ABSTRACT
Objectives:
High-risk multiple myeloma (HRMM) is associated with poor survival, despite many advances in antimyeloma strategies. Autologous followed by allogeneic stem cell transplantation (auto-allo-SCT) has yielded controversial results compared to tandem autologous stem cell transplantation (auto-SCT) in patients with HRMM. We conducted this meta-analysis to compare the efficacy and safety of auto-allo-SCT and tandem-auto-SCT in patients with HRMM.
Methods:
Embase, Cochrane Library, and PubMed databases were searched until March 2023. Prospective or retrospective studies comparing the effects of auto-allo-SCT and tandem-auto-SCT were included. Hazard ratios (HRs) and 95% confidence intervals (CIs) for time-to-event outcomes, and odds ratios (ORs) and 95%CIs for dichotomous outcomes were pooled using random-effects models.
Results:
Three studies involving 491 patients were included. Despite auto-allo-SCT seemed to be associated with improvements in progression-free survival (PFS) (HR [95%CI], 0.71 [0.51–1.00]) and complete response (CR) (OR [95%CI], 3.16 [1.67–5.99]), and reduced relapse/progression rates (47% vs. 55%) in comparison with tandem-auto-SCT, no marked improvement in overall survival (OS). In comparison to tandem-auto-SCT, patients assigned to auto-allo-SCT exhibited a higher risk of transplant-related mortality (TRM) (11.9% vs. 4.1%) and non-relapse mortality (NRM) (12.3% vs. 3.1%).
Conclusion:
Auto-allo-SCT seemed to be associated with improvements in PFS and CR when compared to tandem-auto-SCT in patients with HRMM, but it did not lead to a significant improvement in OS. Furthermore, patients in the auto-allo-SCT group were at a higher risk of developing TRM and NRM. Auto-allo-SCT transplantation should not be routinely incorporated into HRMM therapy but rather should be considered investigational.
Introduction
Multiple myeloma (MM) is characterized by the presence of neoplastic cells within the bone marrow and the secretion of monoclonal M proteins in urine and blood [Citation1]. MM accounts for 1% of all cancers and 10% of all hematologic malignancies [Citation2]. It is considered incurable because disease-free intervals are increasingly shortened due to the development of drug resistance, until the patient eventually dies [Citation3]. In 2022, MM will account for 1.8% of all new cancer cases and 2.1% of all cancer-related deaths [Citation3]. The 5-year (2012–2018) relative survival rate was 57.9% [Citation3]. Few patients live for more than 20 years after being diagnosed, and most live with relapsed or refractory disease [Citation3]. The heterogeneous clinical course of MM, which is closely linked to its biology, can be predicted using certain prognostic factors at the time of diagnosis.
Numerous factors are associated with poor outcomes, including high β2-microglobulin and lactate levels, gene expression profiles, and recurrent cytogenetic abnormalities [Citation4]. For example, the presence of t(4;14) or t(14;16) is generally accepted as a marker of poor prognosis and has been incorporated into the Revised International Staging System (R-ISS) MM. Additionally, other aberrations may be present, such as the gain of 1q [gain(1q)], which is present in approximately 35–40% of patients and is associated with a worse prognosis [Citation5], and the 17p deletion [del(17p)], which is found in 8–10% of cases and is also considered a high-risk marker for reduced survival [Citation3,Citation6]. Thus, high-risk MM (HRMM) has varied definitions, but refers to patients with a first progression-free survival (PFS) of less than 2–3 years [Citation7]. Approximately 15–20% of newly diagnosed MM (NDMM) patients and up to 30% of relapsed/refractory MM (RRMM) patients are classified as high-risk [Citation8].
Autologous stem cell transplantation (auto-SCT) remains the standard of care for eligible patients as a continuation of first-line treatment, including patients with HRMM, even in the era of novel drugs [Citation9]. Contrary to standard-risk patients who may delay their first auto-SCT, patients with HRMM are encouraged to undergo auto-SCT. Auto-SCT prolongs PFS and overall survival (OS) in patients with NDMM. Nevertheless, nearly all patients eventually relapse [Citation10]. Tandem-auto-SCT proved to be more beneficial than single auto-SCT for HRMM patients who did not respond to bortezomib induction therapy (median PFS 42.0 vs 21.0 months, 5-year OS: 70% vs. 17%) [Citation11]. However, a recent randomized phase III trial by the Blood and Marrow Transplant Clinical Trials Network compared three different transplant strategies in patients with MM: (1) tandem-auto-SCT, (2) single auto-SCT followed by lenalidomide/bortezomib/dexamethasone consolidation, and (3) single auto-SCT, with all three groups followed by lenalidomide maintenance [Citation12]. In the intent-to-treat analysis of HRMM, the 6-year PFS and OS rates were the same for tandem-auto-SCT (43.9% and 73.1%, respectively) and for single auto-SCT (40.9% and 76.4%, respectively). In contrast, in the as-treated analysis, patients with HRMM showed significantly greater 6-year PFS after tandem-auto-SCT than after single auto-SCT (43.6% vs. 26%, respectively; p = 0.03). Thus, the role of tandem auto-SCT in patients with HRMM continues to be debated in light of the available evidence.
Moreover, allogeneic stem cell transplantation (allo-SCT) can potentially benefit from the graft–versus–myeloma effect; however, the risk of non-relapse mortality (NRM) driven by conditioning regimen toxicity and graft-versus-host disease (GVHD) is increased. Patients with HRMM tend to show improved event-free survival (EFS) after allo-SCT; however, the sample size was small [Citation13]. Subgroup analysis in a previous meta-analysis showed that allo-SCT may be strongly considered as the initial course of therapy or in first-relapse HRMM patients [Citation14]; however, subgroup analysis in another meta-analysis did not show an OS benefit from auto-SCT followed by allo-SCT (auto-allo-SCT) over tandem auto-SCT in these patients [Citation10]. Recently, the Deutschen Studiengruppe Multiples Myelom (DSMM) V trial compared the effects of auto-allo-SCT with those of tandem auto-SCT in patients with HRMM and found that PFS were better after auto-allo-SCT than tandem-auto-SCT (median OS 34.5 vs 21.8 months, respectively) [Citation15].
Given these conflicting results, the preferred treatment strategy for patients with HRMM remains unclear. We performed a systematic review and meta-analysis of all published studies comparing the efficacy and safety of auto-allo-SCT and tandem-auto-SCT in patients with newly diagnosed HRMM.
Methods
This systematic review and meta-analysis were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [Citation16].
Search strategy and selection criteria
We conducted a literature search to identify relevant articles in PubMed, the Cochrane Central Register of Trials, and EMBASE from the inception of each database to March 2023. We used a combination of standardized terms, keywords, and controlled vocabulary to reflect the following concepts: ‘multiple myeloma’ AND ‘allogeneic transplantation’ AND ‘autologous transplantation’, without filtering publication type or language. The search strategy is presented in Supplemental Table 1. The references list of the included studies and those of previous reviews and meta-analyses were manually reviewed. We included all prospective or retrospective studies of patients with newly diagnosed HRMM that compared auto-allo-SCT with tandem-auto-SCT, reported OS and/or PFS for both groups, and included at least five patients per group. Patients with HRMM were defined as having extramedullary disease [Citation7,Citation17] and/or chromosomal abnormalities [t(4;14), t(14;16), t(14;20), del(17p), or gain(1q)], and/or R-ISS stage III [Citation18] at diagnosis according to the criteria of the International Myeloma Working Group consensus (IMWG) [Citation19], and Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART; www.msmart.org).
Study screening, selection, data extraction, and quality assessment
All potentially suitable articles were independently evaluated by two reviewers. All conflicts were resolved through discussion and any irreconcilable disagreements were resolved by consulting a third reviewer. After removing duplicates, the titles, and abstracts of all the articles were screened for eligibility. The full texts of all potentially relevant articles were reviewed, and eligible articles were included. A pre-designed Excel spreadsheet was used to extract the following study data: year of publication, first author, country of origin, sample size, age, sex, treatment regimen, follow-up duration, and study outcomes (OS, PFS, response rates, relapses, and mortality). The quality of the included studies was examined using the Risk-of-Bias in Non-randomized Studies of Interventions (ROBINS-I) tool [Citation20], which assesses seven domains of bias, classified as low, moderate, serious, critical risk of bias, or no information. These domains included confounding bias, selection bias, bias in the measurement classification of interventions, bias due to deviations from intended interventions, bias due to missing data, bias in the measurement of outcomes, and bias in the selection of reported results.
Definition of outcomes
The primary outcomes were OS and PFS achieved with auto-allo-SCT versus tandem auto-SCT. The secondary outcomes included relapse/progression rates, transplant-related mortality (TRM), NRM, and complete response (CR). If multiple publications were available, the publication with the longest follow-up period was used to extract the summary effect.
Statistical analysis
For the time-to-event outcomes, OS, PFS, hazard ratios (HRs), and 95% confidence intervals (CIs) were extracted when reported. If the HR was not reported, it was estimated using published Kaplan–Meier survival curves using the method outlined by Guyot et al. [Citation21]. Time-to-event outcomes were pooled using generic inverse variance under the random-effects model. If possible, estimates were pooled across studies for the OS and PFS endpoints for both intention-to-treat and per-protocol populations. For dichotomous outcomes, data were combined across studies using random-effects meta-analytical methods to construct overall estimates of odds ratios (ORs) and their 95%CIs. We used Cochran’s Q and I2 statistics to assess study-level heterogeneity. I2 values of approximately 25%, 50%, and 75% were defined as mild, moderate, and high heterogeneity, respectively [Citation22,Citation23]. Substantial heterogeneity was investigated using appropriate sensitivity and subgroup analyses. Finally, we used a funnel plot of the reported effect estimates to evaluate the risk of publication bias if at least 10 studies were included, and Egger’s regression test was also used to assess publication bias. Statistical analyses were performed using Review Manager (RevMan) version 5.4; (the Cochrane Collaboration, 2020). All p-values were two-sided and considered statistically significant at p < 0.05.
Results
Search results and study selection
We retrieved 1994 articles from electronic databases and citation searches and excluded 397 duplicate studies. The remaining 1597 articles were manually screened after reading their abstracts and titles. Of these, 1567 were excluded because they were irrelevant. After retrieving 30 articles for full-text review, 26 articles were excluded due to inappropriate article types (n = 4), no comparison with tandem-auto-SCT (n = 9), relapsed/refractory MM (n = 2), unspecified outcomes for HRMM (n = 7), and not in line with the definition of HRMM (high risk was defined as having deletion 13q or β2-microglobulin levels greater than 4 mg/L, which is not in line with the definition of high risk according to the criteria of R-ISS, IMWG and mSMART) (n = 4 manuscripts for two studies) [Citation24–27]. Finally, four relevant reports provided information on three studies that met the eligibility criteria for inclusion in the analysis () [Citation15,Citation28–30]. One study reporting patients with deletion 13q was included in our meta-analysis because most patients were also confirmed by the presence of t(4;14), del(17p), or gain(1q) [Citation15,Citation30].
Characteristics of the included trials
The auto-allo-SCT and tandem auto-SCT groups comprised 229 and 262 patients, respectively. The median age of the patients ranged from 52 to 59 years. Female patients accounted for 41% to 47.6% of the patients. The median follow-up duration of survivors ranged from 49 to 91 months. One study compared auto-allo-SCT and tandem-auto-SCT in patients with NDMM with extramedullary disease [Citation28]. All the patients in the auto-allo-SCT group underwent reduced-intensity allo-SCT. The characteristics of the individual trials and entire population included in the analysis are shown in .
Table 1. Details of studies included in the meta–analysis.
Quality assessment
Supplemental Table 2 shows the risk of bias in the included studies based on the ROBINS-I tool. All studies had a moderate risk of bias. The most consistent source of bias in these studies was the confounding factors.
Primary outcomes
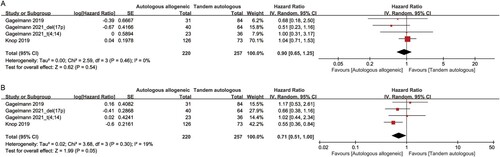
All three studies reported OS and PFS data for auto-allo-SCT compared to tandem auto-SCT in patients with newly diagnosed HRMM. Estimates were pooled across these studies for OS and PFS endpoints for the intention-to-treat populations because most studies did not report per-protocol population outcomes. Pooling these studies using the most recent follow-up data, the OS was not significantly different between the auto-allo-SCT and tandem-auto-SCT groups (HR [95%CI], 0.90 [0.65–1.25], p = 0.54) ((A)). Auto-allo-SCT appeared to be associated with improved PFS compared with tandem-auto-SCT (HR [95%CI], 0.71 [0.51–1.00], p = 0.05) ((B)). OS showed no statistically significant heterogeneity (I2 = 0%) and PFS showed mild heterogeneity (I2 = 19%).
Secondary outcomes
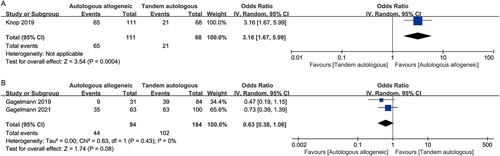
CR data were reported in only one study [Citation15,Citation30]. This study used the European Group for Blood and Marrow Transplantation (EBMT) criteria for the response assessment [Citation31]. The result showed a statistically significant difference in CR between auto-allo-SCT and tandem-auto-SCT (OR [95%CI], 3.16 [1.67–5.99], p = 0.0004) ((A)). Two studies reported relapse/progression rates [Citation28,Citation29]. Although a lower relapse/progression rate was achieved with auto-allo-SCT than with tandem auto-SCT (47% vs. 55%, respectively), the pooled results showed no significant difference (OR [95%CI], 0.63 [0.38–1.06], p = 0.08) ((B)).
Figure 3. Forest plot of the odds ratios comparing auto-allo-SCT versus tandem-auto-SCT in patients with HRMM: (A) CR; (B) relapse/progression rates.
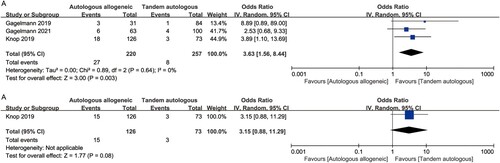
All three studies provided sufficient information to contribute to the NRM analysis [Citation15,Citation28–30]. Pooled results from these studies showed that NRM was significantly worse with auto-allo-SCT than with tandem auto-SCT (12.3% vs 3.1%; OR [95%CI], 3.63 [1.56–8.44], p = 0.003) ((A)). No heterogeneity was observed in the included studies (I2 = 0%). Similarly, the results from only one study [Citation15,Citation30] showed that auto-allo-SCT appeared to be associated with a higher risk of TRM, but there was no statistically significantly difference between auto-allo-SCT and tandem auto-SCT (11.9% vs 4.1%; OR [95%CI], 3.15 [0.88–11.29], p = 0.08) ((B)).
Figure 4. Forest plot of the odds ratios comparing auto-allo-SCT versus tandem-auto-SCT in patients with HRMM: (A) NRM; (B) TRM.
Auto-allo-SCT, autologous followed by allogeneic stem cell transplantation; CI, confidence interval; CR, complete response rate; HRMM, high-risk multiple myeloma; IV, inverse variance; NRM, non-relapse mortality; OS, overall survival; PFS, progression-free survival; SE, standard error; tandem-auto-SCT, tandem autologous stem cell transplantation; TRM, transplant-related mortality.
Publication bias
Only three studies were included in this meta-analysis; therefore, funnel plots and Egger’s regression tests were not reported.
Discussion
In this systematic review and meta-analysis, we found that despite lower relapse/progression rates, higher CR rates, and improved PFS following auto-allo-SCT, there was no apparent improvement in the OS of patients with newly diagnosed HRMM. We found higher TRM (11.9%) and NRM (12.3%) in patients assigned to auto-allo-SCT, although there was no statistically significant difference in TRM between auto-allo-SCT and tandem-auto-SCT. These findings are of direct clinical relevance and may provide evidence for the treatment of newly diagnosed HRMM.
Auto-SCT has become the standard up-front therapy for younger patients with MM. The patients continued to relapse with no survival plateau [Citation32]. Allo-SCT, primarily based on graft-versus-myeloma effects, can offer long-term disease control and probability of cure. The potential superiority of auto-allo-SCT was suggested by Bruno et al. [Citation33,Citation34]. Despite the lengthy follow-up, their study was suspected to be due to, among other reasons, the inconsistency of the conditioning regimen, the high dropout rate between the first and second transplants, and the extraordinarily poor performance of the control group. A diverging result emerged from a previous meta-analysis of trials with biological assignments, which showed that autologous plus reduced-intensity conditioning allogeneic transplantation was associated with higher TRM and CR, without PFS or OS improvement, compared to tandem auto-SCT [Citation35]. Another early systematic review and meta-analysis similarly indicated that despite the higher CR rates, OS did not improve with auto-allo-SCT, in addition to this approach led to higher NRM in patients with NDMM [Citation36]. Our results also demonstrated that patients with HRMM who underwent auto-allo-SCT had a higher CR rate, but there was no apparent improvement in OS. In addition, auto-allo-SCT was associated with higher NRM and TRM. These findings are consistent with those of the aforementioned meta-analyses. However, these studies included both standard- and high-risk patients. Considering that HRMM continues to portend worse outcomes despite advances in antimyeloma therapeutics, our meta-analysis was limited to patients with HRMM. Hence, this study provides more pertinent and accurate evidence concerning auto-allo-SCT than tandem auto-SCT in patients with HRMM.
In contrast, a meta-analysis that evaluated the role of allo-SCT based on studies from 2007 to 2017 did not recommend allo-SCT as a standard care option for standard-risk newly diagnosed or relapsed patients with MM; however, for HRMM patients with a poor long-term prognosis, allo-SCT may be considered in the initial therapy course or in the first relapse post-chemotherapy [Citation14]. Nevertheless, in that meta-analysis, patients with HRMM after auto-allo-SCT had similar OS and PFS to those with standard-risk MM, and the pooled effect measure for OS and PFS was expressed as a risk ratio. In contrast to their study, our meta-analysis pooled all published studies directly comparing auto-allo-SCT with tandem auto-SCT and is expected to help clinicians choose an optimal treatment regimen for patients with HRMM. Additionally, due to censoring, time-to-event outcomes are not amenable to standard statistical procedures used for the analysis of continuous outcomes, and the CONSORT guidelines suggest that the effect measure for survival time could be the HR or the difference in median survival time [Citation21]. The Cochrane Handbook also recommends that the effect measurement for time-to-event outcomes be expressed in terms of HR [Citation21]. Hence, in our meta-analysis, time-to-event outcomes, such as OS or PFS, were analysed using HRs, which consider the number and timing of events, since OR/RR, which measures only the number of events without considering when they occur, is appropriate for measuring dichotomous outcomes but less so for analysing time-to-event outcomes [Citation37]. Costa et al. performed a pooled analysis of individual-patient data to determine whether long-term follow-up affected the comparison between auto-allo-SCT and tandem auto-SCT for NDMM. The authors also conducted a subgroup comparison of OS and PFS between the two transplantation strategies for HRMM. However, their results were ambiguous as PFS was superior in the auto-allo-SCT arm as compared to the tandem-auto-SCT (HR 0.72, 95%CI [0.53–0.97], p = 0.028), but OS was similar between the strategies (HR 0.84, 95%CI [0.60–1.17], p = 0.293). Our study also indicated that auto-allo-SCT appeared to be associated with improved PFS compared to tandem-auto-SCT, but there was no significant improvement in OS. Nevertheless, β2-microglobulin level ≥4 mg/L and/or presence of deletion 13q by metaphase karyotyping were defined as high-risk patients in Costa et al. study. Our meta-analysis included the latest studies and aligned with the contemporary definition of high risk. High risk was defined as the presence of del(17p), t(4;14), t(14;16), t(14;20), or gain(1q) because the presence of deletion 13q is not in line with the current definition of high-risk abnormalities [Citation9,Citation38]. Thus, our study provides novel and accurate evidence regarding the efficacy of auto-allo-SCT and tandem auto-SCT in patients with HRMM.
Only one study involving patients with extramedullary disease in our meta-analysis showed a trend towards better outcomes using a tandem approach than with single auto-SCT, but there was no significant difference between auto-allo-SCT and tandem-auto-SCT [Citation28]. However, these results were limited by the small number of patients receiving auto-allo-SCT (n = 31). Given the poor survival rates of patients with double- or triple-hit myeloma, a previous study suggested that tandem ASCT should be considered [Citation9]. Two studies involving the small number of patients with more than one high-risk abnormality were included in our study. Gagelmann et al. included a small number of patients with both del(17p) and t(4;14), and their results showed that there was no significant difference in the 5-year OS and PFS between patients with t(4;14), del(17p), or both abnormalities [Citation29]. Another study involving patients with more than one high-risk abnormality who received single auto-SCT, auto-allo-SCT, or tandem-auto-SCT showed no difference in OS and PFS between the number of high-risk abnormalities categorized as isolated and >1 high-risk abnormality [Citation28]. However, we were unable to perform further analysis because these studies did not specifically provide the outcomes of auto-allo-SCT versus tandem-auto-SCT in patients with more than one high-risk abnormality. Taken together, for NDMM patients with extramedullary disease, double- or triple-hit, the tandem approach (either auto-allo-SCT or tandem-auto-SCT) should be investigated further.
The present study indicated that despite the lack of statistically significant differences in the relapse/progression rates between auto-allo-SCT and tandem-auto-SCT in patients with HRMM, a lower overall rate of relapse/progression was achieved with auto-allo-SCT than with tandem-auto-SCT (47% vs. 55%), which is consistent with a previous meta-analysis of auto-allo-SCT and tandem-auto-SCT for patients with NDMM [Citation10]. MM is a heterogeneous disease, and patients with HRMM are more likely to experience relapse or progression. We hypothesized that patients who underwent allo-SCT might have a lower risk of relapse/progression due to the graft-versus-myeloma effect. However, OS did not improve markedly, possibly due to insufficient long-term follow-up in some of the included studies. Allo-SCT may lead to deeper responses and may carry long-term benefits due to the existence of graft-versus-myeloma effects. Hence, long-term follow-up is important [Citation10,Citation39]. We observed a higher risk of TRM and NRM in patients with HRMM after auto-allo-SCT was observed in our study. These findings are consistent with those of previous studies of auto-allo-SCT for NDMM (including standard- and high-risk patients) [Citation10,Citation35,Citation36]. The TRM rate was 11.9% in autologous followed by reduced intensity allo-SCT in our study, which was significantly lower than that of fully myeloablative allo-SCT (approximately 30%) [Citation32]. However, this finding is limited by the fact that only one study provided TRM results [Citation15,Citation30].
This meta-analysis has several limitations. First, the total number of clinical trials contributing to this meta-analysis was small (only three studies); therefore, the conclusions may be inaccurate. Secondly, cytogenetically high risk was defined as the presence of del(17p) and/or t(4;14) in most studies. Our findings may be limited to patients with other cytogenetic abnormalities [such as t(14;16), t(14;20) or gain(1q)]. Therefore, auto-allo-SCT should be considered investigational and further assessed for different cytogenetic abnormalities in future trials, especially for extramedullary diseases and double- or triple-hit NDMM. In addition to patients with high-risk cytogenetics, several studies have highlighted that which may be the best treatment strategy for functionally high-risk patients [Citation40]. However, we were unable to assess the outcome of auto-allo-SCT versus tandem auto-SCT in these patients because we did not find a study concerning tandem SCT for functional high-risk patients. Finally, we were unable to assess some important outcomes, such as GVHD and post-relapse survival, as most of the included studies did not report these results. Further subgroup analysis was impossible because only four studies were included, most of which did not provide subgroup information. Only estimates were pooled across these studies for OS and PFS endpoints for intention-to-treat populations as most studies did not report outcomes with per-protocol populations.
Conclusion
In conclusion, we included the latest studies that compared the efficacy and safety of auto-allo-SCT with those of tandem auto-SCT in patients newly diagnosed with HRMM. We found no apparent improvement in OS in patients with newly diagnosed HRMM after auto-allo-SCT, despite it appeared to be associated with improved PFS and CR. Furthermore, auto-allo-SCT was associated with a higher risk of TRM and NRM; however, it may also be associated with a lower risk of relapse/progression. Auto-allo-SCT transplantation may not be routinely incorporated into HRMM therapy and should be considered investigational. Nevertheless, our findings should be interpreted with caution because there are several limitations to this meta-analysis. A larger number of prospective trials are required to confirm these findings.
Ethics statement
Ethical approval is not required, because this manuscript is a meta-analysis.
Supplemental Material
Download MS Word (17.6 KB)Acknowledgement
We would like to thank Editage (www.editage.cn) for English language editing.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Jakubowiak AJ, Kumar S, Medhekar R, et al. Daratumumab improves depth of response and progression-free survival in transplant-ineligible, high-risk, newly diagnosed multiple myeloma. Oncologist. 2022 Jul 5;27(7):e589–e596. doi:10.1093/oncolo/oyac067
- Rajkumar SV. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. 2022 Aug;97(8):1086–1107. doi:10.1002/ajh.26590
- Marcon C, Simeon V, Deias P, et al. Experts’ consensus on the definition and management of high risk multiple myeloma. Front Oncol. 2022;12:1096852. doi:10.3389/fonc.2022.1096852
- Giri S, Grimshaw A, Bal S, et al. Evaluation of daratumumab for the treatment of multiple myeloma in patients with high-risk cytogenetic factors: a systematic review and meta-analysis. JAMA Oncol. 2020 Nov 1;6(11):1759–1765. doi:10.1001/jamaoncol.2020.4338
- Barila G, Bonaldi L, Grassi A, et al. Identification of the true hyperdiploid multiple myeloma subset by combining conventional karyotyping and FISH analysis. Blood Cancer J. 2020 Feb 17;10(2):18. doi:10.1038/s41408-020-0285-6
- Manier S, Salem KZ, Park J, et al. Genomic complexity of multiple myeloma and its clinical implications. Nat Rev Clin Oncol. 2017 Feb;14(2):100–113. doi:10.1038/nrclinonc.2016.122
- Derman BA, Kosuri S, Jakubowiak A. Knowing the unknowns in high risk multiple myeloma. Blood Rev. 2022 Jan;51:100887. doi:10.1016/j.blre.2021.100887
- Mohyuddin GR, Sigle M, Chandrasekar VT, et al. Impact of anti-CD38 therapy in multiple myeloma with high-risk cytogenetics: systematic review and meta-analysis. Leuk Lymphoma. 2020 Oct;61(10):2519–2522. doi:10.1080/10428194.2020.1772475
- Goldman-Mazur S, Kumar SK. Current approaches to management of high-risk multiple myeloma. Am J Hematol. 2021 Jul 1;96(7):854–871. doi:10.1002/ajh.26161
- Costa LJ, Iacobelli S, Pasquini MC, et al. Long-term survival of 1338 MM patients treated with tandem autologous vs. autologous-allogeneic transplantation. Bone Marrow Transplant. 2020 Sep;55(9):1810–1816. doi:10.1038/s41409-020-0887-4
- Cavo M, Salwender H, Rosiñol L, et al. Double vs single autologous stem cell transplantation after bortezomib-based induction regimens for multiple myeloma: an integrated analysis of patient-level data from phase European III studies. Blood. 2013;122(21):767. doi:10.1182/blood.V122.21.767.767
- Hari P, Pasquini MC, Stadtmauer EA, et al. Long-term follow-up of BMT CTN 0702 (STaMINA) of postautologous hematopoietic cell transplantation (autoHCT) strategies in the upfront treatment of multiple myeloma (MM). J Clin Oncol. 2020 May 20;38(15_suppl[May 20, 2020]):8506–8506. 10.1200/JCO.2020.38.15_suppl.8506.
- Schilling G, Hansen T, Shimoni A, et al. Impact of genetic abnormalities on survival after allogeneic hematopoietic stem cell transplantation in multiple myeloma. Leukemia. 2008 Jun;22(6):1250–1255. doi:10.1038/leu.2008.88
- Yin X, Tang L, Fan F, et al. Allogeneic stem-cell transplantation for multiple myeloma: a systematic review and meta-analysis from 2007 to 2017. Cancer Cell Int. 2018;18:62. doi:10.1186/s12935-018-0553-8
- Knop S, Engelhardt M, Liebisch P, et al. Allogeneic transplantation in multiple myeloma: long-term follow-up and cytogenetic subgroup analysis. Leukemia. 2019 Nov;33(11):2710–2719. doi:10.1038/s41375-019-0537-2
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol. 2021 Jun;134:178–189. doi:10.1016/j.jclinepi.2021.03.001
- Hagen P, Zhang J, Barton K. High-risk disease in newly diagnosed multiple myeloma: beyond the R-ISS and IMWG definitions. Blood Cancer J. 2022 May 30;12(5):83. doi:10.1038/s41408-022-00679-5
- Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised international staging system for multiple myeloma: a report from international myeloma working group. J Clin Oncol. 2015 Sep 10;33(26):2863–2869. doi:10.1200/JCO.2015.61.2267
- Chng WJ, Dispenzieri A, Chim CS, et al. IMWG consensus on risk stratification in multiple myeloma. Leukemia. 2014 Feb;28(2):269–277. doi:10.1038/leu.2013.247
- Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Br Med J. 2016 Oct 12;355:i4919. doi:10.1136/bmj.i4919
- Guyot P, Ades AE, Ouwens MJ, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012 Feb 1;12:9. doi:10.1186/1471-2288-12-9
- Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, et al. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006 Jun;11(2):193–206. doi:10.1037/1082-989X.11.2.193
- Higgins JP, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. Chichester (UK): John Wiley & Sons; 2019.
- Garban F, Attal M, Michallet M, et al. Prospective comparison of autologous stem cell transplantation followed by dose-reduced allograft (IFM99-03 trial) with tandem autologous stem cell transplantation (IFM99-04 trial) in high-risk de novo multiple myeloma. Blood. 2006 May 1;107(9):3474–3480. doi:10.1182/blood-2005-09-3869
- Giralt S, Costa LJ, Maloney D, et al. Tandem autologous-autologous versus autologous-allogeneic hematopoietic stem cell transplant for patients with multiple myeloma: long-term follow-up results from the blood and marrow transplant clinical trials network 0102 trial. Biol Blood Marrow Transplant. 2020 Apr;26(4):798–804. doi:10.1016/j.bbmt.2019.11.018
- Krishnan A, Pasquini MC, Logan B, et al. Autologous haemopoietic stem-cell transplantation followed by allogeneic or autologous haemopoietic stem-cell transplantation in patients with multiple myeloma (BMT CTN 0102): a phase 3 biological assignment trial. Lancet Oncol. 2011 Dec;12(13):1195–1203. doi:10.1016/S1470-2045(11)70243-1
- Moreau P, Garban F, Attal M, et al. Long-term follow-up results of IFM99-03 and IFM99-04 trials comparing nonmyeloablative allotransplantation with autologous transplantation in high-risk de novo multiple myeloma. Blood. 2008 Nov 1;112(9):3914–3915. doi:10.1182/blood-2008-07-168823
- Gagelmann N, Eikema DJ, Koster L, et al. Tandem autologous stem cell transplantation improves outcomes in newly diagnosed multiple myeloma with extramedullary disease and high-risk cytogenetics: a study from the chronic malignancies working party of the European society for blood and marrow transplantation. Biol Blood Marrow Transplant. 2019 Nov;25(11):2134–2142. doi:10.1016/j.bbmt.2019.07.004
- Gagelmann N, Eikema DJ, de Wreede LC, et al. Upfront stem cell transplantation for newly diagnosed multiple myeloma with del(17p) and t(4;14): a study from the CMWP-EBMT. Bone Marrow Transplant. 2021 Jan;56(1):210–217. doi:10.1038/s41409-020-01007-w
- Knop S, Liebisch P, Hebart H, et al. Autologous followed by allogeneic versus tandem-autologous stem cell transplant in newly diagnosed FISH-del13q myeloma. Washington (DC): American Society of Hematology; 2014.
- Blade J, Samson D, Reece D, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998 Sep;102(5):1115–1123.
- Rosinol L, Perez-Simon JA, Sureda A, et al. A prospective PETHEMA study of tandem autologous transplantation versus autograft followed by reduced-intensity conditioning allogeneic transplantation in newly diagnosed multiple myeloma. Blood. 2008 Nov 1;112(9):3591–3593. doi:10.1182/blood-2008-02-141598
- Bruno B, Rotta M, Patriarca F, et al. A comparison of allografting with autografting for newly diagnosed myeloma. N Engl J Med. 2007 Mar 15;356(11):1110–1120. doi:10.1056/NEJMoa065464
- Giaccone L, Storer B, Patriarca F, et al. Long-term follow-up of a comparison of nonmyeloablative allografting with autografting for newly diagnosed myeloma. Blood. 2011 Jun 16;117(24):6721–6727. doi:10.1182/blood-2011-03-339945
- Armeson KE, Hill EG, Costa LJ. Tandem autologous vs autologous plus reduced intensity allogeneic transplantation in the upfront management of multiple myeloma: meta-analysis of trials with biological assignment. Bone Marrow Transplant. 2013 Apr;48(4):562–567. doi:10.1038/bmt.2012.173
- Kharfan-Dabaja MA, Hamadani M, Reljic T, et al. Comparative efficacy of tandem autologous versus autologous followed by allogeneic hematopoietic cell transplantation in patients with newly diagnosed multiple myeloma: a systematic review and meta-analysis of randomized controlled trials. J Hematol Oncol. 2013 Jan 4;6:2. doi:10.1186/1756-8722-6-2
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007 Jun 7;8:16. doi:10.1186/1745-6215-8-16
- Kumar SK, Rajkumar SV. The multiple myelomas – current concepts in cytogenetic classification and therapy. Nat Rev Clin Oncol. 2018 Jul;15(7):409–421. doi:10.1038/s41571-018-0018-y
- Lokhorst HM, Schattenberg A, Cornelissen JJ, et al. Donor lymphocyte infusions for relapsed multiple myeloma after allogeneic stem-cell transplantation: predictive factors for response and long-term outcome. J Clin Oncol. 2000 Aug;18(16):3031–3037. doi:10.1200/JCO.2000.18.16.3031
- Mateos MV, Martínez BP, González-Calle V. High-risk multiple myeloma: how to treat at diagnosis and relapse? Hematology Am Soc Hematol Educ Program. 2021 Dec 10;2021(1):30–36. doi:10.1182/hematology.2021000229