ABSTRACT
Objective
Multiple myeloma is a highly heterogenous plasma cell malignancy, commonly seen in older patients. Age is one of the important prognostic factors. However, nearly all the prognostic staging systems are based on clinical trials, where patients were relatively fit and young. It is unknown how the presence of biochemical or cytogenetic prognostic factors and their risk weights changes with older age. To further investigate this question, we retrospectively analyzed the data from a consecutive cohort of patients treated with either bortezomib or thalidomide-based therapy.
Methods
This retrospective study was carried out on a cohort of 1125 newly diagnosed multiple myeloma patients, from January 2008 to December 2019. Patients received bortezomib or thalidomide-based induction and maintenance therapy. Patients accepted hematopoietic stem cell transplantation if eligible. Statistical analysis was conducted by Stata/MP 16.0 and SPSS 26.0.
Results
With age increasing, the proportion of patients with ISS 3, performance status score ≥2, and the incidence rate of gain(1q) significantly increased. We also found that ISS became less important in older patients. However, cytogenetic abnormalities exerted a consistently adverse impact on survival, both in young and old patients. Older patients had an inferior outcome than their young counterparts. All patients in our cohort benefitted more from bortezomib than thalidomide-based induction therapy, except for patients ≥71 years old.
Conclusions
ISS may lose prognostic value in patients ≥71 years old. Older patients had an inferior outcome and needed more effective and less toxic treatment.
Plain Language Summary
Multiple myeloma is a type of blood cancer commonly seen in older people. To treat this disease, genetic abnormality, the poor physical status of patients and the abundance of tumor cells are the main difficulties. We often draw these conclusions from clinical trials. However, clinical trials always enrolled relatively younger patients, so the presence and significance of these factors may vary from clinical trials to the real world. We conducted the study to find out the real risk in both young and old patients. We found that older patients were more likely to have anemia, poor nutritional status and renal function. We also found older patients had more risk of relapse, progression or death than young patients. Frail physical status is the key obstacle to treating older patients, and tumor burden no longer impacts the outcome of these people. Bortezomib is a powerful drug to treat this disease, but patients ≥71 years old had less benefit than younger ones. More studies should focus on older or frail patients as these patients need more effective and less toxic treatment.
Introduction
Multiple myeloma (MM) is a plasma cell malignancy commonly seen in older adults. Due to population growth, global aging and age-related morbidity, the incidence rate of MM increased by 126% from 1990 to 2016 [Citation1]. In people over 71 years old, the incidence of monoclonal gammopathy of undetermined significance (MGUS) increased by 4–5% each year. Among them, 1% of people with MGUS progressed to symptomatic MM [Citation2]. The mortality rate of MM patients gradually increases every five years, reaching 7 per 100,000 in 90–95-year-old patients in China [Citation3,Citation4]. Advancing age indicates poor prognosis for MM patients, and is closely associated with frailty, complex comorbidities and more adverse effects [Citation5].
This is partly because the most commonly used staging systems of MM are based on clinical trials in which patients are relatively fit and young, they do not include age as a prognostic factor. The revised international staging system (R-ISS) comprises serum albumin, β2-MG, LDH, and t(4;14), t(14;16) and del(17p) [Citation6]. The mSMART staging system defines high-risk MM as any presence of high-risk genetic abnormalities (CA), R-ISS 3, high plasma cell S-phase, and high-risk signature [Citation7]. These staging systems put more emphasis on tumor characteristics and tumor burden rather than patient-related factors. A few studies have discussed how the incidence rate of risk factors changes with age. Furthermore, how age affects the risk weight of other prognostic factors is unknown. A study showed that the relative contribution of prognostic factors varied by age [Citation8]. Therefore, it is important to report the incidence rate and risk weight of biochemical or cytogenetic factors in older Chinese patients, to determine the best treatment strategy for these patients.
In this study, we retrospectively analyzed the data from a consecutive cohort of patients hospitalized in our centre. All patients were treated with either a bortezomib or a thalidomide-based regimen. The study aims at clarifying the age-dependent distribution and risk contribution of different prognostic factors in the new drug era.
Methods
This retrospective study was carried out on a cohort consisting of 1125 MM patients treated in our hospital. The patients were treated with the same regimen as previously reported in the BDH2008/02 clinical trial [Citation9]. Patients diagnosed with MM from January 2008 to December 2019 were included in the study. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. The design of our study has been approved by the Institutional Review Board of the Blood Diseases Hospital in Tianjin, China. Patients who died of any reason in two months were excluded. The diagnosis was made based on the IMWG criteria of 2003 [Citation10]. Risk stratifications were adopted according to the ISS, R-ISS and R2-ISS [Citation6,Citation11,Citation12]. Responses to treatment and disease progression were evaluated using the IMWG Uniform Response Criteria [Citation13]. Briefly, patients received four to six cycles of induction therapy with a bortezomib-based or a thalidomide-based triplet regimen, according to their willingness and physical conditions. Patients eligible for transplantation were advised to accept HSCT (hematopoietic stem cell transplantation), otherwise, they received the original regimen consolidation therapy. Subsequently, patients received either thalidomide or lenalidomide in combination with dexamethasone as the maintenance therapy for 1 year, unless intolerant.
Patients received a fluorescence in situ hybridization (FISH) test before the initiation of treatment. Plasma cells were sorted by CD138 magnetic beads. Routine FISH panel included the following probes: RB1/LAMP1, TP53, CKS1B, IgH and the dual-fusion probes targeting IgH/CCND1, IgH/FGFR3, IgH/MAF, IgH/MAFB. The cut-off value was 20% for chromosome deletion or gain, and 10% for chromosome translocation [Citation9]. High-risk IgH translocations were defined as t(4;14), t(14;16) or t(14;20). High-risk CA (HRCA) referred to high-risk IgH translocations, del(17p) and gain(1q). Performance status (PS) was evaluated by ECOG standards [Citation14,Citation15]. Glomerular filtration rate was calculated by an estimated CKD-EPI equation [Citation16]. Renal failure was defined as serum creatinine of more than 2 mg/dl or a creatinine clearance rate of less than 40 mL/min/1.73 m2.
Chi-Square and Fisher’s exact tests for categorical variables were used to evaluate the statistical significance among different groups, and the Kruskal–Wallis test was applied to continuous variables. Overall survival (OS) was calculated from the date of initial therapy to the date of death. Progression-free survival (PFS) was calculated from the date of initial therapy to the date of death or first documented disease progression. Survival curves were plotted using the Kaplan-Meier method and compared by the two-sided log-rank test unless specifically stated. A multivariate Cox proportional-hazards model was developed to assess the variables with significant effects on PFS and OS based on the univariate analysis. The explained variation of variables was quantified by RD2. Statistical analysis was conducted by Stata/MP 16.0 (Stata Corp., TX, USA) and SPSS 26.0 (IBM Corp., Chicago, Illinois, USA).
Results
The median follow-up time of 1125 patients was 49.3 months. The median PFS and OS of all patients were 40.0 (35.8–44.2) and 65.8 (60.2–71.4) months, respectively. 59.1% (665/1125) of patients were male. 54.0% (607/1125), 34.4% (386/1125), and 11.7% (132/1125) of patients were in the ≤60, 61–70 and ≥71-year-old group, respectively. 16.9% (190/1114) of patients had renal impairment. 66.8% of patients received induction therapy based on bortezomib, and the rest received thalidomide-based therapy. 22.2% (250/1125) of patients underwent frontline HSCT. Other baseline characteristic data are listed in Supplemental Table 1.
The impact of age on clinical factor
There was no significant difference in gender and monoclonal Ig type among the three age groups. The incidence rate of renal failure was consistent across different age groups. As age increased, the median value of hemoglobulin (P = 0.011) significantly decreased, and corrected serum calcium significantly increased (P < 0.001). Tumor burden-related factors including bone marrow plasma cell ratio, M-protein, and LDH were similar among different age groups. The absolute neutrophil granulocyte count and absolute lymphocyte count did not change with age. The GFR of patients largely decreased with age advancing (P < 0.001), consistent with a significant increase in serum β2-MG (P < 0.001) and a decrease in serum albumin level (P < 0.001). () The proportion of ISS 3-stage patients also significantly increased with age (A). 40.8%, 52.3% and 59.5% of ≤60, 61–70 and ≥71-year-old patients were classified as ISS 3 stage, respectively (P < 0.001). As for R-ISS, patients ≥71-year-old were more likely to be classified into R-ISS 2, compared to ≤60, 61–70-year-old patients (79.0% vs. 61.3% and 66.1%, B). The older patients also had higher ECOG PS scores than younger patients (P < 0.001). 24.1%, 29.1% and 43.9% of patients with ECOG PS ≥2 were ≤60, 61–70 and ≥71-year-old (C). The number of patients who received bortezomib-based induction therapy significantly decreased in the ≥71-year-old group (40.2%, 53/132).
Figure 1. Proportion of patients classified by ISS (A), R-ISS (B) or ECOG PS (C) score. ISS, International staging system; ECOG PS, Performance status defined by the Eastern Cooperative Oncology Group. (D) The proportion of patients with high-risk cytogenetic abnormalities (CA), including t(4;14), t(14;16), gain(1q), and del(17p). SR = Standard risk, no high-risk CA; HR = High risk, only one high-risk CA; UHiR = Ultra-high risk, more than one high-risk CA. (E) The incidence rate of CA in each age group. (F–H) Correlation between indicated cytogenetic abnormality and age.
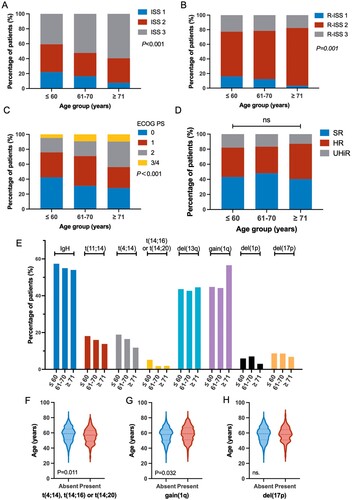
Table 1. Clinical characteristics of patients in different age groups.
The impact of age on cytogenetic abnormalities
All patients had at least one cytogenetic data. 989 patients had complete data for t(4;14), t(14;16), del(17p) and gain(1q). The incidence rate of CA varied among different age groups. The incidence rate of high-risk IgH translocation, namely t(4;14), t(14;16) or t(14;20), was 24.2% (135/557), 18.6% (62/333) and 14.3% (15/105) in ≤60, 61–70 and the ≥71-year-old group, respectively, showing a decreasing trend as age increased. The median age of patients with either t(4;14), t(14;16) or t(14;20) was significantly younger than those without (57 vs. 59 years old, P = 0.011, F). The incidence rate of gain(1q) was 44.8% (263/587), 44.2% (169/382) and 56.6% (73/129) in ≤60, 61–70 and ≥71-year-old groups, respectively, exhibiting an increasing trend with age. The median age of patients with gain(1q) was slightly older than those without(59 vs. 58 years old, P = 0.032, G). The incidence rate of del(17p) (8.7% 52/598, 8.6% 33/382, 6.8% 9/132) and del(13q) (43.6% 261/599, 42.7% 162/379, 44.6% 58/130) did not show association with changed age (E,H). The total number of HRCA did not significantly change with age (D).
The impact of age on the weight of high-risk factors
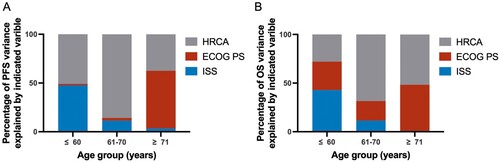
By constructing the Cox proportional-hazards model, we found that ISS, the number of HRCA and ECOG PS exerted a negative impact on patient survival in both univariate and multivariate analyses (Supplemental Table 2). Furthermore, the explained variation analysis showed that the influence of ISS on survival decreased as age increased. Consistently, ECOG PS overweighted ISS on PFS and OS in the advancing age group. HRCA number had the best separation effect on the prognosis in the 61–70 age group and had less effect in the other two age groups (A,B).
The impact of age on survival
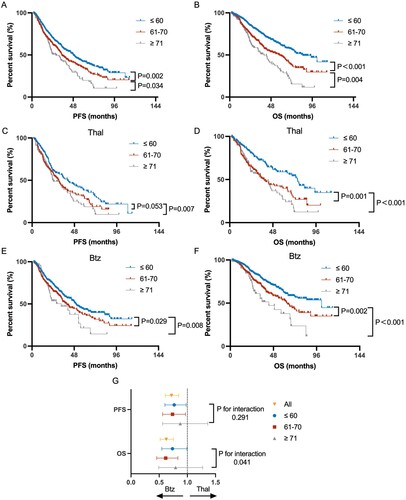
With age increasing, the PFS and OS of patients significantly decreased in a stepwise manner. The median PFS of ≤60, 61–70 and ≥71-year patients was 48.1, 34.7 and 27.8 months, respectively (P < 0.001). The median OS of the three groups was 85, 57.9 and 40.0 months, respectively (P < 0.001) (A,B). The survival difference among different age groups was independent of the treatment regimen (bortezomib- or thalidomide-based) (C–F). In the bortezomib-based induction arm, the median PFS of ≤60, 61–70 and ≥71-year patients was 49.8, 39.4 and 33.3 months, respectively (P = 0.009). The median OS of the three groups was 101.6, 62.6 and 42.9 months, respectively (P<0.001). Tn the thalidomide-based induction arm, the median PFS of ≤60, 61–70 and ≥71-year patients was 40.0, 30.3 and 24.0 months, respectively (P = 0.023). The median OS of the three groups was 75.3, 38.2 and 40.0 months, respectively (P < 0.001).
Figure 3. Kaplan–Meier analysis of PFS and OS of total (A,B), thalidomide-treated (C,D) and bortezomib-treated (E,F) patients in the ≤60, 61–70, ≥71-year-age group. (G) Subgroup analysis of patients in each age group. The Bortezomib arm was regarded as the experimental group. P-value was calculated for interaction.
Treatment benefits of patients in each age group
The response rate to induction treatment gradually decreased with age increasing, especially in the thalidomide-treated patients. The overall response rate of bortezomib-based therapy was 96.3%, 90.6% and 97.5% in ≤60, 61–70 and ≥71-year-old groups, respectively. The overall response rate of thalidomide-based therapy in the three age groups was 88.1%, 79.3% and 63.8%, respectively (Supplemental Figure 1A). Furthermore, the MRD negative rate(10−4) slightly decreased with age advancing, and older patients were more likely to have missing MRD data (Supplemental Figure 1C). Overall, patients benefitted more from bortezomib-based induction therapy with significantly prolonged PFS and OS, compared to patients in the thalidomide arm. In subgroup survival analysis, patients over 71 years old seemed to lose OS benefits from a bortezomib-based regimen (G). For these ≥71-year-old patients, 49.2% accepted two drug induction therapies due to comorbidity and complication. These patients had an inferior OS by the Breslow test (P = 0.012, Supplemental Figure 1E).
Discussion
MM is a monoclonal plasma cell malignancy with a median onset age of ∼70 years old when patients first present symptoms [Citation1,Citation7]. Frailty is more common in older patients and affects treatment tolerability, incidence of side effects, and clinical outcomes [Citation17–19]. A few studies focus on the incidence rate and the relative risk weight of those factors among different age groups [Citation8].
Age is related to unfavorable outcomes [Citation3,Citation5,Citation19–23], and is considered a continuous variable, for which any cutoff to classify patients as young or old seems arbitrary. However, a reasonable criterion for transforming age into a categorical variable is practical in clinical application. Until now, there is no consensus on the prognostic cut-off value of age [Citation17,Citation20,Citation21,Citation23]. Since the lower quartile and median value of age in this cohort is 51 and 59 years, respectively, and transplantation is only eligible to patients under 70 years old, which may exert a strong effect on survival, we stratified patients into ≤60, 61–70, ≥ 71 age subgroups as there was no survival difference between patients younger than 51- and 51–60 years old (data not shown).
We found that β2-MG and correlated serum calcium levels increased with age and GFR and serum albumin levels decreased with age. The level of serum β2-MG is determined by various factors, mainly by GFR and cell turnover rate [Citation24]. As the plasma cell ratio of bone marrow and serum M-protein level did not change significantly with age, another explanation for increasing β2-MG was the natural decline of renal function in older patients. Therefore, the increasing proportion of ISS 3 in older patients can be partially explained by the fact that the parameters used by ISS naturally worsen with aging. Consistently, the separation of Kaplan-Meier curves between each ISS stage becomes less significant with advancing age. The survival prediction efficacy of ISS may be weakened in patients over 71 years old, even stratified by the second revision of ISS by IMWG (Supplemental Figure 2).
The physical status of patients deteriorates with age and is affected by education, personality, and family support. Several studies have incorporated different combinations of factors such as the activity of daily living (ADL), instrumental activity of daily life (IADL), Charlson comorbidity index (CCI), Kamofsky performance status (KPS) to evaluate the frailty of patients [Citation19,Citation25,Citation26]. The IMWG recommends a geriatric assessment system with ADL ≤ 4, IADL ≤ 5, CCI ≥ 1, and 76–80 or over 80 years to stratify patients into fit, intermediate-fit or frail groups (score = 0, 1, ≥ 2), and the 3-year OS in these groups was 84–91%, 76–77% and 47–57%, respectively [Citation17,Citation27]. However, all these questionnaires are time-consuming and may be subjective to the patients themselves. To avoid the time-consuming questionnaire, a simplified frailty scale was conducted with age, CCI and ECOG PS. Frail patients have a ≥2 score and nonfrail patients have a 0–1 score. Frail patients (accounting for 48.8%) experienced a worse response rate and survival compared with nonfrail patients [Citation18]. Our studies showed that a higher ECOG PS score was related to advancing age and indicated inferior outcomes. Different from ADL or IADL questionnaires, the ECOG PS is simple and more commonly collected in clinical trials. As a major shortcoming, ECOG PS can be misleading by severe anemia or bone disease. Further prospective clinical trials should include frailty assessment to evaluate its role in response and outcome prediction.
CA is considered to be a tumor-specific factor of MM and the incidence rate of CA may change with age. Some centers reported that older patients had a lower incidence rate of t(4;14) and a higher rate of gain(1q). However, the age-dependent incidence of del(17p) is not consistent among different studies [Citation8,Citation28]. In this study, we confirmed that the incidence rate of gain(1q) increased with age advancing. The presence of high-risk IgH translocation seems to be negatively related to age. It may be interesting to consider whether aging exerts an impact on the presence of CAs and the pathogenesis of MM. The prognostic value of HRCA was highest in the 61–70 age group and lower in the ≤60 and ≥71 age groups. With age advancing, the ISS gradually lost its prognostic value, while ECOG PS exhibited better prognostic value. In patients over 70 years old, the heterogeneity of this population complicates the prognosis evaluation and treatment decisions. Additionally, older patients were more likely to die in the first year after diagnosis (5.4%, 9.1% and 10.6% in ≤61, 61–70, ≥71 age groups), indicating aging patients are faced with higher risk.
At last, aging always poses obstacles to treatment options and responses. As a major shortcoming of this study, most of the patients accepted either thalidomide- or bortezomib-based induction therapy, whereas current first-line therapy is RVD (lenalidomide, bortezomib and dexamethasone). A major reason is that the study enrolled patients as early as 2008, when lenalidomide was not available, the other reason is that the economic cost and treatment tolerance restricted the wide usage of RVD in China. In this study, we found that older patients had an impaired response to induction treatment. There was a larger difference in ORR among different age groups in the thalidomide arm, compared with the bortezomib arm. Meanwhile, our results indicated that the survival benefit of bortezomib became obscure in older patients. Although not discussed in this study, a few articles have reported that peripheral neuritis, treatment inconvenience and the following high treatment discontinuation rate may impair the extra benefit of bortezomib [Citation29]. We also admit the relatively fewer older patients in this study might partly explain the lacking of benefit of bortezomib. However, some subgroup analyses also suggested that bortezomib failed to benefit older patients. SWOG S0777 trial showed that ≥65-year-old patients did not obtain a significant overall survival benefit from VRd (HR 0.769, 0.520–1.138), compared to the Rd arm [Citation30]. A dose-modified RVD (lenalidomide, bortezomib and dexamethasone) largely improves the drug tolerance of older patients. Peripheral neuropathy rate was 62% and the most (30/31) was grade 1/2 [Citation31]. The result shows a comparable response rate with VRD in SWOG S0777, and a better PFS than continuous Rd of 25.5 months in the FIRST trial [Citation32]. Another comparable combination for transplantation-ineligible patients is a daratumumab-containing induction regimen. For transplantation-ineligible newly diagnosed MM patients, MAIA and ALCYONE clinical trials both showed that daratumumab brought extra survival benefits to all patients. Meanwhile, the adverse effect was mainly hematological events, and the neurotoxicity was acceptable in the daratumumab arm [Citation33–35]. Most importantly, all subgroups of frailty status had PFS advantages [Citation34,Citation35]. Although daratumumab-based induction therapy may be an ideal choice for older patients, the main drawback is the high cost. In light of the above discussion, it is of interest to compare the efficacy, safety and long-term survival benefit of daratumumab and bortezomib in the three-drug lenalidomide-based combination for frail patients. Generally considered, three-drug induction is better than two-drug. Our result also showed a trend for extra OS benefit in ≥71-year-old patients treated with three-drug induction, compared to those treated with two-drug (Supplemental Figure 1E). In other words, the heterogeneity of drug tolerance and comorbidities obscures the formulation of optimal therapy for high-risk older patients [Citation36].
In summary, advancing age was negatively correlated with survival. Anemia, hypercalcemia, hypoalbuminemia and creatinine clearance decline were more common in older patients, and these patients tend to have advanced ISS stage and ECOG PS scores. With age increasing, the incidence of gain(1q) was in an increasing trend, while the probability of developing high-risk IgH translocations might decrease. Moreover, the prognostic value of ISS was reduced in older patients, while the prognostic value of ECOG PS increased with age. For older patients, it is necessary to design a more optimal frailty assessment tool and better utilize the currently available treatment options, including the more effective and less toxic agents such as CD38 monoclonal antibodies.
Authorship
CX.D. analyzed the data and wrote the manuscript. LN. L., JH. L., HS. F., XH. M. collected clinical data and conducted follow-ups. Y. X., WW. S., SH. D., DH. Z., SH. Y., YZ. Z. managed patients and collected samples. CW. L., JW. Z., M. H. performed FISH. LG. Q., G. A. designed the research, revised the manuscript critically and approved the final version. All authors reviewed the manuscript and provided the final approval for submission.
Supplemental Material
Download MS Word (383.6 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- van de Donk N, Pawlyn C, Yong KL. Multiple myeloma. Lancet. 2021;397(10272):410–427. doi:10.1016/S0140-6736(21)00135-5
- Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346(8):564–569. doi:10.1056/NEJMoa01133202
- Ludwig H, Bolejack V, Crowley J, et al. Survival and years of life lost in different age cohorts of patients with multiple myeloma. J Clin Oncol. 2010;28(9):1599–1605. doi:10.1200/JCO.2009.25.2114
- Liu J, Liu W, Mi L, et al. Incidence and mortality of multiple myeloma in China, 2006-2016: an analysis of the Global Burden of Disease Study 2016. J Hematol Oncol. 2019;12(1):136. doi:10.1186/s13045-019-0807-5
- Corre J, Munshi NC, Avet-Loiseau H. Risk factors in multiple myeloma: is it time for a revision? Blood. 2021;137(1):16–19. doi:10.1182/blood.2019004309
- Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised international staging system for multiple myeloma: a report from international myeloma working group. J Clin Oncol. 2015;33(26):2863–2869. doi:10.1200/JCO.2015.61.2267
- Rajkumar SV. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am J Hematol. 2020;95(5):548–567. doi:10.1002/ajh.25791
- Pawlyn C, Cairns D, Kaiser M, et al. The relative importance of factors predicting outcome for myeloma patients at different ages: results from 3894 patients in the myeloma XI trial. Leukemia. 2020;34(2):604–612. doi:10.1038/s41375-019-0595-5
- An G, Xu Y, Shi L, et al. Chromosome 1q21 gains confer inferior outcomes in multiple myeloma treated with bortezomib but copy number variation and percentage of plasma cells involved have no additional prognostic value. Haematologica. 2014;99(2):353–359. doi:10.3324/haematol.2013.088211
- International Myeloma Working G. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121(5):749–757. doi:10.1046/j.1365-2141.2003.04355.x
- D'Agostino M, Cairns DA, Lahuerta JJ, et al. Second revision of the international staging system (R2-ISS) for overall survival in multiple myeloma: A European myeloma network (EMN) report within the HARMONY project. J Clin Oncol. 2022;40(29):3406–3418. doi:10.1200/JCO.21.02614
- Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412–3420. doi:10.1200/JCO.2005.04.242
- Rajkumar SV, Harousseau JL, Durie B, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117(18):4691–4695. doi:10.1182/blood-2010-10-299487
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol. 1982;5(6):649–655. doi:10.1097/00000421-198212000-00014
- Blagden SP, Charman SC, Sharples LD, et al. Performance status score: do patients and their oncologists agree? Br J Cancer. 2003;89(6):1022–1027. doi:10.1038/sj.bjc.6601231
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi:10.7326/0003-4819-150-9-200905050-00006
- Palumbo A, Bringhen S, Mateos MV, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood. 2015;125(13):2068–2074. doi:10.1182/blood-2014-12-615187
- Facon T, Dimopoulos MA, Meuleman N, et al. A simplified frailty scale predicts outcomes in transplant-ineligible patients with newly diagnosed multiple myeloma treated in the FIRST (MM-020) trial. Leukemia. 2020;34(1):224–233. doi:10.1038/s41375-019-0539-0
- Engelhardt M, Domm AS, Dold SM, et al. A concise revised Myeloma Comorbidity Index as a valid prognostic instrument in a large cohort of 801 multiple myeloma patients. Haematologica. 2017;102(5):910–921. doi:10.3324/haematol.2016.162693
- Cook G, Royle KL, Pawlyn C, et al. A clinical prediction model for outcome and therapy delivery in transplant-ineligible patients with myeloma (UK Myeloma Research Alliance Risk Profile): a development and validation study. Lancet Haematol. 2019;6(3):e154–ee66. doi:10.1016/S2352-3026(18)30220-5
- Ludwig H, Durie BG, Bolejack V, et al. Myeloma in patients younger than age 50 years presents with more favorable features and shows better survival: an analysis of 10 549 patients from the International Myeloma Working Group. Blood. 2008;111(8):4039–4047. doi:10.1182/blood-2007-03-081018
- Bringhen S, Mateos MV, Zweegman S, et al. Age and organ damage correlate with poor survival in myeloma patients: meta-analysis of 1435 individual patient data from 4 randomized trials. Haematologica. 2013;98(6):980–987. doi:10.3324/haematol.2012.075051
- Chretien ML, Hebraud B, Cances-Lauwers V, et al. Age is a prognostic factor even among patients with multiple myeloma younger than 66 years treated with high-dose melphalan: the IFM experience on 2316 patients. Haematologica. 2014;99(7):1236–1238. doi:10.3324/haematol.2013.098608
- Heegaard NH. beta(2)-microglobulin: from physiology to amyloidosis. Amyloid. 2009;16(3):151–173. doi:10.1080/13506120903151775
- Nakazato T, Hagihara M, Sahara N, et al. Phase II clinical trial of personalized VCD-VTD sequential therapy using the Vulnerable Elders Survey-13 (VES-13) for transplant-ineligible patients with newly diagnosed multiple myeloma. Ann Hematol. 2021;100(11):2745–2754. doi:10.1007/s00277-021-04592-y
- Cruz-Jentoft AJ, Gonzalez B, de la Rubia J, et al. Further psychometric validation of the GAH scale: Responsiveness and effect size. J Geriatr Oncol. 2017;8(3):211–215. doi:10.1016/j.jgo.2016.12.008
- Engelhardt M, Dold SM, Ihorst G, et al. Geriatric assessment in multiple myeloma patients: validation of the International Myeloma Working Group (IMWG) score and comparison with other common comorbidity scores. Haematologica. 2016;101(9):1110–1119. doi:10.3324/haematol.2016.148189
- Avet-Loiseau H, Hulin C, Campion L, et al. Chromosomal abnormalities are major prognostic factors in elderly patients with multiple myeloma: the intergroupe francophone du myelome experience. J Clin Oncol. 2013;31(22):2806–2809. doi:10.1200/JCO.2012.46.2598
- Barth P, Giri S, Reagan JL, et al. Outcomes of lenalidomide- or bortezomib-based regimens in older patients with plasma cell myeloma. Am J Hematol. 2021;96(1):14–22. doi:10.1002/ajh.25996
- Durie BGM, Hoering A, Sexton R, et al. Longer term follow-up of the randomized phase III trial SWOG S0777: bortezomib, lenalidomide and dexamethasone vs. lenalidomide and dexamethasone in patients (Pts) with previously untreated multiple myeloma without an intent for immediate autologous stem cell transplant (ASCT). Blood Cancer J. 2020;10(5):53. doi:10.1038/s41408-020-0311-8
- O'Donnell EK, Laubach JP, Yee AJ, et al. A phase 2 study of modified lenalidomide, bortezomib and dexamethasone in transplant-ineligible multiple myeloma. Br J Haematol. 2018;182(2):222–230. doi:10.1111/bjh.15261
- Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371(10):906–917. doi:10.1056/NEJMoa1402551
- Facon T, Kumar SK, Plesner T, et al. Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(11):1582–1596. doi:10.1016/S1470-2045(21)00466-6
- Mateos MV, Dimopoulos MA, Cavo M, et al. Daratumumab plus bortezomib, melphalan, and prednisone versus bortezomib, melphalan, and prednisone in transplant-ineligible newly diagnosed multiple myeloma: frailty subgroup analysis of ALCYONE. Clin Lymphoma Myeloma Leuk. 2021;21(11):785–798. doi:10.1016/j.clml.2021.06.005
- Facon T, Cook G, Usmani SZ, et al. Daratumumab plus lenalidomide and dexamethasone in transplant-ineligible newly diagnosed multiple myeloma: frailty subgroup analysis of MAIA. Leukemia. 2022;36(4):1066–1077. doi:10.1038/s41375-021-01488-8
- Palumbo A, Bringhen S, Ludwig H, et al. Personalized therapy in multiple myeloma according to patient age and vulnerability: a report of the European Myeloma Network (EMN). Blood. 2011;118(17):4519–4529. doi:10.1182/blood-2011-06-358812