ABSTRACT
Background:
Venous thromboembolism (VTE) can occur in children with COVID-19, and the efficacy and safety of prophylactic anticoagulant therapy are uncertain. This study aimed to assess the incidence of VTE in pediatric patients with COVID-19, the association of D-dimer with thrombus formation, and the effectiveness and safety of prophylactic anticoagulation treatment.
Methods:
We systematically searched databases from January 2020 to February 2023. A systematic review and meta-analysis were conducted to determine the incidence of VTE in children and evaluate the efficacy and safety of prophylactic anticoagulant therapy.
Results:
Thirteen cohort studies and one clinical trial were included. The pooled incidence rate of VTE in affected children was 1.5% (95% CI 0.4–2.9%). Children with D-dimer levels five times higher than normal had a higher risk of VTE (OR 4.92, 95% CI 1.60–15.11). Prophylactic anticoagulant therapy did not significantly reduce the risk of VTE (OR 1.35, 95% CI 0.74–2.49). The safety of prophylactic anticoagulant therapy was relatively high, with major bleeding and all-cause mortality rates below 0.1% (95% CI 0.0–0.2%).
Conclusions:
The incidence of VTE in children with COVID-19 is low, and prophylaxis based on ISTH standards is reasonable. Low-molecular-weight heparin (LMWH) for VTE prevention has a high level of safety. However, more high-quality studies are needed to understand the impact of anticoagulant therapy on VTE incidence in pediatric patients with COVID-19.
Introduction
Coronavirus Disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). COVID-19 can induce a prothrombotic state characterized by elevated levels of D-dimer and fibrin degradation products, prolonged prothrombin time (PT), activated partial thromboplastin time (aPTT), and decreased platelet count. In adults, this hypercoagulable state can lead to progressive pulmonary and renal disease, pulmonary embolism (PE), venous thromboembolism (VTE), recurrent catheter obstruction, and stroke [Citation1,Citation2].
On January 20, 2020, Shenzhen reported the first case of a child infected with COVID-19 [Citation3]. Children with COVID-19 usually experience milder symptoms than adults, and most children are asymptomatic and do not require any special treatment. Most children with mild or moderate coronavirus disease do not develop severe or critical illnesses. However, a small proportion of children with COVID-19 may require hospitalization and intensive care unit (ICU) treatment. In critically ill pediatric patients, a characteristic manifestation is dysregulated inflammatory response accompanied by pronounced activation of the coagulation system, including elevated plasma D-dimer levels, which are risk factors for thrombus formation [Citation4,Citation5]. In addition, some children can develop Multisystem Inflammatory Syndrome in Children (MIS-C), a severe complication of COVID-19 that can affect multiple organs and require intensive care. MIS-C is associated with a higher risk of venous thromboembolism due to the intense inflammation and hypercoagulable state [Citation6]. To date, there are no authoritative guidelines based on randomized controlled trial analysis at the international level, and only a hypothetical consensus has been published. Recently, the International Society on Thrombosis and Hemostasis (ISTH) released ‘consensus-based clinical recommendations and research priorities for anticoagulant thromboprophylaxis in children hospitalized for COVID-19-related illnesses.’ [Citation7] The recommendations mainly rely on D-dimer levels and prethrombotic risk factors to determine the risk of thrombus formation in pediatric patients. According to the ISTH guidelines, prophylactic anticoagulation therapy is recommended for children hospitalized with COVID-19-related illnesses (including MIS-C) if their D-dimer level is five times more than the normal value. For patients with a D-dimer level less than five times the normal value, prophylactic anticoagulation treatment is also recommended if they have one or more venous thromboembolism risks factors such as obesity (BMI > 95th percentile), a central venous catheter, or mechanical ventilation. The recommended prophylactic anticoagulant medication and administration method is low-dose, low-molecular-weight heparin administered subcutaneously twice daily, aiming for an anti-Xa activity level of 0.2–<0.5 U/mL 4 h post-administration [Citation7]. The formation of ISTH recommendations is primarily based on expert consensus rather than multicenter cohort studies, registries, or retrospective research that elucidates the risk of venous thromboembolism (VTE) and identifies VTE risk factors in children with COVID-19-related diseases (including MIS-C), particularly in hospitalized children. These recommendations do not address the effectiveness or safety issues associated with prophylactic anticoagulation treatment. Some researchers believe that D-dimer testing in children with COVID-19 is affected by confounding factors. Although D-dimers are widely considered biochemical markers in VTE diagnosis, they lack specificity as they tend to increase under all inflammatory conditions [Citation8]. Therefore, these scholars argue that clinical thromboprophylaxis should not strictly adhere to the ISTH recommendations.
This study conducted a meta-analysis of the existing literature to assess the incidence of venous thromboembolism in pediatric patients with COVID-19. Additionally, it aimed to investigate the association between D-dimer levels and the risk of thrombus formation, as well as to evaluate the effectiveness and safety of prophylactic anticoagulation treatment. The study was motivated by the current lack of robust clinical evidence in this area.
Methods
To guarantee the superior quality of this study, we adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement, an updated guideline for documenting systematic reviews [Citation9]. Two investigators possessing varying levels of expertise (D.X. and J.Y.W.) independently completed all steps. In instances of disagreement, the reviewers held discussions or sought the opinion of a third reviewer (W.X.Q.) for resolution. The review protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO; registration number CRD42023414338) [Citation10].
Search strategy
We searched the PubMed, EMBASE, and Cochrane Library databases to identify all relevant articles published between January 2020 and February 2023. Additionally, we searched the World Health Organization (WHO) database and medRxiv.org for potentially related publications, including unpublished articles that have been accepted. The specific search strategy can be found in the Supplementary Materials. Furthermore, the reference lists of pertinent articles were manually examined.
Study selection
The inclusion criteria were as follows: (1) case–control, cohort studies, or clinical trials; (2) a control group with participants not receiving anticoagulants; (3) studies that reported the number of thrombotic cases, bleeding events, and deaths; (4) research involving hospitalized pediatric patients with COVID-19; (5) sample size greater than 10; (6) studies with sufficient data for risk assessment; and (7) studies written in English.
The exclusion criteria were as follows: (1) studies without extractable data and (2) non-original research (letters, comments, and editorials).
Outcomes
The primary outcome assessed the cumulative incidence of venous thromboembolism during hospitalization, with the incidence rate serving as the exploratory efficacy endpoint. Secondary outcomes concentrated on safety, specifically clinically related bleeding within 30 days post-enrollment, as defined by the ISTH (i.e. major bleeding plus clinically relevant non-major bleeding) [Citation11]. Major bleeding encompassed (1) fatal bleeding, (2) clinically overt bleeding correlated with a decrease in hemoglobin (Hgb) of at least 2 g/dL within 24 h, (3) retroperitoneal, pulmonary, intracranial, or other central nervous system bleeding, and (4) bleeding requiring surgical intervention in the operating room. Clinically relevant non-major bleeding involved (1) overt bleeding necessitating blood product usage, not directly attributed to the patient's underlying condition, and (2) bleeding requiring pharmacological or surgical intervention for hemostasis outside the operating room.
Data extraction and quality assessment
Using a predesigned form, two authors (D.L. and Y. Z.) independently performed data extraction. Any discrepancies were resolved by a senior researcher (L.H.M). Demographic and outcome data were also extracted. The demographic data of the included studies comprised study design, sample size, mean age, gender, number of thrombotic cases, bleeding event count, and reported mortality rate. We assessed the quality of the included studies and their potential risk of bias using the risk of bias in non-randomized studies of interventions (ROBINS-I) tool [Citation12].
Statistical analysis
The R programming software (meta-package, version 4.2.3; R Core Team, Vienna, Austria) [Citation13] was used for data analysis. Binary variables were compared using odds ratios (OR) with 95% confidence intervals (CI). For all meta-analyses, Cochrane Q p-values and I2 statistics were used to assess the heterogeneity. A random-effects model was employed to combine results when significant heterogeneity was present, as indicated by a p-value <0.05 or I2 > 50%. Otherwise, a fixed effects model was used [Citation14,Citation15]. P-values less than 0.05 were considered statistically significant.
Results
Search results
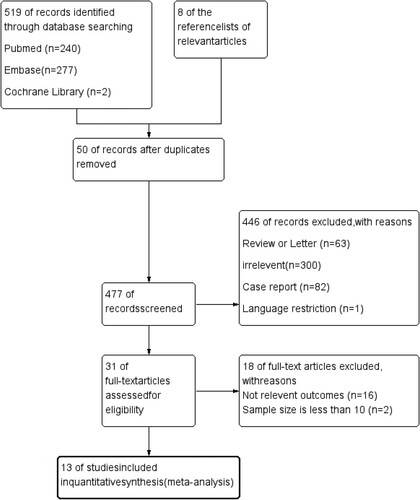
From the database and manual search of the reference lists of relevant articles, 527 studies on venous thromboembolism formation and prevention were obtained. Among these, 50 duplicate studies were excluded. After screening by title and abstract, 477 studies were excluded, and an additional 18 studies were excluded during full-text screening (). Finally, our meta-analysis included 13 studies, consisting of 1 clinical trial [Citation16], 11 retrospective cohort studies [Citation8,Citation17–26], and 1 prospective cohort study [Citation27]. presents the characteristics of the included studies. Among these, 8 studies were conducted in the United States, 1 in Canada, 2 in Europe, 1 in Iran, and 1 in Egypt. The number of pediatric patients with COVID-19 ranged from 14 to 4,063, the average age of the participants ranged from 0.95 to 15.4 years old, and the proportion of males ranged from 36% to 73%.
Table 1. Characteristics of the included studies.
Quality assessment
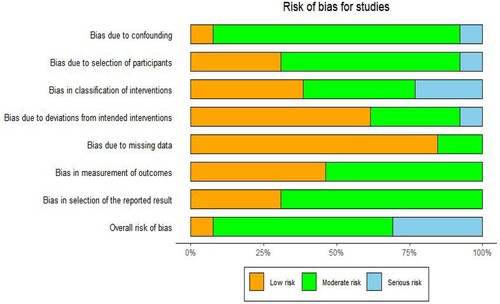
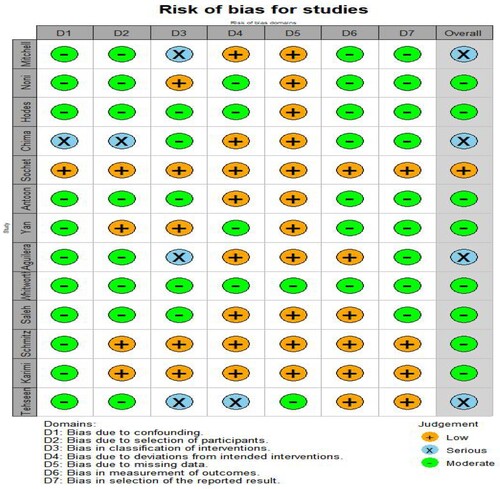
Based on the ROBINS-I tool assessment, we identified 1 study with a low bias risk [Citation16], 8 studies with a moderate bias risk [Citation8,Citation17,Citation18,Citation20,Citation22–25,Citation27], and 4 studies with a serious bias risk [Citation17,Citation19,Citation21,Citation26] ( and ).
Venous thromboembolism incidence
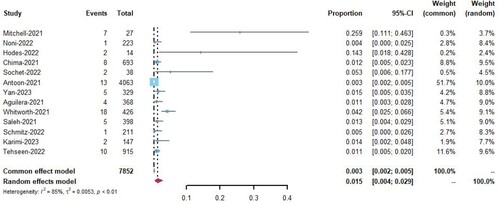
Thirteen studies [Citation8,Citation16–27] reported the incidence of venous thromboembolism in pediatric patients with COVID-19, with rates ranging from 1% to 25%. Among the 7,852 hospitalized patients, 87 developed venous thromboembolism (VTE). Meta-analysis was performed using a random-effects model and applied the Freeman-Tukey double-arcsine transformation [Citation28]. The analysis showed that the overall incidence of VTE among all hospitalized pediatric patients was 1.5% (95% CI 0.4–2.9%, I2 = 85%; ).
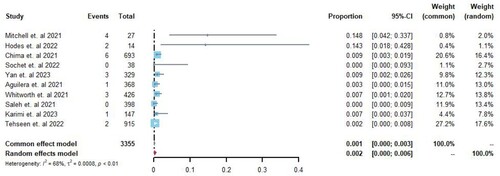
Due to the higher incidence of pulmonary embolism (PE) in COVID-19 cases, the rates of PE and deep vein thrombosis (DVT) were reported separately. Ten studies [Citation8,Citation16–19,Citation21–23,Citation25,Citation26] reported the occurrence of PE in pediatric patients with COVID-19, with rates ranging from 0% to 14.8%. Among 3355 hospitalized patients, 22 cases of PE were identified. A random-effects model was used for meta-analysis, and the Freeman-Tukey double arcsine transformation was applied. The analysis revealed an overall incidence rate of PE of 0.2% (95% CI 0–0.6%, I2 = 68%; ) among all hospitalized pediatric patients.
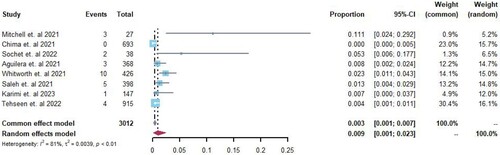
Eight studies [Citation16,Citation17,Citation19,Citation21–23,Citation25,Citation26] reported the incidence rate of deep vein thrombosis (DVT) in pediatric patients with COVID-19, with rates ranging from 0% to 11.1%. Among 3012 hospitalized patients, 28 cases of DVT were identified. A random-effects model was used for meta-analysis, and the Freeman-Tukey double arcsine transformation was applied. The analysis revealed an overall incidence rate of DVT of 0.9% (95% CI 0.01–0.23%, I2 = 81%; ) among all hospitalized pediatric patients.
Impact of D-dimer levels higher than five times the normal value on venous thromboembolism
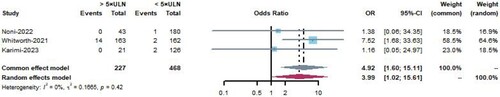
Three studies [Citation22,Citation25,Citation27] showed the incidence of venous thromboembolism in hospitalized pediatric patients with COVID-19 with D-dimer levels five times higher than the normal value and those with D-dimer levels five times lower than the normal value. A total of 685 patients were included, of whom 227 had D-dimer levels five times higher than the normal value, with 14 cases of venous thromboembolism, and 468 patients had D-dimer levels five times lower than the normal value, with 5 cases of venous thromboembolism. The meta-analysis was conducted using a fixed-effects model, and the results indicated that D-dimer levels five times higher than the normal value are a risk factor for venous thromboembolism formation (OR = 4.92, 95%CI 1.60–15.11, I2 = 0, P = 0.42, ).
Effectiveness of prophylactic anticoagulation therapy
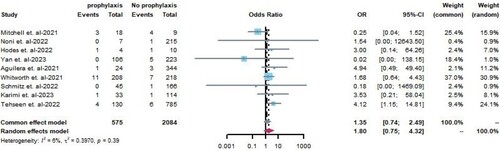
This analysis included nine studies [Citation8,Citation17,Citation18,Citation21,Citation22,Citation24–27] comprising 575 pediatric patients with COVID-19 who received prophylactic anticoagulation therapy and 2,084 patients who did not receive prophylactic anticoagulation therapy. Meta-analysis was conducted using a fixed-effects model. Overall, there was no statistically significant difference in the risk of thrombus formation between patients with COVID-19 exposed and unexposed to prophylactic anticoagulants (OR = 1.35, 95%CI 0.74–2.49, P = 0.39, I2 = 6%; ). Prophylactic anticoagulation treatments included prophylactic-intensity low molecular weight heparin (LMWH) subcutaneous injections, therapeutic-intensity LMWH subcutaneous injections, unfractionated heparin (UFH), and oral anticoagulant medications. Most patients used prophylactic-intensity LMWH subcutaneous injections of prophylactic-intensity LMWH as prophylactic anticoagulation treatment.
Safety of prophylactic anticoagulation therapy
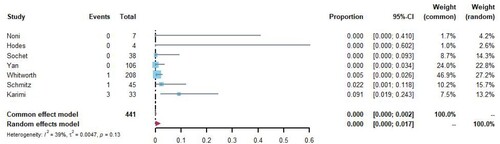
Seven studies [Citation8,Citation16,Citation18,Citation22,Citation24,Citation25,Citation27] reported the number of major bleeding events and all-cause mortality in patients with COVID-19 receiving prophylactic anticoagulation therapy, with 441 patients undergoing anticoagulation treatment and five patients experiencing major bleeding or death. The meta-analysis was conducted using a random-effects model, and Freeman-Tukey double arcsine transformation was applied. The analysis showed that the rate of major bleeding and all-cause mortality for patients receiving prophylactic anticoagulation therapy was <0.1% (95%CI 0.0–0.2%, I2 = 39%; ).
Discussion
To the best of our knowledge, this systematic review and meta-analysis is the first to examine the incidence of venous thromboembolism in pediatric patients with COVID-19 and the effectiveness and safety of anticoagulation therapy. In this study, we consolidate findings on venous thromboembolism incidence rates in children affected by COVID-19 and assess the clinical significance and safety of the ISTH recommendations for venous thromboembolism prevention. It should be noted that these results may primarily pertain to symptomatic Venous Thromboembolism (VTE) as not all the included studies clearly delineated how VTE was diagnosed. Furthermore, the follow-up periods varied across the studies, which could have impacted the results.
Our research found that the incidence of venous thromboembolism in children with COVID-19 was low, with an overall morbidity rate of 1.5%. Children with D-dimer levels five times higher than the normal value had a 4.92 times greater risk of venous thromboembolism compared to those with D-dimer levels less than five times the normal value. Prophylactic anticoagulation therapy demonstrated relatively high safety, as treatment-related major bleeding and all-cause mortality rates were below 0.1%. However, the findings suggested that prophylactic anticoagulation therapy may not be significantly linked to a reduced incidence of venous thromboembolism.
D-dimer levels are generally elevated in children with COVID-19 and tend to be higher in children with MIS-C and severe symptoms [Citation29]. The high levels of D-dimer and fibrin degradation products in severely affected patients may have been caused by inflammation-induced endothelial cell damage in COVID-19 [Citation30]. In a retrospective study, elevated D-dimer levels were found to be very common in children with COVID-19, and there was no significant difference in the incidence of venous thromboembolism between patients with abnormal and normal D-dimer levels. In another study using the criterion of D-dimer greater than 10×upper limit of normal (ULN), the venous thromboembolism incidence rate in children with D-dimer >10×ULN was not significantly different from that in children with D-dimer <10× ULN [Citation17]. This finding may be because although D-dimer is an important biomarker for diagnosing VTE, it lacks specificity, and its expression levels can be affected by various factors such as inflammation. Therefore, we believe that the ISTH recommendation to use D-dimer levels >5× ULN as a criterion for determining whether to use prophylactic anticoagulation therapy is comparatively reasonable.
Unfortunately, our results indicated that prophylactic anticoagulation therapy may not effectively prevent venous thromboembolism associated with COVID-19. Research has shown that some children with COVID-19 still experience thrombotic events after receiving prophylactic doses of anticoagulant drugs, similar to the outcomes of prophylactic anticoagulation therapy in adult patients with COVID-19. These findings highlight the presence of other known or unknown risk factors that may lead to thrombus formation [Citation31]. In addition, there were differences regarding the dosage of anticoagulant drugs. Some researchers have directly used therapeutic doses of anticoagulants to prevent venous thromboembolism in patients with multiple risk factors for thrombus formation [Citation17,Citation24,Citation25]. Therefore, it is necessary to adjust the dose of LMWH based on the clinical condition and hematological or inflammatory biomarker levels of patients.
The evaluation of the benefits of pharmacological prophylaxis for preventing venous thromboembolism must be based on the consideration of potential bleeding complications. The probability of major bleeding and all-cause mortality in children receiving pharmacological prophylaxis was <0.1%, indicating that prophylactic anticoagulation therapy was relatively safe. These results suggest that LMWH is a safe choice for prophylactic anticoagulation therapy in pediatric patients with COVID-19.
Limitation
Our study has two key limitations. First, the included studies primarily analyzed data from early in the COVID-19 pandemic, and the characteristics of the disease have since evolved. Recent evidence suggests that current COVID-19 cases may be less prothrombotic. As a result, our findings should be interpreted cautiously and may not fully apply to present-day patients. Given the changing nature of COVID-19, prophylactic anticoagulation could now be more effective at managing thrombotic risk. Further prospective studies are needed to investigate this.
Second, most studies were retrospective in nature, with only one prospective study and one clinical trial included. The heterogeneous study populations likely contributed to significant variability in results. In our study, there was no difference in venous thromboembolism rates between patients who received prophylactic anticoagulation and those who did not, which may have been due to the diverse range of COVID-19 patients and differences in country of origin, anticoagulant exposure definitions, and study designs across the included studies.
In summary, while our study provides initial evidence on the role of prophylactic anticoagulation, the evolving nature of COVID-19 and limitations in study design suggest findings should be interpreted cautiously. Larger prospective studies are necessary to determine the effectiveness of prophylactic anticoagulation in the current COVID-19 population. Continued research will help ensure recommendations remain up to date with this emerging disease.
Conclusion
Summarily, the results of this systematic review and meta-analysis indicate that the incidence of venous thromboembolism in children with COVID-19 is low. The ISTH criterion of a D-dimer level >5× ULN for venous thromboembolism prevention is reasonable. LMWH therapy is relatively safe for the prevention of venous thrombosis. More high-quality studies are required to understand the risk of venous thromboembolism in hospitalized pediatric patients with COVID-19 and the impact of anticoagulation therapy on the incidence of venous thromboembolism.
Statement of Ethics
The study did not require ethical approval because the meta-analysis was based on published research, and the original data were anonymous.
Supplemental Material
Download ()Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Hematology ASo. COVID-19 and coagulopathy: frequently asked questions 2020. Available from: https://www.hematology.org/covid-19/covid-19-and-coagulopathy.
- Song JC, Wang G, Zhang W, et al. Chinese expert consensus on diagnosis and treatment of coagulation dysfunction in COVID-19. Mil Med Res. 2020;7(1):19. [published Online First: 20200420]. doi:10.1186/s40779-020-00247-7
- Cao Q, Chen YC, Chen CL, et al. SARS-CoV-2 infection in children: transmission dynamics and clinical characteristics. J Formos Med Assoc. 2020;119(3):670–673. [published Online First: 20200302]. doi:10.1016/j.jfma.2020.02.009
- Wang Y, Zhu F, Wang C, et al. Children hospitalized With severe COVID-19 in Wuhan. Pediatr Infect Dis J. 2020;39(7):e91–e94. doi:10.1097/inf.0000000000002739
- Giannis D, Barish MA, Goldin M, et al. Incidence of venous thromboembolism and mortality in patients with initial presentation of COVID-19. J Thromb Thrombolysis. 2021;51(4):897–901. [published Online First: 20210305]. doi:10.1007/s11239-021-02413-7
- Karimi M, Bozorgi H, Zarei T, et al. Antithrombotic prophylaxis in children and adolescents’ patients with SARS-CoV-2 (COVID-19) infection: a practical guidance for clinicians. Acta Biomed. 2020;91(4):e2020170. [published Online First: 20201110]. doi:10.23750/abm.v91i4.10720
- Goldenberg NA, Sochet A, Albisetti M, et al. Consensus-based clinical recommendations and research priorities for anticoagulant thromboprophylaxis in children hospitalized for COVID-19-related illness. J Thromb Haemost. 2020;18(11):3099–3105. doi:10.1111/jth.15073
- Yan A, Parsons C, Caplan G, et al. Improving guideline-concordant thromboprophylaxis prescribing for children admitted to hospital with COVID-19. Pediatr Blood Cancer. 2023;70(2):e30112. [published Online First: 20221210]. doi:10.1002/pbc.30112
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89. [published Online First: 20210329]. doi:10.1186/s13643-021-01626-4
- Research NIfH. International prospective register of systematic reviews (PROSPERO). Available from: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=414338.
- Mitchell LG, Goldenberg NA, Male C, et al. Definition of clinical efficacy and safety outcomes for clinical trials in deep venous thromboembolism and pulmonary embolism in children. J Thromb Haemost. 2011;9(9):1856–1858. doi:10.1111/j.1538-7836.2011.04433.x
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Br Med J. 2016;355:i4919. [published Online First: 20161012]. doi:10.1136/bmj.i4919.
- The R Foundation for Statistical Computing Version 4.2.3. [program], 2023.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi:10.1002/sim.1186
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Br Med J. 2003;327(7414):557–560. doi:10.1136/bmj.327.7414.557.
- Sochet AA, Morrison JM, Jaffray J, et al. Enoxaparin thromboprophylaxis in children hospitalized for COVID-19: A phase 2 trial. Pediatrics. 2022;150(1). doi:10.1542/peds.2022-056726
- Mitchell WB, Davila J, Keenan J, et al. Children and young adults hospitalized for severe COVID-19 exhibit thrombotic coagulopathy. Pediatr Blood Cancer. 2021;68(7):e28975. [published Online First: 20210304]. doi:10.1002/pbc.28975
- Hodes AD, Villasana-Gomez G, Traube L, et al. A comparison of pulmonary embolism in pediatric and adult patients with acute COVID-19. Clin Imaging. 2022;85:10–13. doi:10.1016/j.clinimag.2022.02.015
- Chima M, Williams D, Thomas NJ, et al. COVID-19-Associated pulmonary embolism in pediatric patients. Hosp Pediatr. 2021;11(6):e90–e94. [published Online First: 20210330]. doi:10.1542/hpeds.2021-005866
- Antoon JW, Grijalva CG, Thurm C, et al. Factors associated With COVID-19 disease severity in US children and adolescents. J Hosp Med. 2021;16(10):603–610. doi:10.12788/jhm.3689
- Aguilera-Alonso D, Murias S, Martínez-de-Azagra Garde A, et al. Prevalence of thrombotic complications in children with SARS-CoV-2. Arch Dis Child. 2021;106(11):1129–1132. [published Online First: 20210430]. doi:10.1136/archdischild-2020-321351
- Whitworth H, Sartain SE, Kumar R, et al. Rate of thrombosis in children and adolescents hospitalized with COVID-19 or MIS-C. Blood. 2021;138(2):190–198. doi:10.1182/blood.2020010218
- Saleh NY, Aboelghar HM, Salem SS, et al. The severity and atypical presentations of COVID-19 infection in pediatrics. BMC Pediatr. 2021;21(1):144. [published Online First: 20210325]. doi:10.1186/s12887-021-02614-2
- Schmitz AH, Wood KE, Burghardt EL, et al. Thromboprophylaxis for children hospitalized with COVID-19 and MIS-C. Res Pract Thromb Haemost. 2022;6(5):e12780. [published Online First: 20220807]. doi:10.1002/rth2.12780
- Karimi M, Sanaei Dashti A, Haghpanah S, et al. Thromboprophylaxis outcome in childhood SARS-CoV-2 infection: A single-center experience. J Pediatr Hematol Oncol. 2023;45(1):e97–e102. [published Online First: 20220927]. doi:10.1097/mph.0000000000002557
- Tehseen S, Williams S, Robinson J, et al. Thrombosis and hemorrhage experienced by hospitalized children with SARS-CoV-2 infection or MIS-C: results of the PICNIC registry. Pediatric Blood Cancer. 2022;69(9):e29793.
- Noni M, Koukou DM, Tritzali M, et al. Coagulation abnormalities and management in hospitalized pediatric patients With COVID-19. Pediatr Infect Dis J. 2022;41(7):570–574. [published Online First: 20220607]. doi:10.1097/inf.0000000000003545.
- Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi:10.1093/oxfordjournals.epirev.a036298
- Razavi A, Davoodi L, Shojaei L, et al. COVID-19 in children: a narrative review. Open Access Maced J Med Sci. 2020;8(T1):23–31. doi:10.3889/oamjms.2020.4714
- Levi M, Thachil J, Iba T, et al. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7(6):e438–ee40. [published Online First: 20200511]. doi:10.1016/s2352-3026(20)30145-9
- Llitjos JF, Leclerc M, Chochois C, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18(7):1743–1746. [published Online First: 20200527]. doi:10.1111/jth.14869