ABSTRACT
Objectives: We aimed to investigate relationships of platelet glycoprotein (GP) specific antibody with therapeutic efficacy of high-dose dexamethasone (HD-DXM) and bleeding score in primary immune thrombocytopenia (ITP) adults. Methods: A retrospective study was carried out to analyze relationships of polymorphism of GP specific antibody with initial therapeutic efficacy of HD-DXM and bleeding score of newly diagnosed ITP adults between 1 June, 2016 and 31 January, 2020. Results: 59 patients were involved in the study, with 33 cases of responders and 26 cases of non-responders between June 2016 and January 2020. At admission, there were 31 (52.5%) GP antibody-positive patients. Initial therapy of HD-DXM was effective for 78.6% GP antibody-negative patients and 35.5% GP antibody-positive patients, with a better therapeutic efficacy in patients with anti-GP Ib/IX antibody or anti-GP IIb/IIIa antibody but not in those with anti-GP Ib/IX antibody plus anti-GP IIb/IIIa antibody. Notably, therapeutic efficacy is much worse for minority (Uyghur) patients compared with corresponding Han patients. Similarly, it was much lower in GP antibody-positive patients compared with corresponding negative ones at low and medium bleeding score, with no response in GP antibody-positive patients at high bleeding score. Furthermore, there was a moderate negative correlation between therapeutic efficacy and GP-specific antibody (p < 0.05), but no obvious linear relationship between clinical bleeding degree and GP-specific antibody (p > 0.05). Conclusion: Collectively, the newly diagnosed ITP adults with GP-specific antibody have a poor response to short-term HD-DXM, especially in minority (Uyghur) patients with GP-specific antibody in China.
Introduction
Primary immune thrombocytopenia (ITP), an acquired autoimmune disorder, is characterized by isolated thrombocytopenia and is frequently associated with an increased bleeding risk. Annual incidence rate of ITP among adults is estimated to be 5–10 cases per 100,000 persons in the general population [Citation1], with two peaks in the age distribution [Citation2]. Clinical manifestations of ITP range from asymptomatic to mild bruising, mucosal bleeding, or even frank hemorrhage from any site, and intracranial hemorrhage is the most serious complication [Citation1]. The diagnosis of ITP remains one of exclusion of other causes of isolated thromobocytopenia based on patient history, physical examination, blood count, and evaluation of the peripheral blood film [Citation3]. Owning to cardiovascular disease, infection, and bleeding, ITP adults have a 1.3- to 2.2-fold higher mortality than general population [Citation4]. Clinically, the major therapeutic goal for primary ITP adults is to provide a safe platelet count to reduce or resolve bleedings, rather than a normal platelet count [Citation1]. Nowadays, the most common rescue therapy of corticosteroids has been recommended to newly diagnosed ITP adults who are asymptomatic or have minor mucocutaneous bleeding [Citation5], frequently with a prednisone tapering schedule (1 mg/kg daily for 6–8 weeks) or a high-dose dexamethasone (HD-DXM) (40 mg daily for 4 continuous days for 1–4 cycles) [Citation6,Citation7]. Due to discontinuation of corticosteroid therapy, nearly 30–50% of ITP adults achieve a sustained response, and 60–80% of patients have a partial response [Citation8,Citation9]. Except for corticosteroids, intravenous immunoglobulin (IVIG) is recommended as another common first-line or rescue therapy, especially to those at high hemorrhagic risk [Citation10,Citation11]. However, relapse following a brief initial response to the corticosteroids is common and about half of these patients require second-line therapy [Citation12]. Recently, accumulating evidence has documented that IVIG or anti-D Ig can be used for personalized treatment with or without steroids in cases where immediate platelet count elevation is desired, especially for patients with active bleeding [Citation5,Citation13]. Given a wide variation of presentation and clinical efficacy based on ethnicity, it is highly complex to decide whether the patients should be observed or resort to second-line therapy when they are failing to initial therapy.
Autoantibodies against platelet glycoproteins (GP) have long been accepted as a major pathophysiologic mechanism in ITP [Citation14], and they increase the bleeding risk of patients via impairing platelet production and destroying platelets [Citation1,Citation15–18]. As demonstrated in a majority of ITP patients, the autoantibodies mainly directed to the platelet membrane GP complexes, during which the main receptor GP IIb/IIIa may involve the Fc-receptor-mediated clearance of platelets via the reticuloendothelial system and the GP Ib/IX is closely related to the progress of platelet desialylation via Fc-independent release of sialidase from α granules of platelets [Citation6,Citation19,Citation20]. Although anti-platelet autoantibody testing is less sensitive for diagnosis [Citation21,Citation22], accumulating murine models and large cohort human studies have verified that antibody specificity (i.e. anti-GP IIb/IIIa and anti-GP Ib/IX) may play a significant role in assessing response to ITP therapy, such as glucocorticoids [Citation23], IVIG [Citation24] or corticosteroids [Citation23,Citation24]. Even with the availability of many drug therapies for ITP at present than in even the relatively recent past, the expression of platelet-specific antibody associated with the failure of HD-DXM in ITP adults remains poorly understood, especially in minority populations. Herein, we tended to analyze relationships of GP-specific antibody with initial therapeutic efficacy of short-term HD-DXM and bleeding score of ITP adults from two different ethnic backgrounds in China.
Materials and methods
Study design
A retrospective, single-center study was carried out at a tertiary care hospital to analyze relationships between the heterogeneity of GP-specific antibody and initial therapeutic efficacy of short-term HD-DXM together with bleeding score of newly diagnosed ITP adults from 1 June 2016 to 31 January 2020. The study was approved by the Ethics Committee of First Affiliated Hospital of Xinjiang Medical University (Approval number: 20200320-38), and informed consent was obtained from all participants in accordance with ethical principles of the Declaration of Helsinki. The newly diagnosed ITP adults who underwent the detection of platelet GP-specific antibody at admission were involved in our present study, whereas those with (1) secondary, relapsed or refractory ITP, (2) administration of corticosteroids in the past 3 months for other diseases, or (3) missing follow-up information were excluded from the study.
Treatment and monitoring
Therapy should be tailored to ITP patients based upon the presence of bleeding, desired platelet count increase, lifestyle that may predispose patients to trauma, side effects of therapy, and patient preferences [Citation10]. According to an International Consensus Report on the investigation and management of primary ITP [Citation25,Citation26], the newly diagnosed ITP adults received HD-DXM regimen timely at the dose of 40 mg daily for four consecutive days, during which blood pressure and blood glucose levels were closely monitored, and all adverse events were documented and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v4.0 [Citation27,Citation28].
Classification and response criteria for ITP
In clinical practice, a response to ITP-specific treatments represents the best confirmation of the diagnosis of ITP [Citation29]. According to the following clinical phases, the International Working Group (IWG) on ITP defines ITP as newly diagnosed within the first three months post-diagnosis, persistent within 3-12 months post-diagnosis, and chronic for over 12 months post-diagnosis [Citation12,Citation30]. As for refractory ITP, several definitions have been proposed over the past years and it was generally based on the absence of response or failure of splenectomy before the advent of medical alternatives [Citation25].
The IWG on ITP defines ITP therapy as complete response (CR) at a platelet count of greater than 100 × 109/L measured for more than seven days apart, partial response (PR) at a platelet count of more than 30 × 109/L or double from the baseline and absence of active bleeding manifestations, no response (NR) at a platelet count of less than 30 × 109/L or less than double from the baseline and presence of active bleeding manifestations requiring another line of therapy. Response duration was defined as time duration from the day of receiving HD-DXM to the day of documented relapse; relapse was defined as loss of CR or PR during the follow-up; and overall response (OR) was calculated by the sum of CR and PR [Citation11,Citation31,Citation32].
Grouping
After receiving short-term HD-DXM regimen, the patients who achieved CP or PR were classified as responder group and others were classified as non-responder group according to IWG on ITP recommended outcome criteria for treatment responses [Citation25]. Bleeding scale was used to quantify bleeding situation and risk assessment of patients according to Chinese consensus guidelines for diagnosis and treatment of adult primary ITP (2018 edition) [Citation1,Citation7], and the highest score among all bleeding scores was calculated by the sum of age scale and bleeding manifestation scale. After that, each domain was further divided into different scoring items, as illustrated in Supplementary Table 1. Considering total score of age and bleeding symptoms, the patients were divided into three grades: 0–2 score (low bleeding risk), 3–5 score (medium bleeding risk), and 6–8 score (high bleeding risk).
Monoclonal antibody-specific immobilization of platelet antigen (MAIPA) assay
Commercial immunoassay kits (BD Pharmingen TM) were routinely performed to detect platelet GP-specific antibody using a modified MAIPA assay as previously published [Citation33,Citation34].
Statistical analysis
Quantitative variables were expressed as mean (standard deviation) or median (25th and 75th percentiles, interquartile range [IQR]) and analyzed using two independent sample t test or Mann–Whitney U test as appropriate. Qualitative variables were summarized as percentage and analyzed using Chi-square or the Fisher’s exact test as appropriate. Moreover, Mantel-Haenszel Chi-square statistic and Pearson correlation analysis were carried out to estimate linear relationships between therapeutic efficacy of short-term HD-DXM and heterogeneity of platelet GP-specific antibody. SPSS software version 23.0 was used for statistical analysis and p-value of less than 0.05 was considered statistically significant.
Results
Baseline characteristics of patients
Demographic and clinical characteristics for the overall study cohort were summarized in . Among 79 screened patients, 59 newly diagnosed ITP adults were eligible, and there were 22 (38.6%) males and 37 (61.4%) females with mean age of 53.1 ± 17.0 years (range: 13.0–83.0 years) at ITP onset. After receiving short-term HD-DXM regimen, 33 ITP adults were categorized as responder group and 26 were non-responder group. The median of baseline platelet count and frequency of positive GP-specific antibody were lower in responder group than those in non-responder group (all p < 0.05). A higher proportion of Han patients and a lower proportion of Uyghur patients were observed in responder group than those in non-responder group (all p < 0.05). However, there were no significant differences in other baseline data between two groups (p > 0.05).
Table 1. Baseline characteristics of newly diagnosed ITP adults between responder and non-responder groups.
Therapeutic efficacy of short-term HD-DXM regimen on newly diagnosed ITP adults
Among 59 ITP adults, there were 31 (52.5%) patients with positive GP-specific antibody, including anti-GP IIb/IIIa antibody (n = 7), anti-GP Ib/IX antibody (n = 14), and anti-GP IIb/IIIa antibody plus anti-GP Ib/IX antibody (n = 10). At initial evaluation, the frequency of anti-GP Ib/IX antibody and anti-GP IIb/IIIa antibody plus anti-GP Ib/IX antibody was lower in responder group compared with non-responder group (p < 0.05), but there was no significant difference in the frequency of anti-GP IIb/IIIa antibody between two groups (p > 0.05) (). In the responder subgroups, merely 11 (35.5%) GP-specific antibody-positive ITP adults and 22 (78.6%) GP-specific antibody-negative ITP adults were effective to HD-DXM regimen (p = 0.001). Notably, therapeutic efficacy was better in patients with anti-GP Ib/IX antibody or anti-GP IIb/IIIa antibody than in those with anti-GP IIb/IIIa antibody plus anti-GP Ib/IX antibody (p = 0.003). Mantel-Haenszel Chi-square statistic showed a linear relationship between therapeutic efficacy and GP-specific antibody (p = 0.001) and Pearson correlation analysis further suggested a moderate negative correlation between them (r = −0.534, p = 0.001).
Table 2. Relationship between therapeutic efficacy of short-term HD-DXM regimen and newly diagnosed ITP adults with GP-specific antibody.
Therapeutic efficacy of short-term HD-DXM regimen on different ethnic ITP adults with platelet GP-specific antibody
In the ethnic ITP subgroups, only 7.7% (1/13) GP-specific antibody-positive Uyghur patients and 55.6% (10/18) GP-specific antibody-positive Han patients were response to short-term HD-DXM regimen, and there was a significant difference between them (p = 0.004). All these data suggest that the therapeutic efficacy of short-term HD-DXM is much worse for minority (Uyghur) ITP patients with platelet GP-specific antibody compared with corresponding Han patients ().
Table 3. Relationship between therapeutic efficacy of short-term HD-DXM regimen and different ethnic ITP adults with platelet GP-specific antibody.
Changes of baseline platelet count in newly diagnosed ITP adults after therapy
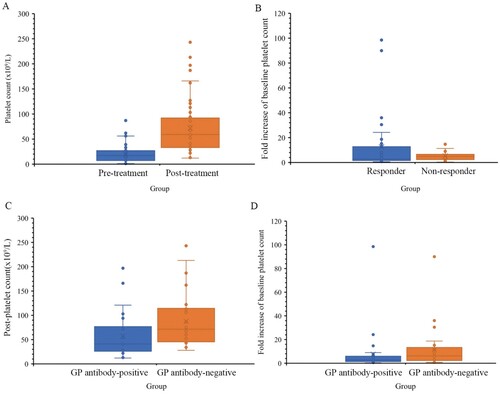
Administration of short-term HD-DXM regimen improved levels of post-platelet count and there was a significant difference between pre-treatment group and post-treatment group (p < 0.001) ((A)), and the fold increase of baseline platelet count was higher in responder group compared with non-responder group (p < 0.001) ((B)). Notably, mean levels of post-platelet count and fold increase of baseline platelet count were lower in GP-specific antibody-positive group compared with corresponding negative group (all p < 0.05) ((C,D)).
Figure 1. The levels of platelet count and fold increase of baseline platelet count after receiving short-term HD-DXM regimen. GP platelet glycoproteins, HD-DXM high-dose dexamethasone. (A) Comparison of platelet count between post-treatment (n = 59) group and pre-treatment (n = 59) group. (B) Comparison of fold increase of baseline platelet count between responder group (n = 33) and non-responder group (n = 26). (C) Comparison of platelet count between GP-specific antibody-positive group (n = 31) and GP-specific antibody-negative group (n = 28). (D) Comparison of fold increase of baseline platelet count between GP-specific antibody-positive group (n = 31) and GP-specific antibody-negative group (n = 28).
Relationship between clinical bleeding degree and newly diagnosed ITP adults
11 (37.9%) patients with bleeding disorders mainly occurred under 65 years, and they were manifested as petechiae and ecchymosis in both lower limbs or venous blood collection sites in low bleeding risk group. In medium bleeding risk group, 13 (46.4%) patients were often accompanied by epistaxis or gingival bleeding. Notably, in the high bleeding risk group, 2 (100%) patients appeared visible urinary blood, but no clinical manifestations of central nervous system bleeding.
Therapeutic efficacy was much lower in GP antibody-positive patients compared with corresponding negative ones at low (p < 0.001) and medium (p = 0.002) bleeding score, with no response in 2 cases of anti-GP Ib/IX antibody-positive patients at high bleeding score (). Moreover, Mantel-Haenszel Chi-square statistic showed no obvious linear relationship between clinical bleeding degree and GP-specific antibody (χ2 = 0.126, p = 0.722).
Table 4. Relationship between clinical bleeding degree and newly diagnosed ITP adults with GP-specific antibody.
Discussion
Consistent with phenotype of typical ITP patients [Citation35], there were 59 cases of newly diagnosed ITP adults in our final study. Administration of short-term HD-DXM regimen elevated levels of post-platelet count and fold increase of baseline platelet count, and both of them were lower in GP antibody-positive ITP adults compared with corresponding negative ones. We also observed a relief of bleeding manifestations in most responders at low and medium bleeding risk score. This is consistent with other studies [Citation36,Citation37]. These data suggest that short-term HD-DXM regimen may be an effective initial therapy for newly diagnosed ITP adults. Clinically, subsequent treatments for patients who fail the initial therapy or refractory include thrombopoietic agents, rituximab, fostamatinib, splenectomy, and several older immunosuppressive agents [Citation38]. The optimized subsequent treatment strategy, such as oral aitopopal and haitopopal, was tailored to the patients in our study. This is similar to a previous study from a developing country that use of eltrombopag was well-tolerated and yielded excellent overall response among adult ITP patients who have progressed following treatment with at least one prior line of therapy [Citation13]. In addition, it has recently been highlighted that splenectomy is very good second line treatment in low to middle-income countries where most of patients are not affordable for thrombopoietin agonists and unable to do regular follow up [Citation28]. However, splenectomy is not feasible in a not negligible portion of ITP patients, particularly in the elderly and/or in those with multiple/significant comorbidities [Citation29].
Due to no gold standard and lack of a known biomarker specific for ITP diagnosis, platelet autoantibody testing is widely used to evaluate the thrombocytopenic patients [Citation14]. Compared with the MAIPA assay for measuring anti-GP IIb/IIIa antibody, the enzyme-linked immunospot (ELISPOT) method for detecting the frequency of circulating B cells secreting anti-GP IIb/IIIa antibody and flow cytometry for assessing the percentage of platelet GP IIb/IIIa had higher sensitivity but with equal specificity [Citation3,Citation39]. A retrospective study on 176 newly diagnosed acute ITP showed responses to HD-DXM therapy in most patients with anti-GPIIb/IIIa antibody or without detectable antibody, but in a minority of patients with anti-GPIbα antibody (26.5%) or anti-GPIbα antibody plus anti-GPIIb/IIIa antibody (29.6%) [Citation23]. Notably, thrombocytopenia was more severe in patients with anti-GPIb autoantibody than those without, but not in those with anti-GPIIb/IIIa autoantibody [Citation40]. Our results of modified MAIPA assay showed that the positive rate of GP antibody was 52.5% in ITP adults, and initial therapeutic efficacy was much worse in ITP adults with anti-GP Ib/IX antibody plus anti-GP IIb/IIIa antibody than that in those with anti-GP Ib/IX antibody or anti-GP IIb/IIIa antibody, implying that initial therapeutic efficacy may be influenced by the heterogeneity of platelet GP-specific antibody. Simultaneously, ethnicity-based evaluation of therapeutic efficacy was conducted in different ethic population and it was indeed much worse in GP-specific antibody-positive Uyghur patients compared with corresponding Han patients, with a moderate negative correlation of therapeutic efficacy with GP-specific antibody. All these data suggest that platelet GP antibody may be associated with HD-DXM failure in minority patients with ITP. Although the exact pathophysiology of ITP in these patients remains unclear, it seems to be possible that interaction of human macrophages with autologous platelets results in scavenger-receptor-mediated platelet uptake and clearance via the reticuloendothelial system [Citation3,Citation28,Citation41], which may be one of the main reasons why ITP patients with GP-specific antibody have poor treatment efficacy. Accordingly, our data and others suggest that GP-specific antibody-positive ITP adults may partly predict a poor response to the short-term HD-DXM, especially in those with positive GP-specific antibody. However, apart from ethnicity, other possible factors including genetic polymorphisms, education degree and environment, should be further studied to explore the divergent response to HD-DXM. More recently, it has been documented that corticosteroid non-response overall was rare (13% of ITP patients), but the majority (82%) of corticosteroid non-responders were positive for GPIa/IIa in a comprehensive retrospective study [Citation14]. Similarly, a lower steroid response rate was detected in patients with antibodies against GP Iba or both GP Iba and GP IIb/IIIa, but not in those with anti-GP IIb/IIIa antibody [Citation23]. Therefore, it is also needed to explore other relevant anti-GP specificities (i.e. GPIa, GP Ia/IIa, GPIba, and GPV [Citation42]) in our newly diagnosed ITP patients in the future.
Up to now, the relationship between serology and severity or prognosis of ITP has been investigated, but the relation between the number of glycoprotein-specific autoantibody and disease severity has not been fully explored [Citation14]. Using a murine model of ITP, it was confirmed that animals with anti-GP Ib/IX autoantibody always showed a more serious bleeding tendency [Citation43]. In our current study, bleeding scores were assessed at admission and most patients with petechiae were at low and medium bleeding risk score. After receiving short-term HD-DXM regimen, the patients had no serious adverse events during the study period; the degree of common bleeding manifestations may be influenced by different GP antibodies, but no obvious linear relationship between bleeding score and GP-specific antibody was detected. Conversely, there was a remarkable increased bleeding score in GP antibody-positive patients [Citation44], but its relevance to patients is questionable due to only 13.9% GP antibody-positive rate in a single-center observational study of 108 ITP patients [Citation14]. All these data suggest that it is still controversial whether there is a relationship of bleeding risk score and GP-specific antibody. There are several possible factors for this. One is that it only contains data from different single center. Another is interlaboratory difference in sensitivity and specificity of EDTA-anticoagulated MAIPA protocol, merely with a range of sensitivity from 30% to 60% [Citation45,Citation46]. A third possibility is the influence of ethical characteristics. Finally, ITP diagnosis is still one of exclusion, and there are no clinical or laboratory criteria for prognosis [Citation47]. Nevertheless, there was general uniformity in the results that the degree of common bleeding manifestations in newly diagnosed ITP patients was influenced by the heterogeneity of platelet GP-specific antibody, yet a longer follow-up period should be required to determine the true therapeutic efficacy.
The weakness of this study is a retrospective design with a small size number of patients, and the therapeutic efficacy of short-term HD-DXM was based on parts of platelet GP-specific antibody. Despite these limitations, our study provides valuable clinical information that there exists difference in the efficacy of initial corticosteroid therapy to newly diagnosed ITP patients from two different ethnic backgrounds, which is absent from the existing published literature. Prospective clinical trials are needed to further confirm these initial findings in future studies.
Taken together, the newly diagnosed ITP adults with GP-specific antibody have a poor response to short-term HD-DXM, especially in minority (Uyghur) patients with GP-specific antibody in China.
Authorship contributions
M S, X W, and X G conceived the idea of study. M S, X W, M S, LW, X W, Y L, W F, and Q L performed the data collection. M S, and M S contributed to data analysis. M S wrote the draft of paper. X G reviewed the final version of the manuscript, and all authors read and approved the final manuscript.
Ethical approval
The design and execution of this study received approval from the hospital ethics committee of Xinjiang Medical University in accordance with the ethical standards of the Declaration of Helsinki. And all participants or their family members signed an informed consent form prior to the involvement.
Supplemental Material
Download MS Word (44.8 KB)Acknowledgements
We would like to thank all participating patients and their families, whose contribution made research possible.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability
Data are available from corresponding author X G upon reasonable request.
Additional information
Funding
References
- Liu XG, Bai XC, Chen FP, et al. Chinese guidelines for treatment of adult primary immune thrombocytopenia. Int J Hematol. 2018;107:615–623. doi:10.1007/s12185-018-2445-z
- Cooper N, Ghanima W. Immune thrombocytopenia. N Engl J Med. 2019;381(10):945–955. doi:10.1056/NEJMcp1810479
- Chen JF, Yang LH, Chang LX, et al. The clinical significance of circulating B cells secreting anti-glycoproteinIIb/IIIa antibody and platelet glycoprotein IIb/IIIa inpatients with primary immune thrombocytopenia. Hematology. 2012;17(5):283–290. doi:10.1179/1607845412Y.0000000014
- Frederiksen H, Maegbaek ML, Nørgaard M. Twenty-year mortality of adult patients with primary immune thrombocytopenia: a Danish population-based cohort study. Br J Haematol. 2014;166(2):260–267. doi:10.1111/bjh.12869
- Neunert C, Terrell DR, Arnold DM, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3(23):3829–3866. doi:10.1182/bloodadvances.2019000966
- Wei Y, Ji XB, Wang YW, et al. High-dose dexamethasone vs prednisone for treatment of adult immune thrombocytopenia: a prospective multicenter randomized trial. Blood. 2016;127(3):296–302. quiz 370, doi:10.1182/blood-2015-07-659656
- Thrombosis and Hemostasis Group. Chinese Society of Hematology, Chinese Medical Association. [Chinese guideline on the diagnosis and management of adult primary immune thrombocytopenia (version 2020)]. Zhonghua Xue Ye Xue Za Zhi. 2020;41(8):617–623. Chinese, doi:10.3760/cma.j.issn.0253-2727
- Frederiksen H, Ghanima W. Response of first line treatment with corticosteroids in a population-based cohort of adults with primary immune thrombocytopenia. Eur J Intern Med. 2017;37:e23–e25. doi:10.1016/j.ejim.2016.09.001
- Xu J, Zhang X, Feng S, et al. Clinical efficacy of high-dose dexamethasone with sequential prednisone maintenance therapy for newly diagnosed adult immune thrombocytopenia in a real-world setting. J Int Med Res. 2021;49(4):3000605211007322, doi:10.1177/03000605211007322
- Khan AM, Mydra H, Nevarez A. Clinical practice updates in the management of immune thrombocytopenia. P T. 2017;42(12):756–763. PMID: 29234214.
- Song F, Al-Samkari H. Management of adult patients with immune thrombocytopenia (ITP): a review on current guidance and experience from clinical practice. J Blood Med. 2021;12:653–664. doi:10.2147/JBM.S259101
- Mishra K, Kumar S, Singh K, et al. Real-world experience of anti-D immunoglobulin in immune thrombocytopenia. Ann Hematol. 2022;101(6):1173–1179. doi:10.1007/s00277-022-04829-4
- Mishra K, Kumar S, Jandial A, et al. Real-world experience of eltrombopag in immune thrombocytopenia. Am J Blood Res. 2020;10(5):240–251. PMID: 3322456.
- Al-Samkari H, Rosovsky RP, Karp Leaf RS, et al. A modern reassessment of glycoprotein-specific direct platelet autoantibody testing in immune thrombocytopenia. Blood Adv. 2020;4(1):9–18. doi:10.1182/bloodadvances.2019000868
- Zhang J, Zhang Q, Li Y, et al. Immune dysregulation in primary immune thrombocytopenia patients. Hematology. 2018;23(8):510–516. doi:10.1080/10245332.2018.1435021
- Caserta S, Zaccuri AM, Innao V, et al. Immune thrombocytopenia: options and new perspectives. Blood Coagul Fibrinolysis. 2021;32(7):427–433. doi:10.1097/MBC.0000000000001058
- Cines DB, Bussel JB, Liebman HA, et al. The ITP syndrome: pathogenic and clinical diversity. Blood. 2009;113(26):6511–6521. doi:10.1182/blood-2009-01-129155
- Chen Y, Hu J, Chen Y. Platelet desialylation and TFH cells-the novel pathway of immune thrombocytopenia. Exp Hematol Oncol. 2021;10(1):21, doi:10.1186/s40164-021-00214-5
- Zheng SS, Ahmadi Z, Leung HHL, et al. Antiplatelet antibody predicts platelet desialylation and apoptosis in immune thrombocytopenia. Haematologica. 2022;107(9):2195–2205. doi:10.3324/haematol.2021.279751
- Sirotich E, Guyatt G, Gabe C, et al. Definition of a critical bleed in patients with immune thrombocytopenia: communication from the ISTH SSC subcommittee on platelet immunology. J Thromb Haemost. 2021;19(8):2082–2088. doi:10.1111/jth.15368
- Hirokawa M, Fujishima N, Togashi M, et al. High-throughput sequencing of IgG B-cell receptors reveals frequent usage of the rearranged IGHV4-28/IGHJ4 gene in primary immune thrombocytopenia. Sci Rep. 2019;9(1):8645, doi:10.1038/s41598-019-45264-2
- Lambert MP, Gernsheimer TB. Clinical updates in adult immune thrombocytopenia. Blood. 2017;129:2829–2835. doi:10.1182/blood-2017-03-754119
- Zeng Q, Zhu L, Tao L, et al. Relative efficacy of steroid therapy in immune thrombocytopenia mediated by anti-platelet GPIIbIIIa versus GPIbα antibodies. Am J Hematol. 2012;87(2):206–208. doi:10.1002/ajh.22211
- Peng J, Ma SH, Liu J, et al. Association of autoantibody specificity and response to intravenous immunoglobulin G therapy in immune thrombocytopenia: a multicenter cohort study. J Thromb Haemost. 2014;12(4):497–504. doi:10.1111/jth.12524
- Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386–2393. doi:10.1182/blood-2008-07-162503
- Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115(2):168–186. doi:10.1182/blood-2009-06-225565
- Atkinson TM, Ryan SJ, Bennett AV, et al. The association between clinician-based common terminology criteria for adverse events (CTCAE) and patient-reported outcomes (PRO): a systematic review. Support Care Cancer. 2016;24:3669–3676. doi:10.1007/s00520-016-3297-9
- Mishra K, Kumar S, Sandal R, et al. Safety and efficacy of splenectomy in immune thrombocytopenia. Am J Blood Res. 2021;11(4):361–372. PMID: 34540344.
- Vianelli N, Auteri G, Buccisano F, et al. Refractory primary immune thrombocytopenia (ITP): current clinical challenges and therapeutic perspectives. Ann Hematol. 2022;101(5):963–978. doi:10.1007/s00277-022-04786-y
- Terrell DR, Neunert CE, Cooper N, et al. Immune thrombocytopenia (ITP): current limitations in patient management. Medicina (Kaunas. 2020;56(12):667, doi:10.3390/medicina56120667
- Provan D, Arnold DM, Bussel JB, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3(22):3780–3817. doi:10.1182/bloodadvances.2019000812
- Du Y, Yang C, Chen M, et al. Tacrolimus is effective in relapsed/refractory autoimmune cytopenias: results of a single-center retrospective study. Hematology. 2020;25(1):478–483. doi:10.1080/16078454.2020.1852763
- Hou M, Peng J, Shi Y, et al. Mycophenolate mofetil (MMF) for the treatment of steroid-resistant idiopathic thrombocytopenic purpura. Eur J Haematol. 2003;70(6):353–357. doi:10.1034/j.1600-0609.2003.00076.x
- George NP, Wei Q, Shin PK, et al. Staphylococcus aureus adhesion via Spa, ClfA, and SdrCDE to immobilized platelets demonstrates shear-dependent behavior. Arterioscler Thromb Vasc Biol. 2006;26(10):2394–2400. doi:10.1161/01.ATV.0000237606.90253.94
- Fogarty PF, Segal JB. The epidemiology of immune thrombocytopenic purpura. Curr Opin Hematol. 2007;14(5):515–519. doi:10.1097/MOH.0b013e3282ab98c7
- Cheng Y, Wong RS, Soo YO, et al. Initial treatment of immune thrombocytopenic purpura with high-dose dexamethasone. N Engl J Med. 2003;349(23):831–836. doi:10.1056/NEJMoa.030254
- Matschke J, Müller-Beissenhirtz H, Novotny J, et al. A randomized trial of daily prednisone versus pulsed dexamethasone in treatment-naïve adult patients with immune thrombocytopenia: EIS 2002 study. Acta Haematol. 2016;136(2):101–107. doi:10.1159/000445420
- Liu X, Hou Y, Hou M. How we treat primary immune thrombocytopenia in adults. J Hematol Oncol. 2023;16(4), doi:10.1186/s13045-023-01401-z
- Kuwana M, Okazaki Y, Kaburaki J, et al. Detection of circulating B cells secreting platelet-specific autoantibody is useful in the diagnosis of autoimmune thrombocytopenia. Am J Med. 2003;114(4):322–325. doi:10.1016/s0002-9343(02)01522-x
- Nomura S, Yanabu M, Soga T, et al. Analysis of idiopathic thrombocytopenic purpura patients with antiglycoprotein IIb/IIIa or Ib autoantibodies. Acta Haematol. 1991;86(1):25–30. doi:10.1159/000204794
- Scull CM, Hays WD, Fischer TH. Macrophage pro-inflammatory cytokine secretion is enhanced following interaction with autologous platelets. J Inflamm (Lond). 2010;7:53, doi:10.1186/1476-9255-7-53
- Vollenberg R, Jouni R, Norris PAA, et al. Glycoprotein V is a relevant immune target in patients with immune thrombocytopenia. Haematologica. 2019;104(6):1237–1243. doi:10.3324/haematol.2018.211086
- Nieswandt B, Bergmeier W, Rackebrandt K, et al. Identification of critical antigen-specific mechanisms in the development of immune thrombocytopenic purpura in mice. Blood. 2000;96(7):2520–2527. PMID: 11001906. doi:10.1182/blood.V96.7.2520
- Campbell K, Rishi K, Howkins G, et al. A modified rapid monoclonal antibody-specific immobilization of platelet antigen assay for the detection of human platelet antigen (HPA) antibodies: a multicenter evaluation. Vox Sang. 2007;93(4):289–297. doi:10.1111/j.1423-0410.2007.00989.x.
- Grimaldi D, Canouï-Poitrine F, Croisille L, et al. Antiplatelet antibodies detected by the MAIPA assay in newly diagnosed immune thrombocytopenia are associated with chronic outcome and higher risk of bleeding. Ann Hematol. 2014;93(2):309–315. doi:10.1007/s00277-013-1855-5
- Arnold DM, Santoso S, Greinacher A, et al. Recommendations for the implementation of platelet autoantibody testing in clinical trials of immune thrombocytopenia. J Thromb Haemost. 2012;10(4):695–697. doi:10.1111/j.1538-7836.2012.04664.x
- Rodeghiero F, Marranconi E. Management of immune thrombocytopenia in women: current standards and special considerations. Expert Rev Hematol. 2020;13(2):175–185. doi:10.1080/17474086.2020.1711729
