ABSTRACT
Follicular dendritic cell sarcoma (FDCS) is a rare low-intermediate grade malignant neoplasm. To date, published data on FDCS clinical courses are sparse, and no conditional survival study has been performed. Thus, we retrospectively analyzed 187 patients diagnosed with FDCS from the Surveillance, Epidemiology, and End Results (SEER) database. In this study, the median age at diagnosis was 50 years and 91 (48.7%) patients were male. The most common primary location was the abdomen/pelvis (82, 43.9%). The 1-year, 3-year, and 5-year overall survival (OS) were 88.7%, 69.0%, and 59.8%, respectively. The 5-year conditional overall survival increased from 65.7% at baseline to 83.8% in 5-year survivors. The 3-year FDCS-specific death rate was 26.7% and the rate of death from other reasons was 3.7%. In addition, the annual death hazard was the highest in the first four years after diagnosis and increased again in the 7th and 8th years. Age > 60 years at diagnosis, metastatic disease, and FDCS in thoracic organs were associated with shorter OS and FDCS-specific survival. In addition, FDCS patients, with either local or metastatic disease, could benefit from surgery therapy. In addition, adjuvant radiotherapy or chemotherapy for local disease provided no significant improvement in overall survival or FDCS-specific survival. We hope these findings may guide treatments and surveillance strategies for FDCS patients in clinical practice.
1. Introduction
Follicular dendritic cells (FDCs) are stromal-derived cells that capture and present antigens, retain immunocomplexes, modulate B-cell activation, and shape the immune response [Citation1–3]. In addition, follicular dendritic cells can prevent autoimmunity, provide scaffolds to lymphoid tissues, and affect the removal of cell debris from the germinal center [Citation4–6]. Follicular dendritic cell sarcoma (FDCS) is a rare low-intermediate grade malignant neoplasm that occurs in lymph nodes and extranodal sites [Citation7]. Follicular dendritic cell sarcoma was first reported in 1986 by Monda et al. and was shown to be characterized by the proliferation of oval and spindle cells with follicular dendritic cell differentiation [Citation8]. Subsequently, the World Health Organization classified it as a subtype of histiocytic and dendritic cell neoplasms.
Follicular dendritic cell sarcoma is prone to be misdiagnosed because of its rarity, variable clinical presentation, and morphologic similarities with other tumors. The differential diagnoses of FDCS include interdigitating dendritic cell sarcoma, Langerhans cell histiocytosis, spindle cell carcinoma, inflammatory myofibroblastic tumor, inflammatory pseudotumor, lymphoma, sarcoma, melanoma, undifferentiated carcinomas, thymoma, Schwannoma, and gastrointestinal stromal tumor [Citation5, Citation7, Citation9]. Immunohistochemical studies are crucial in the diagnosis of FDCS. Tumor cells are typically positive for CD21, CD23, CD35, clusterin, CXCL13, and podoplanin, which are specific immunohistochemical markers of follicular dendritic cell differentiation [Citation10, Citation11].
Follicular dendritic cell sarcomas mainly affect middle-aged people (median age 49 years) and have no gender predilection [Citation12]. Most follicular dendritic cell sarcomas (79.4%) occur in extranodal sites and the most frequent primary extranodal locations are the liver and spleen [Citation7]. There is no uniform therapeutic protocol yet. For local disease, complete surgical excision is the best treatment strategy. Adjuvant radiotherapy did not have a significant impact on overall survival [Citation12, Citation13]. The optimal chemotherapy regimen for advanced disease is uncertain [Citation14]. The effectiveness of kinase inhibitors and immune checkpoint inhibitors warrants further investigation [Citation7]. A pooled analysis indicated that the 2-year survival rates of early localized and locally advanced disease were 82.4% and 80%, respectively. However, for distant metastatic disease, the 2-year survival rate was only 42.8% [Citation12].
Conditional survival refers to the probability of living an additional number of years considering that the patient has survived a specified time since diagnosis [Citation15]. Conditional survival can provide more accurate information to patients and clinicians during follow-up. However, to date, published data on FDCS clinical courses are sparse, and no conditional survival study has been performed. The Surveillance, Epidemiology, and End Results (SEER) database includes information on incidence, mortality and other cancer-associated statistics for malignancy patients in the United States. In this retrospective study, we aimed to assess the conditional survival and annual death hazards of patients with FDCS based on the SEER database and explore clinical characteristics associated with overall survival (OS) and FDCS-specific survival.
2. Method
2.1 Study population and data sources
In this retrospective cohort study, patient data were extracted from the SEER database by SEER*STAT software version 8.4.0. Cases of follicular dendritic cell sarcoma were identified according to the third edition of the International Taxonomy of Oncology (ICD-O-3) histological code 9758/3. The inclusion criteria were as follows: FDCS diagnosed between 2001–2015; FDCS as the first malignancy; complete data on main covariates (age, race, stage, and survival month); and the exact cause of death. Finally, 187 patients were included in the statistical analysis. The detailed screening process is shown in .
2.2 Variables and outcomes
Based on previous studies and data availability, patients’ demographic characteristics, tumor characteristics, treatment data, and survival data were collected, including age at diagnosis, sex, race, year of diagnosis, marital status, primary site, stage, surgery, radiotherapy, chemotherapy, survival time, vital status and cause of death. The stage was divided into local and distant. In accordance with previous publications, surgery was defined as applicable for patients who received any type of surgery, and primary sites were partitioned into the following categories: extremities/spine, head and neck, thorax, abdomen/pelvis, and others. Among them, thoracic organs were defined as including the lung, thymus, intrathoracic lymph nodes, mediastinum, and other soft tissue.
OS, conditional overall survival (COS), and FDCS-specific survival were the outcomes of this study. OS was calculated from the initial FDCS diagnosis to the date of all-cause death (event) or to the date of last follow-up (censored patients). FDCS-specific survival was measured as the period from initial FDCS diagnosis to the date of FDCS-related death (event of interest) or to the date of death from other causes (competing events) or the date of last follow-up (censored patients). According to the concept of conditional survival, we calculated 1-year, 3-year, and 5-year COS for 1, 2, 3, 4, and 5-year survivors in this study.
2.3 Statistical analysis
Continuous variables are expressed as medians (minimum and maximum) and categorical variables are reported as numbers (percentages). Survival rates were analyzed by the Kaplan-Meier method and compared by the log-rank test. The Cox proportional hazards regression model was performed to assess hazard ratios (HRs) and 95% confidence intervals (CIs) of clinical variables on the risk of OS. Fine and Gray’s subdistribution hazards model was used to estimate subdistribution hazard ratios (SHRs) and corresponding 95% CIs of clinical factors on the risk of FDCS-related death, taking into account death from other causes as the competing risk [Citation16]. Conditional survival was calculated as: CS(y/x) = S(y + x)/S(x), where CS(y/x) is the probability of the patient surviving for additional y years, given that they had already survived for x years [Citation15]. The annual hazard was defined as the annual incidence of death. P values less than .05 were considered statistically significant. All statistical analyses were conducted in SPSS version 23.0, R software version 4.1.2, and GraphPad Prism 9.4.1.
3. Result
3.1. Demographic and clinicopathological characteristics
Finally, 187 patients were included in the analysis, 144 (77.0%) patients with local disease and 43 (23.0%) with metastatic disease. The characteristics of these patients and treatment information are summarized in . The median age at diagnosis is 50 years (range: 8–87 years). 91 (48.7%) patients are male, and 96 (51.3%) are female. The most common primary location the is abdomen/pelvis (82, 43.9%), followed by thorax (37, 19.8%) and head and neck (36, 19.3%). In addition, of 187 patients, 137 (73.3%) underwent surgery, 66 (35.3%) received radiation therapy, and 66 (35.3%) were treated with chemotherapy.
Table 1. Characteristics of patients with follicular dendritic cell sarcoma (FDCS).
3.2. Overall survival and conditional overall survival
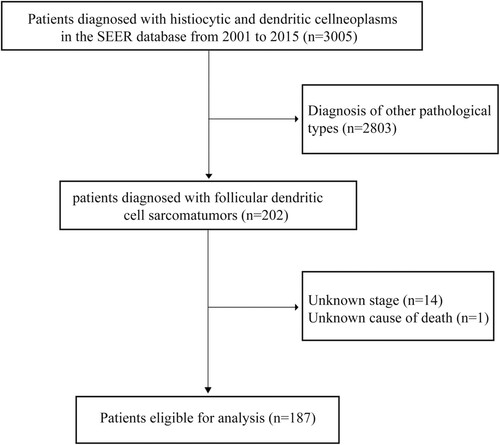
During a median of 58 months of follow-up, 89 (47.6%) deaths occurred. The 1-year, 3-year, and 5-year OS are 88.7%, 69.0%, and 59.8%, respectively. The median survival in all patients is not reached. For patients with local disease, the 1-year, 3-year, and 5-year OS are 89.6%, 73.4%, and 67.3%, respectively. The median OS is not reached. While, for patients with distant disease, the 1-year, 3-year, and 5-year OS are 85.6%, 53.8%, and 34.2%, respectively. The median survival is 39 months.
The 1-year COS for 1-, 2-, 3-, 4-, and 5-year survivors are 88.9%, 87. 4%, 88.4%, 98.0%, and 97.4%, respectively; the 3-year COS are 68.8%, 75.8%, 84.5%, 87.9%, and 83.8%; and the 5-year COS are 65.7%, 67.9%, 72.6%, 82.1% and 83.8% (). The annual death hazards for 1-, 2-, 3-, 4-, 7-, and 8-year survivors are 11.2%, 11.0%, 12.3%, 11.4%, 7.8%, and 6.3%, respectively. The univariate regression analysis suggests that age > 60 years at diagnosis, metastatic disease (P = 0.001), and FDCS in thoracic organs are risk factors for OS. Multivariable Cox regression indicates that except for age > 60 years at diagnosis (hazard ratio [HR]: 2.907; 95% CI: 1.545–5.470; P = 0.001), metastatic disease (HR: 2.625; 95% CI: 1.616–4.263; P < 0.001), and FDCS in thoracic organs (HR: 3.428; 95% CI: 1.258–9.342; P = 0.016), black ethnicity (HR: 2.052; 95% CI: 1.140–3.694; P = 0.017) is also associated with a higher risk of all-cause death ().
Figure 2. Survival of the whole follicular dendritic cell sarcoma (FDCS) cohort. A. Kaplan-Meier survival curve; B. 1-year, 3-year, and 5-year conditional overall survival (COS); and C. the annual rate of death for patients with FDCS.
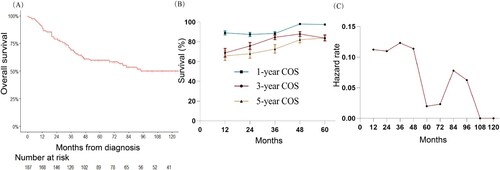
Table 2. Univariable and multivariable Cox regression analyses for OS.
3.3. Disease-specific survival
In this study, 78 (41.7%) patients died due to FDCS, and 11 (5.9%) patients died due to other reasons. Deaths for other reasons mainly occurred within the first three years after diagnosis. The 3-year FDCS-specific death rate is 26.7% and the rate of death from other reasons is 3.7%.
The univariate competing risk analysis shows that age > 60 years at diagnosis, metastatic disease, FDCS in thoracic organs, and time of diagnosis between 2010 and 2015 are indicators for FDCS-specific survival. However, the multivariable competing risk analysis suggests that only age > 60 years at diagnosis (hazard ratio [HR]: 2.907; 95% CI: 1.545–5.470; P = 0.001), metastatic disease (HR: 2.588; 95% CI: 1585–4.230; P < 0.001), and FDCS in thoracic organs (HR: 4.903; 95% CI: 1.152–20.87; P = 0.031) are related to shorter FDCS-specific survival. Of note, race is not associated with the risk of FDCS-specific deaths ( and ).
Figure 3. A. Cumulative incidence of FDCS-specific death by age; B. stage; C. race; and D. primary site.
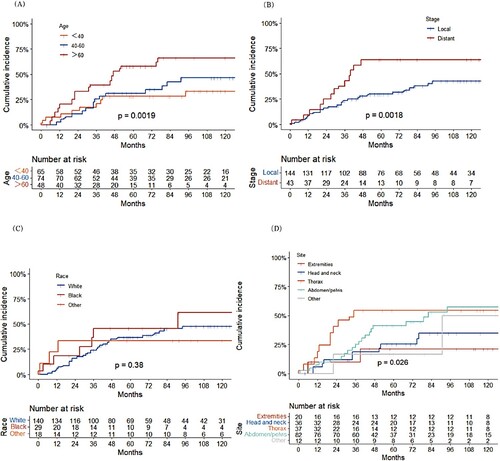
Table 3. Univariable and multivariable Fine-Gray regression analyses for FDCS-specific survival.
3.4. Treatment
Among 144 patients with local disease, 57 (39.6%) underwent surgery only; 40 (27.8%) were treated with surgery combined with radiation; 12 (8.3%) received surgery combined with chemotherapy; and 35 (24.3%) received other therapies. As shown in , compared with surgery alone, surgery combined with radiation has no significant effect on overall survival or FDCS-specific survival, but surgery combined with chemotherapy results in shorter overall survival and a higher risk of FDCS-specific deaths.
Figure 4. A. Overall survival and B. cumulative incidence of FDCS-specific death by treatment of FDCS patients with local disease; C. Overall survival and D. cumulative incidence of FDCS-specific death by treatment of FDCS patients with metastatic disease.
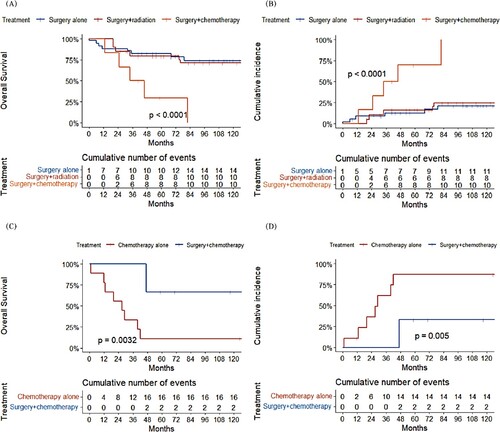
Of 43 patients with metastatic disease, 18 (41.9%) were treated with chemotherapy alone, 6 (14.0%) with surgery combined with chemotherapy, and 19 (44.2%) with other therapies. A significant difference in OS and FDCS-specific deaths was observed between chemotherapy alone and surgery combined with chemotherapy.
4. Discussion
Follicular dendritic cell sarcoma is an extremely rare malignant neoplasm. Since FDCS was first proposed in 1986 by Monda et al, a number of cases have been reported. These cases have been reviewed in a few studies. However, data from large cohorts are lacking, and there is no attempt to assess the conditional survival and annual hazards for FDCS. Therefore, we conducted this population-based study based on the SEER database.
The results of our study suggested that follicular dendritic cell sarcomas mainly affect middle-aged people (median age 50 years) and have no gender predilection, which is in accordance with previous studies [Citation5, Citation7, Citation9, Citation12, Citation17]. In addition, in this study, the most frequent primary site was the abdominal and pelvic region, consistent with the studies by Stephanie et al., Lan et al. and Mrinal et al. and 89.3% of follicular dendritic cell sarcomas occurred in extranodal sites, which was slightly higher than that (79.4%) reported by Fabio et al. [Citation7, Citation13, Citation18, Citation19]. Information on the survival of FDCS is limited. In a pooled analysis by Caner et al., the 2-year survival rates in cases of early localized and locally advanced disease were 82.4% and 80%, respectively. For distant metastatic diseases, the 2-year survival rate was only 42.8% [Citation12]. In the review by Mrinal et al., the 5-year survival rates of localized and metastatic disease were 55% and 38%, respectively [Citation18]. In this study, for patients with local disease, the 2-year and 5-year survival rates were 81.2% and 67.3%, respectively. Regarding patients with distant disease, the 2-year and 5-year survival rates were 70.9% and 34.2%, respectively. The 2-year survival rate of metastatic disease and the 5-year survival rate of local disease in our study were different from those previously reported. A possible explanation is the difference in the number of patients. Our study included 144 patients with local disease and 43 with metastatic disease. However, in the pooled analysis by Caner et al., 27 patients with FDCS had distant metastases and only 19 patients had sufficient follow-up data [Citation12]. The review by Mrinal et al. included only 23 FDCS patients with localized or locally advanced disease [Citation18].
Our study indicated that the 1-year COS in 1-year, 2-year, and 3-year survivors were similar and the 1-year COS increased from 88.4% in 3-year survivors to 97.4% in 5-year survivors; the 3-year COS increased from 65.7% at baseline to 87.9% in 4-year survivors and slightly decreased to 83.8% in 5-year survivors; the 5-year COS increased from 65.7% at baseline to 83.8% in 5-year survivors. In addition, the overall annual death hazard was the highest in the first four years after diagnosis and increased again in the 7th and 8th years. High mortality in the first four years after diagnosis might be due to distant metastases at diagnosis, comorbid conditions, and complications of treatment. The annual death hazard increased in the 7th and 8th years after diagnosis, which may be associated with local recurrences or metastases. Prior publications noted that the rates of local recurrence and distant metastasis are 28% and 27%, respectively [Citation7, Citation12, Citation20]. Conditional overall survival and annual death hazard, reflecting the changes in survival probability over time, provide more information for clinicians during follow-up in routine clinical practice.
Similar to the review by Mrinal et al, localized disease was found to be a predictor of better survival in this study [Citation18]. In contrast to some reports, our study showed that intra-abdominal involvement was not associated with poor prognosis, which was consistent with the pooled analysis by Caner et al. [Citation12, Citation21]. Instead, with univariate and multivariate analyses in this study, FDCS in thoracic organs was demonstrated to be related to adverse outcomes. In addition, we also found that age > 60 years at diagnosis was a prognostic parameter. Of interest, in the Cox regression analysis, black ethnicity was found to be related to a higher risk of all-cause mortality. However, the competing risk analysis indicated that there was no association between black ethnicity and FDCS-specific deaths. In previous studies, there was no evidence indicating that race is a risk factor for OS [Citation12, Citation13, Citation19, Citation21–23]. One explanation is that some individuals of black ethnicity died from non-FDCS-related causes, which may be related to their economic status. In addition, only 29 (15.5%) black patients were included in this study. The number was limited.
There are no uniform guidelines on FDCS treatment yet. Previous studies indicated that for local disease, complete surgical excision is the optimal therapeutic option and adjuvant radiotherapy provides no significant improvement in terms of overall survival [Citation9, Citation12, Citation13, Citation17]. Concordantly, there was no significant difference in the survival curves between the surgery-alone group and the surgery combined with radiotherapy group in our study. Of note, overall survival and FDCS-specific survival were significantly shorter in the surgery combined with chemotherapy group than in the surgery alone and surgery combined with radiotherapy group. We think one potential explanation is that complications of chemotherapy may affect the survival of FDCS patients. For the management of metastatic disease, chemotherapy is the major therapy [Citation12, Citation14, Citation24]. However, the optimal chemotherapy regimen is uncertain. In our study, surgery combined with chemotherapy was associated with longer survival than chemotherapy alone. Surgery might reduce the tumor load and improve the survival of patients with metastatic disease.
There are some limitations in this study. First, information on the status of the operative margin, specific chemotherapy regimens, and histological features was lacking. According to previous studies, some histological features, such as tumor size, necrosis, and high mitotic count, are prognostic factors [Citation12, Citation19, Citation23]. Second, data on disease progression, recurrence, or metastasis are not included in the SEER database. Thus, we were unable to provide information on progression-free survival in this study. In addition, the OS and FDCS-specific survival are different because of the locations. Owing to the rarity of FDCS, the number of patients was limited. Subgroup analysis was lacking in this study.
5. Conclusion
In conclusion, we conducted this retrospective study to assess the conditional survival and annual death hazards for patients with FDCS and explore clinical characteristics associated with overall survival and FDCS-specific survival. We found that in general, conditional survival rates tended to increase over time, and the annual death hazard was the highest in the first four years after diagnosis. In addition, we identified age > 60 years at diagnosis, metastatic disease and FDCS in thoracic organs as negative prognostic factors. FDCS patients, with either local disease or metastatic disease, were able to benefit from surgery. Adjuvant radiotherapy or chemotherapy provided no significant improvement in overall survival or FDCS-specific survival in cases of local disease. These findings may guide treatments and surveillance strategies for FDCS patients in clinical practice.
Ethics approval and patient consent statement
In this study, the data were obtained from SEER database. Thus, no ethics approval and patient consent statement needed.
Data availability statement
If needed, study data can be obtained by contacting the corresponding author.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Heesters BA, Myers RC, Carroll MC. Follicular dendritic cells: dynamic antigen libraries. Nat Rev Immunol. 2014;14(7):495–504. doi:10.1038/nri3689
- Krautler NJ, Kana V, Kranich J, et al. Follicular dendritic cells emerge from ubiquitous perivascular precursors. Cell. 2012;150(1):194–206. doi:10.1016/j.cell.2012.05.032
- Mabbott NA, Baillie JK, Kobayashi A, et al. Expression of mesenchyme-specific gene signatures by follicular dendritic cells: insights from the meta-analysis of microarray data from multiple mouse cell populations. Immunology. 2011;133(4):482–498. doi:10.1111/j.1365-2567.2011.03461.x
- Aguzzi A, Kranich J, Krautler NJ. Follicular dendritic cells: origin, phenotype, and function in health and disease. Trends Immunol. 2014;35(3):105–113. doi:10.1016/j.it.2013.11.001
- Facchetti F, Lorenzi L. Follicular dendritic cells and related sarcoma. Semin Diagn Pathol. 2016;33(5):262–276. doi:10.1053/j.semdp.2016.05.002
- Wang X, Rodda LB, Bannard O, et al. Integrin-mediated interactions between B cells and follicular dendritic cells influence germinal center B cell fitness. J Immunol. 2014;192(10):4601–4609. doi:10.4049/jimmunol.1400090
- Facchetti F, Simbeni M, Lorenzi L. Follicular dendritic cell sarcoma. Pathologica. 2021;113(5):316–329. doi:10.32074/1591-951X-331
- Monda L, Warnke R, Rosai J. A primary lymph node malignancy with features suggestive of dendritic reticulum cell differentiation. A report of 4 cases. Am J Pathol. 1986;122(3):562–572.
- Wu A, Pullarkat S. Follicular dendritic cell sarcoma. Arch Pathol Lab Med. 2016;140(2):186–190. doi:10.5858/arpa.2014-0374-RS
- Lorenzi L, Doring C, Rausch T, et al. Identification of novel follicular dendritic cell sarcoma markers, FDCSP and SRGN, by whole transcriptome sequencing. Oncotarget. 2017;8(10):16463–16472. doi:10.18632/oncotarget.14864
- Grogg KL, Macon WR, Kurtin PJ, et al. A survey of clusterin and fascin expression in sarcomas and spindle cell neoplasms: strong clusterin immunostaining is highly specific for follicular dendritic cell tumor. Mod Pathol. 2005;18(2):260–266. doi:10.1038/modpathol.3800294
- Saygin C, Uzunaslan D, Ozguroglu M, et al. Dendritic cell sarcoma: a pooled analysis including 462 cases with presentation of our case series. Crit Rev Oncol Hematol. 2013;88(2):253–271. doi:10.1016/j.critrevonc.2013.05.006
- Perkins SM, Shinohara ET. Interdigitating and follicular dendritic cell sarcomas: a SEER analysis. Am J Clin Oncol. 2013;36(4):395–398. doi:10.1097/COC.0b013e31824be22b
- Sasaki M, Izumi H, Yokoyama T, et al. Follicular dendritic cell sarcoma treated with a variety of chemotherapy. Hematol Oncol. 2017;35(4):905–908. doi:10.1002/hon.2364
- Shack L, Bryant H, Lockwood G, et al. Conditional relative survival: a different perspective to measuring cancer outcomes. Cancer Epidemiol. 2013;37(4):446–448. doi:10.1016/j.canep.2013.03.019
- Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. doi:10.1080/01621459.1999.10474144
- Chen T, Gopal P. Follicular dendritic cell sarcoma. Arch Pathol Lab Med. 2017;141(4):596–599. doi:10.5858/arpa.2016-0126-RS
- Gounder M, Desai V, Kuk D, et al. Impact of surgery, radiation and systemic therapy on the outcomes of patients with dendritic cell and histiocytic sarcomas. Eur J Cancer. 2015;51(16):2413–2422. doi:10.1016/j.ejca.2015.06.109.
- Li L, Shi YH, Guo ZJ, et al. Clinicopathological features and prognosis assessment of extranodal follicular dendritic cell sarcoma. World J Gastroenterol. 2010;16(20):2504–2519. doi:10.3748/wjg.v16.i20.2504
- Dalia S, Shao H, Sagatys E, et al. Dendritic cell and histiocytic neoplasms: biology, diagnosis, and treatment. Cancer Control. 2014;21(4):290–300. doi:10.1177/107327481402100405
- Jain P, Milgrom SA, Patel KP, et al. Characteristics, management, and outcomes of patients with follicular dendritic cell sarcoma. Br J Haematol. 2017;178(3):403–412. doi:10.1111/bjh.14672
- Li J, Zhou ML, Zhou SH. Clinical and pathological features of head and neck follicular dendritic cell sarcoma. Hematology. 2015;20(10):571–583. doi:10.1179/1607845415Y.0000000008
- Pang J, Mydlarz WK, Gooi Z, et al. Follicular dendritic cell sarcoma of the head and neck: Case report, literature review, and pooled analysis of 97 cases. Head Neck. 2016;38(Suppl 1):E2241–E2249.
- Soriano AO, Thompson MA, Admirand JH, et al. Follicular dendritic cell sarcoma: a report of 14 cases and a review of the literature. Am J Hematol. 2007;82(8):725–728.