, 0.256 to 0.872; P < 0.05), HVOD (HR, 0.235; 95% CI, 0.081 to 0.683; P < 0.05), pulmonary infection (HR, 0.288; 95% CI, 0.102 to 0.811; P < 0.05) and EBV infection (HR, 2.524; 95% CI, 1.359 to 4.686; P < 0.05) were risk factors for PE ().
Table 3. Univariate and multivariate analyses of risk factors for the onset of pericardial effusion.
Survival outcomes
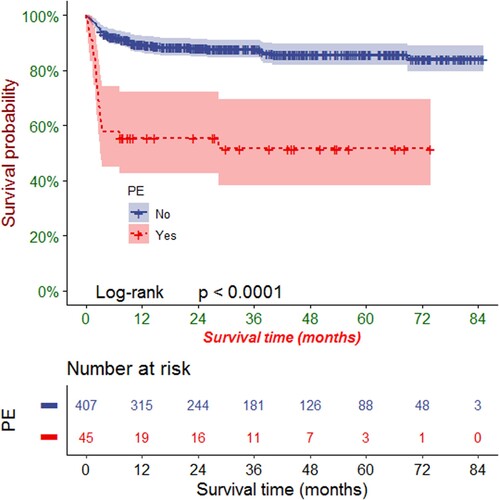
The follow-up deadline was March 2023. During the median follow-up of 28.7 months (range: 11.0-54.0), 73 patients died, resulting in an OS rate of 84% (379/452). Among the deceased were twenty-one patients who experienced PE after HSCT. This group comprised thirteen patients with respiratory and circulatory failure, five patients with bleeding from vital organs (intracranial, pulmonary, or gastrointestinal), two patients with severe GVHD, and one patient with pericardial tamponade. The OS rates during the follow-up period were 53% (24/45) and 87% (355/407) in patients with PE and those without PE, respectively.
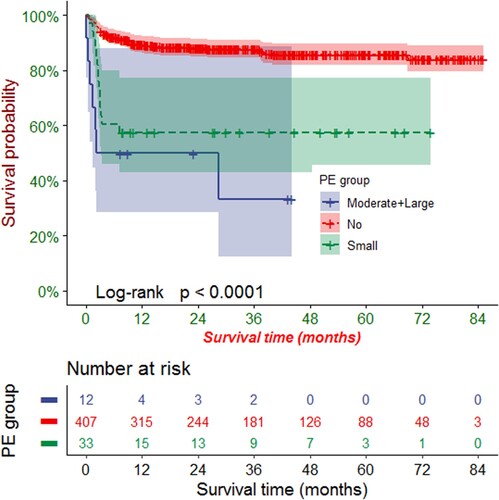
Kaplan-Meier analysis revealed that the OS rates at 1, 3, and 5 years were 86.0% (95% CI: 82.8%-89.3%), 84.2% (95% CI: 80.8%-87.70%), and 82.3% (95% CI: 78.6%-86.3%), respectively. PE had a significant negative effect on the OS rate of patients after HSCT (P < 0.0001, ). According to the stratified analysis of PE size, we found that the more severe the PE, the more obvious the negative impact on OS rate (P < 0.0001, ).
Discussion
Our patients had different risk factors for PE than previously reported children have had, and we also report the first child who died of post-HSCT PE. PE is a common complication after HSCT. The incidence of post-HSCT PE was 0.8-3.1% in adults [Citation13,Citation14] and 4.4-37.8% in children [Citation2–5,Citation8–11,Citation20]. In our study, the incidence of post-HSCT PE was 10%, which was consistent with reports abroad. The occurrence time of PE in our study was 25 (10.5-44) days after HSCT. Ninety-three percent of PE cases occurred within 100 days after HSCT, which was consistent with the time of PE reported in related studies [Citation4,Citation11]. This showed that post-HSCT PE occurred early, and regular follow-up within 100 days after HSCT by echocardiography can be used to detect PE in a timely manner to prevent life-threatening PE.
Our study showed that HLA type, GVHD grade, pre-HSCT ECG, HVOD, pulmonary infection and EBV infection were significantly correlated with PE, which was different from the results of other studies [Citation2,Citation8,Citation9,Citation20]. The discrepancy may be related to region, race, type of disease and other factors. The causes of post-HSCT PE are not clear, and more prospective studies are needed. Our multivariate analysis showed that GVHD grade, abnormal pre-HSCT ECG, HVOD, pulmonary infection and EBV infection were risk factors for PE. Previous studies [Citation8,Citation10,Citation20] have shown that GVHD was associated with the occurrence of PE. We also found that more severe GVHD was a risk factor for PE. GVHD is often associated with a cytokine surge, which may be a trigger for PE [Citation21]. Yanagisawa et al. [Citation20] found that the corrected QTD (QTcD) in children with post-HSCT PE was significantly prolonged before HSCT and that prolonged QT dispersion (QTD) and QTcD predicted the occurrence of post-HSCT PE, which was consistent with the results of our study. In our study, abnormal pre-HSCT ECG included intraventricular conduction block, premature contraction, ventricular pre-excitation, T wave or ST segment changes, PR prolongation, QT prolongation, and electrical axis deviation. Due to the variety of abnormal ECGs, the number of cases of each type was small and the statistical power was low, so no further analysis was performed on abnormal ECGs. In addition, our study suggested that HVOD was closely related to PE, which is consistent with a previous report [Citation3]. HVOD is a syndrome of fibrosis and narrowing of hepatic venules and hepatic sinusoids due to radiation and chemotherapy-induced endothelial cell damage in hepatic venules. The development of liver injury led to hypoalbuminaemia, causing capillary leakage and thus PE [Citation22]. Pulmonary infection was also closely related to PE, again consistent with earlier results [Citation23]. Most patients with PE (31/45, 69%) were treated with methylprednisolone, and the use of higher doses and longer periods of systemic hormones increased the risk of pulmonary infection. Although there were many aetiological agents of pulmonary infection in patients in our study, subgroup analyses were not possible due to the small number of cases, leading to a large statistical bias. EBV was a ubiquitous double-stranded DNA herpesvirus. After HSCT, the body's cellular immune reconstitution was slow, leading to reactivation of EBV and, in severe cases, viraemia and PTLD [Citation24]. Our study suggests that EBV infection is a risk factor for PE, while previous studies have shown that cytomegalovirus infection was associated with PE [Citation5,Citation11]. There has been no report on the correlation between EBV infection and PE.
Of the 45 patients with PE, 73% had small PE, 18% had moderate PE and 9% had large PE, which was similar to the results of Chen et al. [Citation12]. The main manifestations of PE in our group were anhelation, chest tightness, palpitation, dizziness, sinus tachycardia, oedema and hepatomegaly. Because of atypical clinical manifestations, clinicians should be vigilant to avoid missed diagnosis. The causes of PE in our study were mainly CLS, infection, hypoalbuminaemia and PTLD. Since some patients had two or more causes and there were few cases in each group, we did not further analyse the differences in the causes of different PE sizes.
At present, there is no systematic and standardized treatment plan for patients with post-HSCT PE. Notably, patients with post-HSCT PE should be actively treated with diuresis, enhanced immunosuppressants and methylprednisolone, and cardiotonic therapy should be given if cardiac insufficiency is complicated. If it is ineffective, the use of calcineurin inhibitors such as CsA, tacrolimus and sirolimus should be discontinued [Citation10,Citation12,Citation20]. In our study, 45 patients were treated with diuresis and albumin infusion, with some receiving hydroxyethyl starch and some receiving methylprednisolone and cardiotonic treatment. The PE of 24 surviving patients disappeared after treatment. Foreign studies [Citation3–6,Citation8–11,Citation20] showed that 17%∼100% of the patients with post-HSCT PE were treated with pericardiocentesis, while only 7% of the patients with PE were treated with pericardiocentesis in our study. The reason may be that most of the patients in our group had small and moderate PE, which dissipates after conservative treatment, and if pericardiocentesis was performed too actively, it would increase the risk of infection and bleeding.
Here, PE had a significant effect on the OS rate in patients with HSCT: the greater the amount of PE, the more it shortened OS. But for most patients, PE was not a direct cause of death. The causes of death in our group were respiratory and circulatory failure, important organ bleeding, and severe GVHD, consistent with relevant reports [Citation5,Citation12]. Other studies [Citation2,Citation7–11,Citation14,Citation17] have shown that PE progresses slowly after HSCT and can subside after active treatment, and no patient with PE as the direct cause of death was found in children. In adults, however, one study reported a patient with a large PE who died of cardiac tamponade 2 days later without emergency pericardiocentesis [Citation16]. In our study, 1 patient (, Patient 4) developed a large amount of PE only 1 d after reinfusion of hematopoietic stem cells. Paediatric patients with PE as the direct cause of death have never been reported before. Our patient who died was a teenager whose primary disease was aplastic anaemia. He received peripheral-blood hematopoietic stem cells from his brother with HLA 10/10. He received CY + FLU + anti-human thymocyte globulin (ATG) as a conditioning regimen and CsA + MTX as a GVHD prophylaxis regimen. The pre-HSCT ECG showed ST segment changes. Before his death, he was in a state of agranulocytosis with severe infection, but he had no complications such as GVHD or EBV infection. We think his rapid progression resulted from a severe infection leading to a dramatic increase in the volume of PE. When we found his PE, we immediately gave diuresis, albumin, methylprednisolone and pericardial puncture and drainage, but none of this helped. The experience with this teenager should remind clinicians that they should actively monitor echocardiography and address PE-related risk factors as early as possible to prevent such deaths if at all possible.
In conclusion, PE is not uncommon in patients after HSCT. Post-HSCT PE is closely related to GVHD grade, abnormal pre-HSCT ECG, HVOD, pulmonary infection and EBV infection. Post-HSCT PE significantly worsens the survival outcomes of patients, and large PE may be fatal. At present, there is no systematic treatment plan. To improve the survival outcomes of patients after HSCT, clinicians should actively treat the risk factors for post-HSCT PE and increase the monitoring frequency of echocardiography within 100 days after HSCT to detect heart-related complications early.
Supplemental Material
Download MS Word (23.1 KB)Acknowledgements
Ying Dou performed the research. Yan Meng, Luying Zhang, Xiaoying Lei, Xianmin Guan and Jie Yu offered detailed materials for this manuscript. Li Xiao provided guidance on statistical methods. Ke Tong wrote the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Zhao Y, He R, Oerther S, et al. Cardiovascular complications in hematopoietic stem cell transplanted patients. J Pers Med. 2022;12(11):1797.
- Tinianow A, Gay JC, Bearl DW, et al. Pericardial effusion following hematopoietic stem cell transplantation in children: incidence, risk factors, and outcomes. Pediatr Transplant. 2020;24(5):e13748.
- Diamond M, Ruiz-Mesa C, Corrales-Medina FF, et al. Incidence and outcome of pericardial effusion in pediatric patients after hematopoietic stem cell transplant: a single-institution experience. J Pediatr Hematol Oncol. 2018;40(2):132–136.
- Jaing TH, Chen SH, Wen YC, et al. Factors affecting survival in children with pericardial effusion after hematopoietic stem cell transplantation. Cell Transplant. 2017;26(11):1792–1797.
- Neier M, Jin Z, Kleinman C, et al. Pericardial effusion post-SCT in pediatric recipients with signs and/or symptoms of cardiac disease. Bone Marrow Transplant. 2011;46(4):529–538.
- Pfeiffer TM, Rotz SJ, Ryan TD, et al. Pericardial effusion requiring surgical intervention after stem cell transplantation: a case series. Bone Marrow Transplant. 2017;52(4):630–633.
- Khandaker MH, Espinosa RE, Nishimura RA, et al. Pericardial disease: diagnosis and management. Mayo Clin Proc. 2010;85(6):572–593.
- Rhodes M, Lautz T, Kavanaugh-Mchugh A, et al. Pericardial effusion and cardiac tamponade in pediatric stem cell transplant recipients. Bone Marrow Transplant. 2005;36(2):139–144.
- Cox K, Punn R, Weiskopf E, et al. Pericardial effusion following hematopoietic cell transplantation in children and young adults is associated with increased risk of mortality. Biol Blood Marrow Transplant. 2017;23(7):1165–1169.
- Versluys AB, Grotenhuis HB, Boelens MJJ, et al. Predictors and outcome of pericardial effusion after hematopoietic stem cell transplantation in children. Pediatr Cardiol. 2018;9(2):236–244.
- Aldoss O, Gruenstein DH, Bass JL, et al. Pericardial effusion after pediatric hematopoietic cell transplant. Pediatr Transplant. 2013;17(3):294–299.
- Chen X, Zou Q, Yin J, et al. Pericardial effusion post transplantation predicts inferior overall survival following allo-hematopoietic stem cell transplant. Bone Marrow Transplant. 2016;51(2):303–306.
- Liu YC, Gau JP, Hong YC, et al. Large pericardial effusion as a life-threatening complication after hematopoietic stem cell transplantation-association with chronic GVHD in late-onset adult patients. Ann Hematol. 2012;91(12):1953–1958.
- Norkin M, Ratanatharathorn V, Ayash L, et al. Large pericardial effusion as a complication in adults undergoing SCT. Bone Marrow Transplant. 2011;46(10):1353–1356.
- Liu YC, Chien SH, Fan NW, et al. Risk factors for pericardial effusion in adult patients receiving allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2015;169(5):737–745.
- Kubo H, Imataki O, Fukumoto T, et al. Risk factors for and the prognostic impact of pericardial effusion after allogeneic hematopoietic stem cell transplantation. Transplant Cell Ther. 2021;27(11):949. e1–9949.e8.
- Johansson T, Partanen J, Saavalainen P. HLA allele-specific expression: methods, disease associations, and relevance in hematopoietic stem cell transplantation. Front Immunol. 2022;13:1007425.
- Schoemans HM, Lee SJ, Ferrara JL, et al. EBMT-NIH-CIBMTR task force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone Marrow Transplant. 2018;53(11):1401–1415.
- Azarbal A, LeWinter MM. Pericardial effusion. Cardiol Clin. 2017;35(4):515–524.
- Yanagisawa R, Ishii E, Motoki N, et al. Pretransplant-corrected QT dispersion as a predictor of pericardial effusion after pediatric hematopoietic stem cell transplantation. Transpl Int. 2015;28(5):565–574.
- Dean RM, Fry T, Mackall C, et al. Association of serum interleukin-7 levels with the development of acute graft-versus-host disease. J Clin Oncol. 2008;26(35):5735–5741.
- Lee CC, Chang HH, Lu MY, et al. The incidence and risk factors of hepatic veno-occlusive disease after hematopoietic stem cell transplantation in Taiwan. Ann Hematol. 2019;98(3):745–752.
- Yang CL, Wang XD, Zhou XH, et al. Clinical characteristics and risk factors of pericardial effusion after hematopoietic stem cell transplantation in children with thalassemia major. Chin J Pediatr. 2022;60(4):323–328.
- Xuan L, Huang F, Fan Z, et al. Effects of intensified conditioning on Epstein-Barr virus and cytomegalovirus infections in allogeneic hematopoietic stem cell transplantation for hematological malignancies. J Hematol Oncol. 2012;5:46.