ABSTRACT
Objective Patients living with myeloproliferative neoplasms (MPNs) suffer from symptom burden that affect quality of life. Due to the differences in cultures, climates, and genetic background, we aimed to investigate the symptom burden of Thai MPN patients Methods A comprehensive survey using the MPN-10 questionnaire was carried out between September 1, 2014, and September 30, 2017. The scores obtained were then correlated with clinical outcomes.. Results A total of 145 patients were enrolled. Nearly 90% of patients reported being symptomatic. The mean MPN-10 score was 13.6 (SD = 11). The mean MPN-10 score was highest in PMF, whereas the mean score and intensity of individual items were surprisingly low in ET and PV. Notably, the mean MPN-10 score was significantly higher in patients with documented splenomegaly compared to those with a normal-sized spleen. However, there were no correlations between MPN-10 scores and the mutation status, disease complications such as thrombosis and hemorrhage, progression to myelofibrosis or leukemia, and mortality. Patients who needed regular transfusions reported a higher MPN-10 score compared to those who did not. Conclusion The MPN-10 score did not predict survival outcomes among Thai MPN patients. Higher MPN-10 was associated with more transfusion. Thai MPN patients reported lower MPN-10 compared to western population especially PV and ET.
Introduction
Myeloproliferative neoplasms (MPNs) are clonal myeloid malignancies characterized by cellular proliferation of one or more hematopoietic lineages without dysplastic features. The classic MPNs or BCR-ABL1-negative MPN include polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF) [Citation1]. Since 2005, a gain-of-function mutation of JAK2V617F in committed myeloid progenitors was discovered as a major driver mutation in the three classic MPNs [Citation2–5]. Several research papers have demonstrated that JAK2V617F is the most prevalent driver mutation in MPNs which is detectable in more than 90% of patients with polycythemia vera (PV) and approximately 50–60% of those with essential thrombocythemia (ET) and primary myelofibrosis (PMF). The lack of JAK2 mutations in approximately half of patients has led to the discovery of MPL [Citation6] and CALR mutations [Citation7]. Other molecular pathogenesis of MPNs including MPL and CALR mutations have also been comprehensively studied [Citation8]. These three driver mutations cover almost all the classic MPNs, whereas 10–15% of ET and PMF which do not harbor these driver mutations are termed triple negative MPNs.
Major causes of morbidity and mortality in MPN are thrombohemorrhagic complications and progression to acute myeloid leukemia [Citation9–11]. Nonetheless, MPNs are now considered to have a favorable prognosis. Although people with MPNs have improved life-expectancy in this era, a substantial proportion of patients suffer from significant constitutional symptoms. MPN-related symptoms have been well-documented by several international surveys [Citation12–14]. MPN symptoms include fatigue, itchiness, night sweats, bone pain, fever, and weight loss. The prevalence and severity of individual symptoms differ among MPN subtypes. Hematopoietic stem cell transplantation is the only curative treatment for MPN. Unfortunately, this option is only reserved for young patients and those with advanced-stage disease. The goal of MPN treatment is to alleviate a patient’s symptom burden especially in those with myelofibrosis. Higher symptom burden is associated with decreased daily activity and poor quality of life. Several studies of JAK inhibitors marked the symptom burden as a surrogate outcome of treatment response in the patient’s point of view in addition to the clinical response [Citation15–20]. Different symptom assessment tools have been used to find links between disease outcomes and MPN symptoms. Several questionnaires have been developed to standardize the evaluation of symptom response in clinical trials, ranging from verbal interviews to patient-reported outcomes. The Myeloproliferative Neoplasm Symptom Assessment Form total symptom score (MPN-SAF TSS) or MPN-10 score [Citation14] consists of an original 17-item Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF) and the 10-item Brief Fatigue Inventory (BFI). This instrument has been validated in more than 20 languages across nearly 30 countries [Citation21]. MPN-10 score has been translated into Thai language to assess patient quality of life. However, the score has not been evaluated in Thai population. Therefore, we aimed to investigate the clinical characteristics and disease-related complications in Thai MPN patients and related patient symptom burden as assessed by MPN-10 score.
Material and methods
Study participants
A cross-sectional survey was conducted at King Chulalongkorn Memorial Hospital (KCMH) from 1 September 2014, to 30 September 2017. Data were collected from PV, ET and MF patients including post PV and post ET-MF (diagnosis according to WHO 2008 criteria [Citation22]) who were 18 years or older. Patients who did not give informed consent were excluded. Baseline demographic data (age, sex, and comorbid diseases), clinical characteristics (presenting sign and symptoms including splenomegaly), complete blood count (CBC) at diagnosis, and driver genetic mutations were retrospectively reviewed and retrieved from electronic medical records at diagnosis or at the time of referral to KCMH. Bone marrow aspiration and biopsy were reviewed by an experienced hematopathologist. The risk categories of patients were classified by age and prior history of thrombosis in PV and ET [Citation23]. The Dynamic International Prognostic Scoring System (DIPSS) [Citation24] or DIPSS-plus [Citation25] was used to classify patients with PMF. Transfusion dependency was defined as patients who needed more than 1 unit of red blood cells per month. Disease-related thrombohemorrhagic complications included thrombosis or bleeding events that occurred at diagnosis or at any time during follow-up at KCMH. All patients were treated under institutional review-approved retrospective protocols and in accordance with the declaration of Helsinki.
MPN related symptom assessment
MPN-SAF TSS and MPN-10 were translated into Thai language by a Thai MPN working group and assessed as the sum of 10 items: worst fatigue, early satiety, abdominal discomfort, inactivity, concentration problems, night sweats, itching, bone pain, fever, and weight loss. The intensity of scores had a range from 0 (absent) to 10 (worst imaginable) for each symptom. The total score had a range from 0 to 100. MPN-10 questionnaire was conducted as an observational cross-sectional study. The participating patients were asked to complete the survey and to provide answers as completely and accurately as possible. If the patients were not able to read but were able to answer the questionnaire, the patients were interviewed using a translated MPN-10 questionnaire by the investigators.
Statistical analyses
Continuous data are described by means (± standard deviations, SD) or medians (± interquartile range, IQR) as appropriate. Qualitative data are presented as frequency and percentage. The Kruskal–Wallis test was used to compare differences between the three groups. Chi-square test or Fisher’s exact test examined relationships between quantitative variables. Cox proportional hazard regression model was used for the multivariable analysis. Overall survival was calculated from date of diagnosis to death or last visit. Event-free survival was calculated from date of diagnosis to thrombotic or hemorrhagic events. Survival analysis according to MPN-10 score was calculated from the date of MPN-10 survey to death or last visit. Survival analysis was done using the Kaplan–Meier survival curve and the comparison was performed using log-rank test. All tests were two-tailed. Statistical significance was met if the p value less than 0.05. All data were analyzed using SPSS version 22 (SPSS Inc., Chicago, IL).
Results
Demographic and clinical characteristics
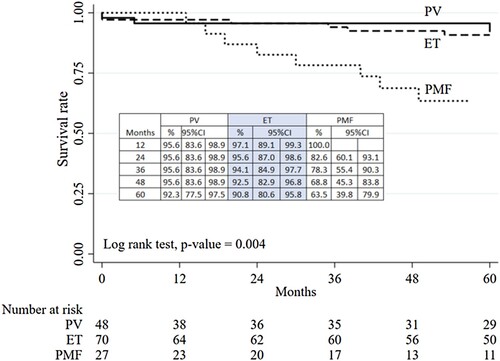
A total of unselected 145 MPN patients were enrolled between 1 September 2014 and 30 September 2017 in this study. Forty-eight patients (33%) were diagnosed with polycythemia vera (PV), 68 (47%) with essential thrombocythemia (ET), and 29 (20%) with myelofibrosis (PMF) (). Median age was 58 years (range 49–69 years), 61 years (44–73 years) and 65 years (56–72 years) in PV, ET and PMF, respectively. For ET, female patients represented a greater proportion (66%). The male percentage was 48% in PV and 41% in PMF. Most patients 64% (93/145) had comorbidities such as hypertension, dyslipidemia, chronic renal insufficiency, or gouty arthritis. History of cardiovascular disease (CVD) including peripheral arterial disease, ischemic heart disease, and cerebrovascular disease was 12% (17/145). CVD history was slightly higher in PV (16%) followed by ET (10%) and PMF (7%). Detectable driver mutations were common to the disease as previously reported (). The most common mutation was JAK2 V617F mutation (PV = 99%; ET = 59% and PMF = 74%). CALR mutations were found 26% (18/70) in ET and 14% (4/29) in PMF. There were 10 patients who had triple-negative ET (14%) and 3 patients with triple-negative PMF (10%). Hemoglobin levels were similar in all types of MPN (PV 13.6 vs. ET 12.8 vs. PMF 12.0 g/dL; P = 0.10), while WBC was higher in PV (PV 13,940/mm3 vs. ET 8260/mm3 vs. PMF 7930/mm3; P < 0.00). ET had the highest median platelet count (PV 623,000/mm3 vs. ET 1,002,000/mm3 vs. PMF 842,000/mm3; P = 0.02). As expected, splenomegaly was the most prevalent in PMF (65%), followed by PV (44%) and ET (22%) (P < 0.001). Patients on therapies included 65%, 80%, and 66% for PV, ET, and PMF, respectively. PMF patients were more likely to be transfusion-dependent and receiving ESA. The clinical characteristics of MPN patients are shown in .
Figure 1. Distribution of driver mutations in myeloproliferative neoplasms based on disease phenotype.
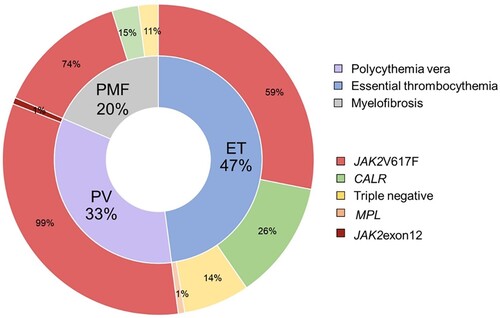
Table 1. Characteristics of patient at diagnosis.
Disease-related complications
The prevalence of thrombosis was 22% (33/145). PV had the highest level of thrombotic complications (29%, 14/48) followed by ET (22%, 15/68) and PMF (14%, 4/29). In PV, venous site thrombosis (Budd-Chiari syndrome, mesenteric vein thrombosis and subclavian vein thrombosis) was slightly more common (57%, 8/14) than arterial site thrombosis (43%, 6/14) (ischemic stroke and transient ischemic attack). For ET, arterial site thrombosis (peripheral arterial disease and ischemic stroke) was more common (73%, 11/15) than venous site thrombosis (26%, 4/15) including Budd-Chiari syndrome, deep vein thrombosis of lower extremity, and splanchnic vein thrombosis as a presentation of the disease. There were four PMF patients who had thrombosis, two of them had portal vein thrombosis and the others had ischemic heart disease and peripheral arterial disease ().
Table 2. Characteristics of patients with thrombotic complications.
Bleeding complications were less common than thrombotic complications with a prevalence of 8.2% (12/145). There were no differences in the prevalence of bleeding complications among PV (12%, 6/48), ET (7.3%, 5/68), and PMF (3%, 1/29). The most common bleeding sites were gastrointestinal bleeding (50%, 6/12), followed by splenic hematoma (16%, 2/12), unspecified hematuria (16%, 2/12), subdural hematoma (8%, 1/12) and hypermenorrhea (8%, 1/12). Only one patient in this cohort was proven to have acquired von Willebrand disease. During the median follow-up of 71 months, progression to secondary myelofibrosis was found in 16% (8/48) of PV and 7% (5/68) of ET. Progression to acute myeloid leukemia (AML) is significantly higher in PMF (20%, 6/29) than in PV (2%, 1/48), (P = 0.01). No cases of ET progressed to secondary AML.
Survival outcomes
With a median follow-up time of 5.9 years (0–20 years), the median overall survival (OS) was not reached in PV, ET and PMF. During the study period, 8% (12/145) of the cohort died. The survival analysis revealed that PMF had inferior OS compared to ET and PV (P < 0.00) () with 100% survival among low-risk PV, ET, and PMF patients. Five-year OS among high-risk PV, ET, and PMF was 88.7%, 86.5%, and 56%, respectively. Univariate analysis found that age > 70 years, diagnosis of PMF, bleeding complications, disease progression, and transfusion dependence were risks for inferior survival. Multivariate analysis showed only age >70 years and transfusion-dependent status predicted poorer outcomes with odds ratios of 2.66 (1.05–6.72; P = 0.04) and 6.23 (2.62–14.82; P < 0.00), respectively ().
Table 3. Multivariate analysis for risk factors associated with mortality.
MPN related symptoms assessed by MPN-10 score and clinical outcomes
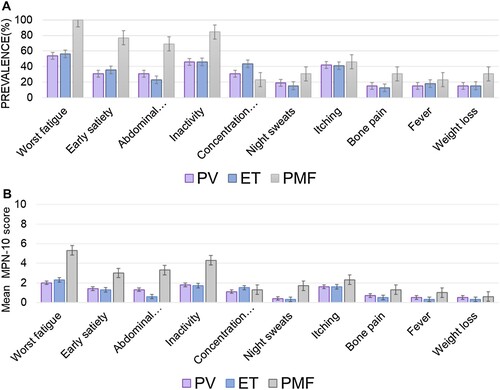
The median time from diagnosis to the score evaluation was 2.3 (0–18.5) years. The MPN-10 scores were calculable in 79 patients (54%). The majority (68%) of patients were evaluated within the first 3 years of diagnosis. Additionally, the median time from the score evaluation to disease progression was 12 months (1–37 months). The proportion of cytoreductive therapy received at the time of survey is described in . The scores were not evaluated in 45% (66/145) due to disabilities (11), death (12), or inability to contact (43). Nearly 90% of evaluable patients were symptomatic (score > 0). The mean MPN-10 score was similar across ages. Female patients with PV reported a non-statistically significant difference in MPN-10-TSS compared to male patients (15 vs. 10, P = 0.24). There was also no gender effect on MPN-10-TSS in ET and PMF. The mean MPN-10 score of all MPN patients was 13.6 (SD,11). The mean MPN-10 score of PMF was 25 (SD,12), which was significantly higher than those of PV (11.7; SD,11), and ET (10.7; SD,9.7) (P < 0.00). The mean intensity of the individual items of MPN-10 was 1.1 (SD, 1.9) in PV, 1.0 (DS,1.7) in ET, and 1.3 (SD, 2.5) in PMF. The most common symptom was fatigue, which had the highest prevalence in PV, ET, and PMF. The other common symptoms were inactivity, itching, early satiety, concentration problems, and abdominal discomfort. In PV, ET, and PMF patients, the two most common symptoms were fatigue and inactivity, while PMF had the highest symptom intensity (mean 5.5; SD 2.2 and mean 4.3; SD 2.8, respectively) The third most common symptom was different among MPNs with itching in PV, concentration problems in ET, and early satiety in PMF. Bone pain was the least reported item among MPN patients. Among ET who had variable mutations, total symptom score (TSS) was not different according to the mutation subtypes. The mean MPN-10 scores of triple negative-ET vs. JAK2V617F-ET vs. CALR-mutated ET were 8.3 (0–19) vs. 10.2 (0–25) (P = 0.99) vs. 12.5 (0–36) (P = 0.42) The prevalence and symptom severity are presented in and .
Table 4. Assessment of patient symptom severity and symptom prevalence by disease type.
Of the patients with an evaluable MPN-10 score, 97% (77 out of 79) had documented spleen size evaluated by ultrasonography or physical examination. It was found that the mean MPN-10 score was significantly higher in patients with documented splenomegaly (21, with a range of 7–30) compared to those with a normal-sized spleen (7.5, with a range of 2–15) (P < 0.001). However, the MPN-10 TSS did not correlate with driver mutations and disease complications including thrombosis, hemorrhage, disease progression to secondary myelofibrosis, AML transformation, and death (Supplementary table S1-S4, Supplementary figure 1). Early satiety was associated with inferior survival in the univariate analysis with an odds ratio of 9.35 (95%CI, 1.16–75.16; P = 0.04), while bone pain did not meet statistical significance (OR 3.63, 95%CI, 0.94–14.07; P = 0.06). None of the individual items reached statistical significance in the multivariate analysis (). Of note, patients who needed regular transfusions (11%, 9/79) reported higher MPN-10 scores, with a total MPN-10 score of 26 (21–31) vs. 8.5(3–19) (P < 0.01) compared to those without transfusion (Supplementary table S5). Transfusion-dependent patients had significantly worse fatigue, early satiety, abdominal discomfort, and inactivity. Patients with bleeding complications experienced a higher intensity of inactivity and greater weight loss compared to those without bleeding events.
Discussion
MPNs are chronic diseases that affect the quality of life of patients. A large study from the Mayo Clinic demonstrated that the median survival of ET and PV are 15 and 18 years, respectively. Among low-risk ET patients, the median overall survival reaches 30 years [Citation26]. Despite the fact that patients with MPN can have long-term survival, the mortality of MPN patients exceeds the age-matched general population [Citation27–29]. Disease-related complications such as inflammation-related symptom burden, cardiovascular disease, and MF/AML progression are serious concerns. Additionally, MPN is pro-inflammatory and can cause debilitating symptoms that result in considerable morbidity. The major treatment goals include prevention of thrombotic complications, decreasing symptom burden, and minimizing disease progression. Understanding disease characteristics, symptom burden, and how the disease impacts patients living with MPN is crucial.
This study investigated clinical characteristics, complications, and symptom burden in a Thai MPN population. The baseline characteristics of patients were consistent with previous reports with the majority diagnosed with ET followed by PV and PMF and a median age of 61 years. Gender-specific distributions were also similar to other MPN studies [Citation21]. PV and PMF were male predominant, while the majority of ET patients were female. The frequency of driver mutations (JAK2, CALR, and MPL) was distributed as expected. More than half of the patients harbored at least one cardiovascular risk factor and approximately 10% had a history of thrombosis prior to MPN diagnosis. Over 65% of patients were high risk and receiving treatment, primarily cytoreductive therapies. Due to limitations in healthcare coverage, only 5% of patients received JAK inhibitor treatment. During the study period, PV had the highest prevalence of thrombosis while bleeding complications were rare among all MPN subtypes. A high disease risk score was a significant risk factor for thrombosis whereas CKD was the only risk factor for bleeding complications in our cohort, contradicting previous studies. With a median follow-up time of 5.9 years, 11% of PV and ET developed secondary myelofibrosis. More than 50% of patients had survived by the end of the study. Mortality was found to be strongly associated with age and transfusion status, consistent with previous findings.
Recognition of symptom burden in MPN patients allows healthcare professionals to identify patients who may benefit from symptom-modifying therapy. This study explores the symptom burden by MPN-10 score, then correlates scores with patient outcomes. PMF had a higher symptom burden than PV and ET as reported in several studies. Not only was the mean MPN-10 score of Thai patients higher in PMF than PV and ET, but the intensity of individual items including fatigue, early satiety, abdominal discomfort, and inactivity was comparable to western populations. Additionally, it is important to consider that the limited number of patients receiving JAK inhibitor treatment could also have an impact on the results of the high MPN-10 score among PMF patients. Of note, the frequency of concentration problems in this study was low. One of the explanations was that MPN-10 scores have been developed from common symptoms in western populations which may be different than Asian populations. Thai people, like many Asian people, have a tendency to not convey their negative emotions openly to other people. People from western cultures are often more comfortable expressing their own feelings and needs. These cultural differences may have affected the results.
There are also differences in occupational structure. For example, concentration problems would be more problematic for executive workers than for laborers. Another reason might be differences in genetic/epigenetic backgrounds. Various mechanisms are responsible for systemic symptoms in MPN patients [Citation30]. Several factors are shown to contribute to symptom burden, interplaying between genetic diversity, inflammatory cytokines, disease phenotypes such as splenomegaly and disease complications. Despite the similar prevalence of driver mutations, the frequency and intensity of individual MPN-10 items of PV and ET patients were lower compared to other international surveys in Caucasians [Citation12, Citation31] or Asian populations [Citation32, Citation33]. In the MERGE study [Citation33], which included Asian and South Asian populations, 10% were from Southeast Asia. The mean MPN-10-TSS of PMF was comparable between the MERGE study and our study (23.5 [17.47] vs. 24.4 [12]). Of note, the mean MPN-10-TSS was lower in PV (16.6 [14.84] vs. 11.7 [11.4]) and ET (14.6 [14.26] vs. 10.7 [9.7]). The common symptoms displayed a similar pattern on individual items, but with less intensity among PV and ET patients.
Our study also demonstrates that the MPN-10 score was correlated with splenomegaly but was not associated with other patient characteristics such as age or gender, nor did it predict clinical outcomes including thrombosis, hemorrhage, secondary myelofibrosis, AML transformation, or death. Contrary to the study from Egypt [Citation32], ElNahass YH et al. showed that MPN-10 was associated with driver mutations and predicts survival in MPN patients. A higher proportion of PMF patients were included in the study compared to our study. MPN-10-TSS scores were also twice as high suggesting that the difference in baseline characteristics of patients may have impacted outcomes. Our study also showed that patients with transfusion dependence reported significantly higher MPN-10-TSS compared to those without transfusions. This may represent an advance phase of the disease. Moreover, transfusion dependence predicted worse survival independent of the diagnosis, disease risk score, and mutational status which was consistent with previous reports [Citation34, Citation35].
This is the first comprehensive long-term follow-up MPN study in Thailand focusing on clinical characteristics and symptom burden related to disease outcomes. The symptom scores may be different in Thai patients due to differences in language, culture, climate, and genetic background. In our study, Thai MPN patients, especially those with PMF, experienced a significant symptom burden. Although MPN-10-TSS did not predict disease-related outcomes such as thrombosis or survival in our sample, a large number of patients struggled with high symptom burden. None of these burdensome symptoms are included in disease prognosis scores. In Thailand, which is a limited-resource country, identifying patients who would benefit most from symptom-alleviating therapy is even more important. From our data, JAK inhibitors would be most beneficial for transfusion-dependent PMF patients.
This study has certain limitations that need to be addressed. First, this is a cross-sectional, single-center retrospective cohort study with a limited sample size with only half of the patients reporting MPN-10 scores. Second, this study did not demonstrate how symptom burden had impacted patient quality of life. Lastly, there is no longitudinal follow-up of MPN-10 score to understand the dynamics of MPN-10 that could provide insights into disease outcomes and early recognition of disease-related complications.
In the conclusion, our study suggested that the MPN-10 score was not found to be predictive of disease-related outcomes in Thai MPN populations. Thai MPN patients reported lower MPN-10 scores compared to what has been previously reported in western populations, especially in the case of PV and ET. Patients who were transfusion-dependent had higher MPN-10 scores compared to those who did not require transfusions. Future research should be conducted on a larger scale to capture the undermanaged symptoms more effectively. Furthermore, the study of cytokines and molecular mechanisms can enhance our understanding of the association between the heterogeneous clinical presentation of MPN symptoms and the underlying pathogenesis of the disease.
Author contributions
NC, collected clinical data, analyzed data, and wrote the draft manuscript. SK, designed research studies, collected clinical data, analyzed data, and wrote and edited the manuscript. CP discussed the results. TC edited the manuscript. PR discussed the results. All authors read and edited the manuscript.
supplementary clean.docx
Download MS Word (28.7 KB)Acknowledgements
This work was supported in parts by grants from Ratchadapisek sompoch research fund (RA60/077). The authors appreciate Jiratchaya Sophonphan for the statistical analysis and Thita Chiaskul for editing the final version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi:10.1182/blood-2016-03-643544
- Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005 Apr 28;352(17):1779–1790. doi:10.1056/NEJMoa051113
- Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005 Apr;7(4):387–397. doi:10.1016/j.ccr.2005.03.023
- Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005 Mar 19-25;365(9464):1054–1061. doi:10.1016/S0140-6736(05)71142-9
- James C, Ugo V, Le Couédic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005 Apr 28;434(7037):1144–1148. doi:10.1038/nature03546
- Pardanani AD, Levine RL, Lasho T, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood. 2006 Nov 15;108(10):3472–3476. doi:10.1182/blood-2006-04-018879
- Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013 Dec 19;369(25):2391–2405. doi:10.1056/NEJMoa1312542
- Vainchenker W, Kralovics R. Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood. 2017;129(6):667–679. doi:10.1182/blood-2016-10-695940
- Finazzi G, Caruso V, Marchioli R, et al. Acute leukemia in polycythemia vera: an analysis of 1638 patients enrolled in a prospective observational study. Blood. 2005 04/01;105(7):2664–2670. doi:10.1182/blood-2004-09-3426
- Nand S, Messmore H, Fisher SG, et al. Leukemic transformation in polycythemia vera: analysis of risk factors. Am J Hematol. 1990;34(1):32–36. doi:10.1002/ajh.2830340108
- Falanga A, Marchetti M. Thrombosis in myeloproliferative neoplasms. Semin Thromb Hemost. 2014 Apr;40(3):348–358. doi:10.1055/s-0034-1370794
- Mesa RA, Niblack J, Wadleigh M, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international internet-based survey of 1179 MPD patients. Cancer. 2007 Jan 1;109(1):68–76. doi:10.1002/cncr.22365
- Mesa R, Miller CB, Thyne M, et al. Myeloproliferative neoplasms (MPNs) have a significant impact on patients’ overall health and productivity: the MPN landmark survey. BMC Cancer. 2016 Feb 27;16:167. doi:10.1186/s12885-016-2208-2
- Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol. 2012 Nov 20;30(33):4098–4103. doi:10.1200/JCO.2012.42.3863
- Vannucchi AM, Kantarjian HM, Kiladjian JJ, et al. A pooled analysis of overall survival in COMFORT-I and COMFORT-II, 2 randomized phase III trials of ruxolitinib for the treatment of myelofibrosis. Haematologica. 2015 Sep;100(9):1139–1145. doi:10.3324/haematol.2014.119545
- Pardanani A, Harrison C, Cortes JE, et al. Safety and efficacy of Fedratinib in patients with primary or secondary myelofibrosis: a randomized clinical trial. JAMA Oncol. 2015;1(5):643–651. doi:10.1001/jamaoncol.2015.1590
- Mascarenhas J, Hoffman R, Talpaz M, et al. Pacritinib vs best available therapy, including ruxolitinib, in patients with myelofibrosis: a randomized clinical trial. JAMA Oncol. 2018;4(5):652–659. doi:10.1001/jamaoncol.2017.5818
- Passamonti F, Kiladjian J-J, Vannucchi AM, et al. ReTHINK: a randomized, double-blind, placebo-controlled, multicenter, phase 3 study of ruxolitinib in early myelofibrosis patients. J Clin Oncol. 2016;34(15_suppl):TPS7080–TPS7080. doi:10.1200/JCO.2016.34.15_suppl.TPS7080
- Verstovsek S, Vannucchi AM, Griesshammer M, et al. Ruxolitinib versus best available therapy in patients with polycythemia vera: 80-week follow-up from the RESPONSE trial. Haematologica. 2016 Jul;101(7):821–829. doi:10.3324/haematol.2016.143644
- Harrison CN, Mead AJ, Panchal A, et al. Ruxolitinib vs best available therapy for ET intolerant or resistant to hydroxycarbamide. Blood. 2017 Oct 26;130(17):1889–1897. doi:10.1182/blood-2017-05-785790
- Geyer H, Mesa RA. Approach to MPN symptom assessment. Curr Hematol Malig Rep. 2017 Oct;12(5):381–388. doi:10.1007/s11899-017-0399-5
- Tefferi A, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia. 2008 Jan;22(1):14–22. doi:10.1038/sj.leu.2404955
- Barbui T, Barosi G, Birgegard G, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol. 2011;29(6):761–770. doi:10.1200/JCO.2010.31.8436
- Passamonti F, Cervantes F, Vannucchi AM, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for myeloproliferative neoplasms research and treatment). Blood. 2010 Mar 4;115(9):1703–1708. doi:10.1182/blood-2009-09-245837
- Gangat N, Caramazza D, Vaidya R, et al. DIPSS plus: a refined dynamic international prognostic scoring system for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol. 2011 Feb 1;29(4):392–397. doi:10.1200/JCO.2010.32.2446
- Szuber N, Mudireddy M, Nicolosi M, et al. 3023 mayo clinic patients with myeloproliferative neoplasms: risk-stratified comparison of survival and outcomes data Among disease subgroups. Mayo Clin Proc. 2019 Apr;94(4):599–610. doi:10.1016/j.mayocp.2018.08.022
- Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013 Sep;27(9):1874–1881. doi:10.1038/leu.2013.163
- Price GL, Davis KL, Karve S, et al. Survival patterns in United States (US) medicare enrollees with non-CML myeloproliferative neoplasms (MPN). PLoS One. 2014;9(3):e90299. doi:10.1371/journal.pone.0090299
- Tefferi A, Guglielmelli P, Larson DR, et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood. 2014 Oct 16;124(16):2507–2513. quiz 2615. doi:10.1182/blood-2014-05-579136
- Geyer HL, Scherber RM, Dueck AC, et al. Distinct clustering of symptomatic burden among myeloproliferative neoplasm patients: retrospective assessment in 1470 patients. Blood. 2014 Jun 12;123(24):3803–3810. doi:10.1182/blood-2013-09-527903
- Brochmann N, Flachs EM, Christensen AI, et al. Health-related quality of life in patients with Philadelphia-negative myeloproliferative neoplasms: a nationwide population-based survey in Denmark. Cancers (Basel). 2020 Nov 28;12(12):3565. doi:10.3390/cancers12123565
- ElNahass YH, Mahmoud HK, Mattar MM, et al. MPN10 score and survival of molecularly annotated myeloproliferative neoplasm patients. Leuk Lymphoma. 2018;59(4):844–854. doi:10.1080/10428194.2017.1365852
- Yassin MA, Taher A, Mathews V, et al. MERGE: a multinational, multicenter observational registry for myeloproliferative neoplasms in Asia, including Middle East, Turkey, and Algeria. Cancer Med. 2020 Jul;9(13):4512–4526. doi:10.1002/cam4.3004
- Bartoszko J, Panzarella T, Lau A, et al. Effect of Red blood cell transfusion dependence on the natural history of myeloproliferative neoplasm-associated myelofibrosis. Clin Lymphoma Myeloma Leuk. 2015 Nov;15(11):e151–e156. doi:10.1016/j.clml.2015.09.001
- Elena C, Passamonti F, Rumi E, et al. Red blood cell transfusion-dependency implies a poor survival in primary myelofibrosis irrespective of IPSS and DIPSS. Haematologica. 2011 Jan;96(1):167–170. doi:10.3324/haematol.2010.031831