ABSTRACT
Objectives
This study investigated the impact of early cyclosporin A (CsA) initiation (day −5) on the risk of acute graft versus host disease (aGvHD) after allogeneic haematopoietic cell transplantation (allo-HSCT).
Methods
Sixty-seven leukaemia patients who underwent allo-HSCT were investigated. The correlation between the CsA level in the first four weeks and the following indices was examined: GvHD, cumulative incidence (CI) of GvHD, CI of relapse at month 18, and non-relapse mortality (NRM) at month 18.
Results
A significant association between aGvHD and CsA level in the fourth week after allo-HSCT was observed, with the incidence of aGvHD in the fourth week in the lower level group being higher than that in the higher level group (p = 0.044). The CI of aGvHD was 30.1% and 9.8% at day +90 and 42.3% and 17.1% at day +180 in the lower level and higher level groups, respectively.
Conclusion
For Chinese patients, early introduction and reaching the target CsA concentration within four weeks after allo-HSCT have a positive effect on preventing GvHD, especially in the fourth week after HSCT. Compared to the Western population, the target CsA concentration is lower and the time required to reach the target (within 4 weeks) is longer in the Chinese population (274.75 ng/mL).
KEYWORDS:
Introduction
Haematopoietic stem cell transplantation (HSCT) is the most curable therapy for a variety of haematological disorders, including haematologic malignancies. It reduces relapse rates when performed in the first complete remission and is the ideal option for recurrent disease. Except for those who are older or cannot undergo transplantation for various reasons, patients in remission should receive the transplant as soon as possible after a period of chemotherapy. However, there an increased risk of donor-cell-mediated graft versus host disease (GvHD) and infection due to post-transplantation immunosuppression [Citation1–3]. Indeed, GvHD is one of the main life-threatening complications after HSCT, and it remains the major cause of morbidity and non-relapse mortality (NRM) after allogeneic haematopoietic cell transplantation.
Under normal circumstances, strategies to prevent GvHD mainly involve cyclosporin A (CsA), together with an immunosuppressant, such as low-dose methotrexate (MTX) or mycophenolic acid (MPA), or anti-thymocyte globulin (ATG) polyclonal antibodies from serum of animals immunized with human thymocytes/lymphocytes. CsA is the basis of GvHD prophylaxis. It has been confirmed that the CsA concentration is related to GvHD after HSCT [Citation4,Citation5]. Achieving a target CsA level early after HSCT is critical to lowering the risk of allogeneic haematopoietic cell transplantation (aGvHD) [Citation6–8]. However, the mechanism by which an early high CsA concentration is beneficial against aGvHD remains to be determined.
To date, studies regarding the influence of the early CsA concentration on GvHD in Chinese leukaemia patients after allogeneic haematopoietic cell transplantation (allo-HSCT) are limited. Accordingly, in this study, we analysed 67 patients who underwent allogeneic haematopoietic cell transplantation to assess the impact of CsA levels on the occurrence of GvHD.
Patients and methods
Patients, anti-GvHD regimen and assessment of GvHD
Patients diagnosed with haematological malignancies in Jiangsu Province Hospital from January 1st, 2020, to April 30th, 2022, were included in this retrospective study. The study was approved by the Ethics Committee of Jiangsu Province Hospital. Informed consent was exempted because of the retrospective design of the study. Our research followed the guidelines outlined in the Helsinki Declaration. Eligibility criteria included (1) diagnosis of haematological malignancy;. (2) undergoing elective allo-HSCT; and (3) age over 16 years. All patients received allogeneic HSCT, including HLA-haploidentical HSCT, HLA-identical sibling HSCT, and unrelated donor HSCT. The conditioning regimen was as follows: BUCY: Busulfan: 3 mg/kg, −7d∼−4d. Cyclophosphamide: 60 mg/kg, −3d∼−2d. FBU: Fludarabine: 0.7 mg/kg, −7d∼−2d, Busulfan: 3 mg/kg, −7d∼−4d. FBU + Decitabine: Fludarabine: 0.7 mg/kg, −7d∼−2d, Busulfan: 3 mg/kg, −7d∼−4d, Decitabine: 0.6 mg/kg, −12d∼−8d. According to the Chinese Expert Consensus on Allogeneic Hematopoietic Stem Cell Transplantation for Hematological Diseases(Ⅲ) – Acute Graft Versus Host Disease (2020 version) [Citation9], preventive regimens were implemented in all patients for GvHD prophylaxis after allogeneic HSCT, including CsA and MPA. The physician decided whether to use MTX and ATG according to the patient’s condition. CsA was administered at 2 mg/kg through intravenous infusion once every 12 h starting from day −5; oral dosing began when the patient tolerated it. CsA doses were adjusted to prevent renal dysfunction. In general, CsA was gradually decreased at 3 months after transplantation and was withdrawn between 6 and 9 months. The application time of CsA should be shortened or extended according to the risk of GvHD and infection. MPA was administered at a fixed dosage of 1 g per day, twice daily, taken orally, starting from day −5, without adjustment. MPA withdrawal between 2 and 3 months after transplantation. MTX was given as follows: day +1 15 mg/m2, day +3, day +6 10 mg/m2, intravenous infusion, rescue with calcium formyl tetrahydrofolate 24 h after the end of each administration. MTX was not implemented for days +11 in patients with severe oral mucositis. The recommended dose of ATG is 7.5∼10 mg/kg, which is infused in batches for −5 ∼ −2 days. Infection prevention and supportive care were the same and implemented regularly for all patients during the whole treatment period. The primary endpoint of the study was to determine the impact of serum CsA concentration on the risk of GvHD.
Acute and chronic GvHD was diagnosed and graded according to the following guidelines and consensus: the revised Glucksberg criteria [Citation10] and the Seattle standard criteria [Citation11]. The judgement of acute and chronic GvHD was confirmed by specialists.
CsA concentration monitoring
During medication, the CsA through concentration were closely monitored and blood samples were collected before medicated in the morning. The mean CsA concentration for each patient was calculated using the different concentrations. Mean CsA blood trough concentrations were calculated per week during the first month and once per month during medication. CsA trough concentrations were assessed by radioimmunoassay. CsA doses were adjusted to achieve the purpose of GvHD prevention and to avoid adverse reactions.
Statistical analysis
All statistical analyses were performed using R (The R Foundation for Statistical Computing; version 3.5.0) and SPSS Statistics (version 22.0). The cut-off value of the CsA trough concentration was assessed by ROC curves at weeks 1, 2, 3 and 4 after allo-HSCT. Comparisons between patients without GvHD and patients with grade I-IV GvHD were calculated using the t test, Mann–Whitney U test, or chi-squared test depending on the type of variable. The cumulative incidence (CI) of GvHD, relapse, and NRM was estimated, and groups were compared using Grey’s test.
Results
Patient characteristics
A total of 67 patients were included in this study. The median age was 43 years (range, 16–57). Diagnoses were myeloid malignancies (37, 55.2%), lymphoid malignancies (25, 37.3%), and other types of leukaemia (5, 7.5%, including mixed-lineage leukaemia and NK-cell leukaemia). Forty-eight (71.6%) patients received a regimen based on busulfan and cyclophosphamide, 37 (58.2%) patients received ATG, and 40 (59.7%) patients received MTX. The median follow-up was 13 months (range, 2–27). Patient characteristics according to the occurrence of GvHD are summarized in .
Table 1. Patients characteristics.
CsA concentration and GvHD
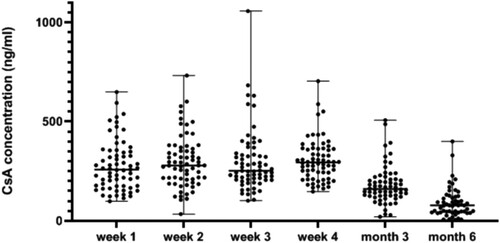
Median CsA concentrations at 1, 2, 3, and 4 weeks and the third and sixth months after allo-HSCT were 258.6 ng/mL (range, 99.4–649.4), 279.5 ng/mL (range, 34.3–731.9), 251.9 ng/mL (range, 102.1–1056.2), 294.4 ng/mL (range, 147.5–703.8), 160.9 (range, 20.6–507.4) and 78.2 (range, 5.4–400.1), respectively (). ROC curve analysis was used to investigate the cut-off value of the CsA level for GvHD. The results indicated the CsA level after allo-HSCT to be a discriminator of the risk of grade I-IV aGvHD. The cut-off values were 280.35, 290.3, 257.95 and 274.75 ng/ml at 1, 2, 3, and 4 weeks after allo-HSCT, respectively. There was a significant association between aGvHD and CsA concentration in the fourth week after allo-HSCT, and the incidence of aGvHD in the fourth week after allo-HSCT in the lower level group was higher than that in the higher level group (p = 0.044). After grouping according to cut-off values, there was a significant difference (p = 0.044) in the incidence of aGvHD between groups only in the fourth week; therefore, 274.75 was determined as the predictive value.
At day +90, the cumulative incidences of aGvHD were 30.1% and 9.8%. At day +180, the cumulative incidences of aGvHD were 42.3% and 17.1%, respectively (p = 0.025, a). After excluding relapse and non-relapse deaths, a competitive risk model was established to estimate cumulative risks of aGvHD in the two groups (< 274.75 vs. ≥274.75). Statistical results showed a significant difference in the CI of aGvHD between the two groups (p = 0.025). However, there was no correlation between CsA level and risk of aGvHD in other periods. After univariate analysis () and multivariate logistic regression (), we found that the CsA level (≥274.75 ng/mL) in the fourth week was the only independent influencing factor correlating with the risk of I-IV aGvHD after allo-HSCT (RR, 95% CI: 0.281 (0.088–0.895), p = 0.032). For cGvHD, we did not observe an effect of CsA level on risk of cGvHD.
Figure 2. (a) Cumulative incidence of aGvHD according to CsA concentration during the fourth week after Allo-HSCT. (b) CI of cGvHD according to CsA concentration during the fourth week after Allo-HSCT. (c) CI of relapse according to CsA concentration during the fourth week after Allo-HSCT. (d) CI of NRM according to CsA concentration during the fourth week after Allo-HSCT.
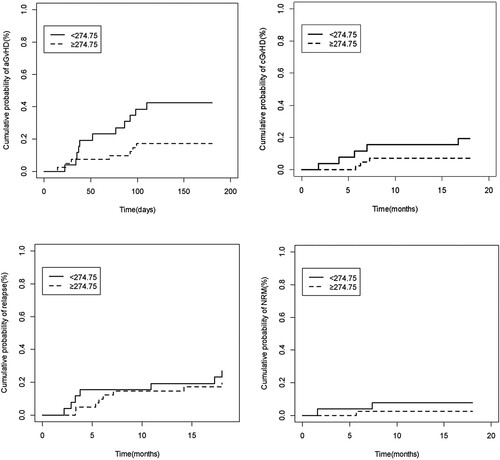
Table 2. Multivariate analysis of aGvHD risk factors.
Clinical outcomes
Clinical outcomes according to CsA concentration in the fourth week after allo-HSCT are shown in . After allo-HSCT, two graft failures occurred in the higher level group. The median time for neutrophil recovery was 11 days (range, 9–28). Finally, there was no statistically significant correlation between CsA level and clinical outcomes, including relapse and NRM. The CIs of cGvHD, relapse and NRM are shown in b-2d.
Table 3. Clinical outcomes after allo-HSCT.
Discussion
The relationship between CsA concentration and risk of GvHD in patients with haematopathy who received HSCT was demonstrated in recent years. Researchers found that higher CsA concentrations early post-HSCT effectively reduce risk of GVHD [Citation12]. As that study progressed, the results supported that early initiation of CsA before haplo-HCT reduces risk of aGvHD [Citation13]. Rogosheske J R et al. [Citation12] found that CsA levels were lower in the week before aGVHD development, with every 50 ng/mL increase in the average CsA concentration leading to a 33% reduction in grade II-IV aGVHD. In our study, we investigated the impact of the serum trough CsA concentration on the incidence of GvHD in patients undergoing allo-HSCT with PBSC and combined use of CsA and MPA as GvHD drug prophylaxis. Our research on Chinese patients with haematological malignancies undergoing HSCT confirmed the previous result. In addition, we found a significant association between aGvHD and CsA concentration in the fourth week after allo-HSCT (p = 0.044). The cumulative incidence of aGvHD on day 90 and day 180 was influenced by the CsA level in the fourth week after allo-HSCT (p = 0.025).
Our findings are similar to some previous studies. According to Stocker N et al. [Citation13], patients with lower CsA levels in the first week after haplo-HSCT had a significantly higher risk of grade II-IV aGvHD. In addition, García Cadenas I et al. [Citation14] found that CsA concentration correlates significantly with grade II-IV aGVHD during the third week after transplantation. With either post-transplant high-dose cyclophosphamide (PTCy) or G-CSF mobilization, intensive immunosuppression in the early stage, ATG use, or combined transplantation of bone marrow and peripheral blood stem cells (GIAC), researchers have suggested that a high CsA concentration after HSCT is associated with a reduced incidence of aGvHD [Citation15]. In contrast to previous results [Citation13], the target CsA concentration of the Chinese population in our study (274.75 ng/mL) was lower than that of the Western population (301 ng/mL), and the time required to reach the target (within 4 weeks) was longer than that of Western people (in the first week). This is a novel finding for Chinese patients. According to Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation [Citation16], higher CsA concentrations in the first weeks post-transplant are associated with a lower frequency of aGVHD, and the target concentration in the first weeks post-transplant should be 200–300 µg/L to efficiently prevent aGVHD. Due to ethnic differences between the East and West, the target CsA level and the time to reach the target for Chinese patients may be different. Our study indicates that the target CsA level does not need to reach 300 µg/L but that the target should be achieved within four weeks after HSCT for Chinese patients. The time required to reach the target was longer than that of the Western population. Thus, our study helps to establish a clear and definite target concentration and time to reach the standard for Chinese patients. However, the starting time of CsA has not yet been unified. The European Society for Blood and Marrow Transplantation stated that CsA is historically started 1 d before transplantation. In addition, retrospective studies show that an earlier start may reduce aGvHD [Citation6,Citation17]. Therefore, more studies are needed to confirm the start time suitable for Chinese patients. The difference in CsA levels between races may be due to ethnic differences in CsA metabolism. CsA is mainly metabolized by liver enzymes such as CYP3A4 and CYP3A5 and is transported and cleared by the multidrug resistance-related gene ABCB1 product. Variability in expression of the above genes between the Chinese and Western populations may be the main reason for the difference in CsA target level [Citation18–20]. For instance, studies have indicated that the ABCB1 C3435 T CC genotype in Asian individuals has a lower C0/D of CsA compared with TT genotype carriers [Citation21,Citation22]. However, this phenomenon was not observed in the Caucasian population. This may explain why the target level of the Chinese population is lower than that of the Western population. At present, the influence of these genes on CsA level has not been fully elucidated, with no clear conclusion, and large-scale studies are still needed.
For cGvHD, the results of our study are the same as those of Rogosheske J R et al. [Citation12], and we did not observe an effect of CsA level the risk of cGVHD or the CI of cGvHD. This is different from the results from Nicolas Stocker et al. [Citation13] and Ruggeri et al. [Citation23], who showed that the CsA concentration after HSCT correlates with the CI of cGvHD and extensive cGvHD. In univariate analysis, we did not find any association between CsA level and the CI of relapse, and the same result for non-relapse mortality was observed. Due to the influence of follow-up time, we did not carry out survival analysis, which will be performed in a follow-up study.
There are some advantages and limitations of the study. This study was completed with a unified transplant team for selection, evaluation and treatment, and analysis of CsA target for Chinese leukaemia is quite limited. However, the scale of this study was small. The present study did not evaluate the correlation between CsA level and II-IV and III-IV aGvHD, though we will continue to explore this in follow-up research. The present study indicates that for Chinese patients, the target level should be achieved within four weeks after HSCT. The time required to reach the target (within 4 weeks) was longer than that of Western people (in the first week). Delayed or inadequate exposure to CsA can be a critical risk factor for developing aGvHD, and reaching the target rapidly may lead to infection caused by immune deficiency as well as ADRs caused by CsA. The target CsA concentration in the Chinese population (274.75 ng/mL) was lower than that in the Western population (301 ng/mL). Our results clarify and define the target period for Chinese patients who receive HSCT. In general, the standard should be reached slowly within four weeks after transplantation in Chinese patients.
Acknowledgements
The authors acknowledge the Department of Hematology of Jiangsu Province Hospital.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Martino R, Valcarcel D, Brunet S, et al. Comparable non-relapse mortality and survival after HLA-identical sibling blood stem cell transplantation with reduced or conventional-intensity preparative regimens for high-risk myelodysplasia or acute myeloid leukemia in first remission[J]. Bone Marrow Transplant. 2008;41(1):33–38. doi:10.1038/sj.bmt.1705879
- Valcárcel D, Martino R, Caballero D, et al. Sustained remissions of high-risk acute myeloid leukemia and myelodysplastic syndrome after reduced-intensity conditioning allogeneic hematopoietic transplantation: chronic graft-versus-host disease is the strongest factor improving survival[J]. J Clin Oncol. 2008;26(4):577–584. doi:10.1200/JCO.2007.11.1641
- Melve GK, Ersvaer E, Kittang AO, et al. The chemokine system in allogeneic stem-cell transplantation: a possible therapeutic target?[J]. Expert Rev Hematol. 2011;4(5):563–576. doi:10.1586/ehm.11.54
- Kanda Y, Hyo R, Yamashita T, et al. Effect of blood CsA concentration on the outcome of hematopoietic stem cell transplantation from an HLA-matched sibling donor[J]. Am J Hematol. 2006;81(11):838–844. doi:10.1002/ajh.20710
- Oshima K, Kanda Y, Nakasone H, et al. Decreased incidence of acute graft-versus-host disease by continuous infusion of cyclosporine with a higher target blood level[J]. Am J Hematol. 2008;83(3):226–232. doi:10.1002/ajh.21087
- Malard F, Szydlo RM, Brissot E, et al. Impact of cyclosporine-A concentration on the incidence of severe acute graft-versus-host disease after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16(1):28–34. doi:10.1016/j.bbmt.2009.08.010
- Martin P, Bleyzac N, Souillet G, et al. Clinical and pharmacological risk factors for acute graftversus-host disease after paediatric bone marrow transplantation from matched-sibling or unrelated donors. Bone Marrow Transplant. 2003;32(9):881–887. doi:10.1038/sj.bmt.1704239
- Martin P, Bleyzac N, Souillet G, et al. Relationship between CsA trough blood concentration and severity of acute graft-versus-host disease after paediatric stem cell transplantation from matched-sibling or unrelated donors. Bone Marrow Transplant. 2003;32(8):777–784. doi:10.1038/sj.bmt.1704213
- Huang X, Wu D. Chinese expert consensus on allogeneic hematopoietic stem cell transplantation for hematological diseases(Ⅲ) – Acute graft versus host disease (2020 version)[J]. Chin J Hematol. 2020;41(7):529–536.
- Przepiorka D, Weisdorf D, Martin P, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15(6):825–828.
- Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204–217. doi:10.1016/0002-9343(80)90380-0
- Rogosheske JR, Fargen AD, DeFor TE, et al. Higher therapeutic CsA levels early post transplantation reduce risk of acute GVHD and improves survival[J]. Bone Marrow Transplant. 2014;49(1):122–125. doi:10.1038/bmt.2013.139
- Stocker N, Duléry R, Battipaglia G, et al. Impact of cyclosporine A concentration on acute graft-vs-host disease incidence after haploidentical hematopoietic cell transplantation[J]. Eur J Haematol. 2019;103(1):10–17. doi:10.1111/ejh.13233
- García Cadenas I, Valcarcel D, Martino R, et al. Impact of cyclosporine levels on the development of acute graft versus host disease after reduced intensity conditioning allogeneic stem cell transplantation[J]. Mediat Inflamm. 2014;2014:1–7.
- Yang X, Yang S, Sun A, et al. Impact of cyclosporine-A concentration in T-cell replete haploidentical allogeneic stem cell transplantation. Clin Transplant. 2018;32(4):e13220. doi:10.1111/ctr.13220
- Penack O, Marchetti M, Ruutu T, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European society for blood and marrow transplantation[J]. The Lancet Haematology. 2020;7(2):e157–e167. doi:10.1016/S2352-3026(19)30256-X
- Kedmi M, Dray L, Grisariu S, et al. The effect of cyclosporine initiation time on the outcome of matched allogeneic stem-cell transplantation following fludarabine-based conditioning[J]. Transpl Int. 2012;25(12):1241–1247. doi:10.1111/j.1432-2277.2012.01559.x
- Tang HL, Ma LL, Xie HG, et al. Effects of the CYP3A5*3 variant on cyclosporine exposure and acute rejection rate in renal transplant patients: a meta-analysis[J]. Pharmacogenet Genomics. 2010;20(9):525–531. doi:10.1097/FPC.0b013e32833ccd56
- Lee JS, Cheong HS, Kim LH, et al. Screening of genetic polymorphisms of CYP3A4 and CYP3A5 genes[J]. The Korean J Physiol Pharmacol: Official J Korean Physiol Soc Korean Soc Pharmacol. 2013;17(6):479. doi:10.4196/kjpp.2013.17.6.479
- Li DY, Teng RC, Zhu HJ, et al. CYP3A4/5 polymorphisms affect the blood level of cyclosporine and tacrolimus in Chinese renal transplant recipients [J]. Int J Clin Pharmacol Ther. 2013;51(6):466–474. doi:10.5414/CP201836
- Li Y, Hu X, Cai B, et al. Meta-analysis of the effect of MDR1 C3435 polymorphism on tacrolimus pharmacokinetics in renal transplant recipients. Transpl Immunol. 2012;27:12–18. doi:10.1016/j.trim.2012.03.006
- Lee J, Wang R, Yang Y, et al. The effect of ABCB 1 C 3435 T polymorphism on cyclosporine dose requirements in kidney transplant recipients: a meta-analysis[J]. Basic Clin Pharmacol Toxicol. 2015;117(2):117–125. doi:10.1111/bcpt.12371
- Ruggeri A, Labopin M, Bacigalupo A, et al. Bone marrow versus mobilized peripheral blood stem cells in haploidentical transplants using posttransplantation cyclophosphamide[J]. Cancer. 2018;124(7):1428–1437. doi:10.1002/cncr.31228