ABSTRACT
Thrombocytopenia is a common and serious complication that can occur following hematopoietic stem cell transplantation (HSCT), and it contributes to increased morbidity and mortality. The mechanisms of post-HSCT thrombocytopenia are multifactorial and complex. There are no clear consensus and guidelines for managing thrombocytopenia post-HSCT. Recently, there has been promising use of thrombopoietin receptor agonists (TPO-RAs), particularly eltrombopag and romiplostim, as treatments for post-HSCT thrombocytopenia. Notably, that this indication is considered off-label, and data in this use are limited. Based on the existing body of evidence, romiplostim emerges as a safe and effective option for individuals with transfusion-dependent thrombocytopenia after HSCT. In this context, we present a summary of our experience at a single center, where romiplostim was used in the management of post-HSCT thrombocytopenia due to poor graft function. Notably, all four cases responded positively to romiplostim treatment, and no significant adverse events were observed.
Introduction
Thrombocytopenia (low platelet level) following hematopoietic stem cell transplantation (HSCT) is a common complication which can lead to increased morbidity and mortality. The mechanisms of thrombocytopenia post-HSCT are generally multifactorial and not well-established. Prolonged isolated thrombocytopenia (PIT) is defined as persistent thrombocytopenia after HSCT despite the recovery of other cell lines and has been reported in up to 20% of HSCT cases [Citation1]. In the other hand, the secondary failure of platelet recovery (SFPR) is defined as platelet counts less than 20,000/mm3 for 7 consecutive days or requirement of transfusion after reaching platelets ≥50,000/mm3 without transfusion for 7 days post-HSCT. It can also occur in up to 20% of HSCT cases [Citation2]. Lastly, poor graft function (PGF) is another entity which might affect up to 20% of HSCT cases and defined as persistent cytopenia in more than one cell lines in the presence hypoplastic/aplastic bone marrow and complete donor chimerism and absence of relapse cases [Citation1,Citation3]. Cases with PGF are usually dependent on blood and/or platelet transfusions and/or growth factor support in the absence of other explanations such as disease relapse, drugs, or infections [Citation4].
Treatment options for post-HSCT thrombocytopenia are unclear and largely based on platelet transfusion. However, transfusion is associated with several adverse effects, such as platelet refractoriness, transfusion reactions, acute lung injury, circulatory overload, and financial burden [Citation1]. Thrombopoietin receptor agonists (TPO-RAs) are drugs that bind to TPO receptors, promoting platelet production [Citation5]. TPO-RAs show significant therapeutic effects in different kinds of thrombocytopenia, including immune thrombocytopenia (ITP), chemotherapy-induced thrombocytopenia (CIT), presurgical thrombocytopenia, and thrombocytopenia related to chronic liver diseases (CLD) and aplastic anemia (AA). Recently, TPO-RAs, specifically eltrombopag and romiplostim, have been used in the treatment of post-HSCT thrombocytopenia with promising results, however, this is an off-label indication and the data on that are limited [Citation5–7]. Informed consent was obtained from the patient to initiate romiplostim therapy, considering the absence of alternative options for post-transplant thrombocytopenia. The patient was thoroughly apprized of both the potential risks and benefits associated with this medication. It was made clear that romiplostim had not received specific approval for the treatment of this condition. In this case series, we aimed to summarize our single center experience in the use of romiplostim in the treatment of post-allogenic HSCT thrombocytopenia. To our knowledge, this is the first report from the state of Qatar.
Case 1
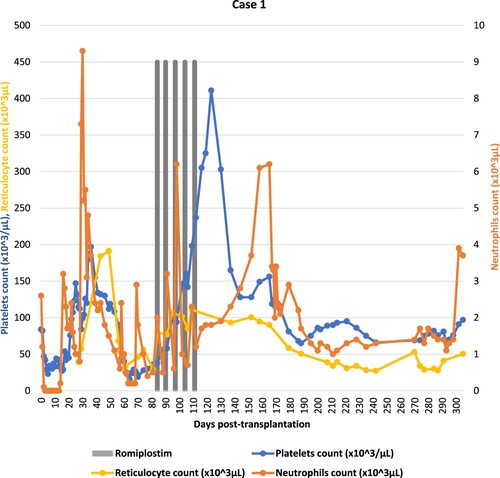
A 29-year-old male intermediate risk acute myeloid leukemia in first complete remission was given an allogeneic peripheral blood stem cell transplantation from his haploidentical mother following fludarabine/total body irradiation conditioning (TBI). Graft-versus-host disease prophylaxis consisted of cyclosporine, mycophenolate mofetil and post-transplant cyclophosphamide. He received a major ABO mismatched graft with CD34 + cell dose of 11.1 × 106/kg. Post-transplantation course was complicated by grade 3 mucositis, and asymptomatic BK virus (BKV) reactivation. Neutrophils engraftment occurred on day +16, and platelets engraftment was on day +19 post-HSCT. Starting from day +56, he developed. Significant pancytopenia requiring transfusion support and growth factor administration. Platelets count decreased until 16 × 103/μL. Bone marrow biopsy showed disease remission with decreased megakaryopoiesis and 10–15% cellularity. CMV-PCR, ADENO-PCR and EBV-PCR were negative and his chimerism study confirmed 100% donor cell engraftment. Laboratory investigations excluded transplant-associated thrombotic microangiopathy (TA-TMA). The patient did not develop GvHD and was off myelosuppressive therapeutic agents. Based on that, the patient was diagnosed with secondary poor graft function. On day +84, the patient was started on romiplostim 4 μg/kg subcutaneously once weekly for five weeks. His platelets recovered to more than 100 × 103/μL on day +95. Treatment was discontinued once the platelets count was over 300 × 103/μL (). Bone marrow biopsy showed variable cellularity ranging between (25–30%) with some more cellular areas of 40–60%. Trilineage hematopoiesis is represented with increased erythropoiesis, mildly decreased granulopoiesis and active megakaryopoiesis. There were no reported adverse events during the treatment with romiplostim.
Case 2
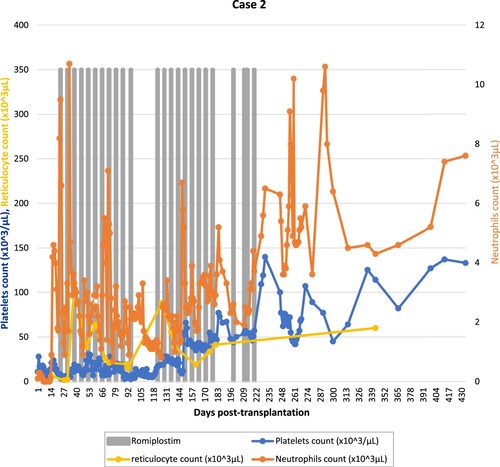
A 40-year-old female with relapsed diffuse large B-cell lymphoma stage IV-E with bone marrow and stomach involvement. She received salvage chemotherapy and autologous stem cell transplantation following BEAM conditioning. Her post-transplant course was complicated by fungal sinusitis, cavernous sinus thrombosis, and left internal carotid artery thrombosis. She never had full hematological recovery and remained transfusion dependent. Four months later, the patient had her second relapse confirmed by PET-CT and bone marrow exam. She received 1 cycle of salvage bendamustine/rituximab, complicated by prolonged bone marrow hypoplasia. She was started on eltrombopag 75 mg orally once daily increased to 150 mg with no response. The patient’s assessment showed significant hepatosplenomegaly. MRI liver iron overload showed T2* time of 0.9 ms indicating severe siderosis. Subsequently, she underwent allo-HSCT from her HLA-matched sibling donor with fludarabine/melphalan reduced-intensity conditioning regimen. She received minor ABO mismatched PBSC graft with a CD34 + cell dose of 11.2 × 106/kg. For GvHD prophylaxis, the patient received cyclosporine and short-course methotrexate. The post-transplantation course was complicated by grade 3 mucositis, neutropenic colitis, pneumonia, and polyserositis. Neutrophils engraftment occurred at day +14; however, her platelet count failed to recover. On day +20, the patient was diagnosed with sinusoidal obstruction syndrome (SOS). She received defibrotide under platelets support. On day +23, once weekly romiplostim 5 μg/kg was started for primary poor graft function. She received 4 doses, then increased to 10 μg/kg for another 6 doses. The patient had no response; therefore, romiplostim was discontinued. Her repeated chimerism studies showed 100% donor cell engraftment. On day +105 post-HSCT, the patient was given PBSC booster with a CD34 + cell dose of 7 × 106/kg from the same donor. She was re-challenged with romiplostim 7 μg/kg subcutaneously once weekly on day +114 due to persistent poor graft function. After 14 doses, she had platelets count recovery to more than 100 × 103/μL on day +226 (). There were no reported adverse events during the treatment with romiplostim.
Case 3
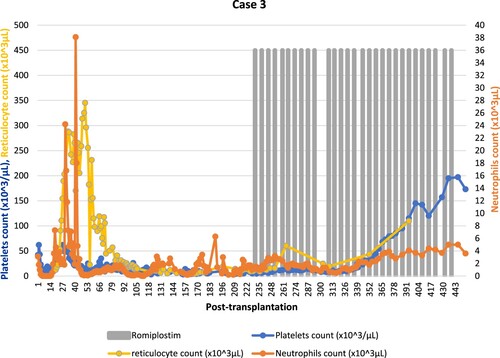
A 48-year-old male patient with relapsed intermediate-risk acute myeloid leukemia (AML) in second complete remission underwent haploidentical allo-HSCT from his brother after fludarabine/TBI conditioning protocol and cyclosporine, mycophenolate mofetil and post-transplantation cyclophosphamide for GvHD prophylaxis. He received a minor ABO mismatched peripheral blood stem cell graft with a CD34 + cell dose of 6.2 × 106/kg. His early post-transplantation course was complicated by cytomegalovirus (CMV) reactivation treated with foscarnet. The patient was also diagnosed with grade 2 acute GvHD with skin and upper GI involvement treated with methylprednisolone 1 mg/kg/day. He had neutrophil and platelet engraftment on day +15 and day +22, respectively. On day +29, defibrotide was initiated for possible TA-TMA. During this period, his platelets count dropped from a 52 × 103/μL baseline to less than 20 × 103/μL requiring transfusion support. Serial bone marrow biopsies showed decreased megakaryocytes and reduced cellularity ranging between 5 and 20%. He had 100% donor cell chimerism. Eltrombopag 50 mg orally once daily was started on day +50 increased gradually to 100 mg. Eculizumab was started for the treatment of TA-TMA on day +98. On day +166, Bone marrow biopsy was hypocellular with decreased trilineage hematopoiesis, increased macrophages/histiocytes and reticulin fibrosis (cellularity range between 5 and 25%) with decreased trilineage hematopoiesis. Megakaryocytes markedly decreased as well. Therefore, eltrombopag was discontinued as it could cause reversible bone marrow fibrosis. He had developed significant pancytopenia requiring regular platelet and RBC transfusion and growth factor administration. Due to persistent thrombocytopenia despite TA-TMA resolution and discontinuation of myelotoxic agents, romiplostim 4 μg/kg subcutaneously once weekly was initiated on day +230 due to secondary poor graft function. At the time of romiplastim start his CBC was showing neutrophils count of 0.2 × 103/μL and platelets count of 11 × 103/μL. After romiplostim initiation platelets requirement decreased to every 72-h and he was transfusion independent after 15 doses. The dose was gradually increased to a final dose of 10 μg/kg. Romiplostim was discontinued once the platelets count reached 195 × 103/μL, a total of 29 doses were given (). There were no reported adverse events during the treatment with romiplostim. Follow-up bone marrow aspirate on day +342 was hypocellular (20–40%) with trilineage hematopoiesis, decreased megakaryopoiesis.
Case 4
A 30-year-old male patient who underwent haploidentical stem cell transplantation for severe aplastic anemia. He received a major ABO mismatched peripheral blood stem cell graft from his brother, with a CD34 + cell dose of 6.8 × 106/kg, following thymoglobulin/cyclophosphamide/fludarabine/TBI conditioning protocol. Graft-versus-host disease prophylaxis consisted of cyclosporine, mycophenolate mofetil and post-transplant cyclophosphamide. His post-transplantation course was complicated by grade 2 acute GvHD with skin involvement. Neutrophils engraftment occurred at day +13; however, his platelets count was persistently below 20 × 103/μL. Bone marrow biopsy on day +27 showed markedly reduced megakaryocytes and 5–20% cellularity. He had 100% donor cell chimerism. CMV-PCR, ADENO-PCR and EBV-PCR were negative, and TA-TMA was excluded. On day +38, the patient was started on romiplostim 500 mcg (6.6 μg/kg) subcutaneously once weekly. Bone marrow aspirate on day +95 was cellular with trilineage hematopoiesis, mildly reduced granulopoiesis, increased erythropoiesis. Following the administration of eight doses with no reported adverse events. His platelets have reached 164 × 103/μL on day +124 (; ).
Figure 4. Summary of cell counts (platelets, neutrophils, reticulocytes) response to romiplostim (Case 4).
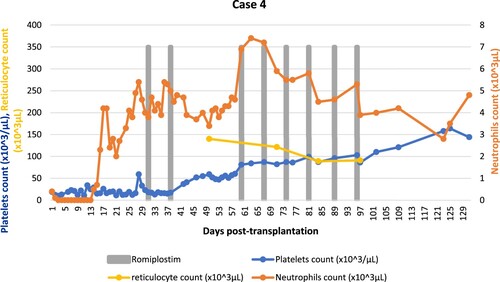
Table 1. Summary of the cases.
Discussion
Thrombopoiesis, the process of platelet production and development, primarily occurs within the bone marrow (BM) and lung. The main regulatory cytokine of thrombopoiesis is TPO and it is produced by the liver. TPO acts by binding to the myeloproliferative leukemia (MPL) receptor which activates several signaling pathways, including the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinases (ERK), PI3K-Akt, and Janus kinase (JAK)/STAT pathways. These pathways have fundamental roles in the proliferation, differentiation, and maturation of megakaryocytes (MKs), the precursor cells of platelets [Citation8]. Romiplostim, which is a TPO-RA, binds to the TPO receptor, also known as the MPL receptor. This binding leads to a conformational change in the TPO receptor, triggering the activation of the JAK2/STAT5 signaling pathway. Consequently, there is an increased proliferation of megakaryocyte progenitor cells and enhanced production of platelets [Citation9].
There are several distinctions between romiplostim and eltrombopag in terms of their mechanism of action. Romiplostim is classified as a peptibody, which directly and competitively binds to the TPO binding site. In contrast, eltrombopag is a small molecule that binds to a transmembrane site. Notably, there are variations in the activation of other signaling pathways within megakaryocytes (MK), such as STAT3, ERK, and AKT. Additionally, romiplostim primarily stimulates mature precursors. Conversely, eltrombopag seems to act earlier in the pathway, promoting the stimulation of MK precursor cells and facilitating MK differentiation [Citation9].
The initial dosing with romiplostim commences at 1 μg/kg per week and is escalated by 1 μg/kg per week until a favorable response is attained. Nevertheless, this incremental method extends over nine weeks to reach the peak dosage of 10 μg/kg per week. A more pragmatic approach would involve commencing at 3 μg/kg per week, especially when urgency in response is required, or using a complete vial of 250 μg. Subsequently, the dosage would be progressively increased on a weekly basis to 5 μg/kg, followed by 7 μg/kg, and ultimately reaching 10 μg/kg per week until the desired response is achieved [Citation10]. Generally, TPO-RAs have been established as safe and well-tolerated. Nevertheless, it's important to acknowledge that a few adverse events have been reported in association with the use of TPO-RAs. Furthermore, research has indicated that the utilization of TPO-RAs is associated with a 2–3 times higher risk of thrombosis. The exact underlying mechanisms accountable for the elevated thrombotic risk linked to TPO-RAs use remain incompletely elucidated and acknowledged as of now. Additional research is needed for a more comprehensive understanding of the pathophysiology behind this phenomenon [Citation9,Citation11]. Nonetheless, in a recent open-label pilot study assessing the use of romiplostim for thrombocytopenia following autologous HCT, only one out of 59 patients experienced a low-risk pulmonary embolism, potentially associated with romiplostim [Citation12].
Thrombocytopenia following HSCT is a prevalent and complex condition. Increasing evidence suggests that TPO-RAs are effective and safe in managing this condition, with minimal occurrence of adverse events. Only a few studies have been published highlighting the role of romiplostim in the treatment of refractory or prolonged thrombocytopenia following HSCT [Citation1,Citation12,Citation13]. In a recent open-label pilot study assessing the use of romiplostim for thrombocytopenia following autologous HCT, romiplostim-treated patients had enhanced platelet recovery to normal values beginning at approximately day +15 compared with those of no romiplostim. Interestingly, enhanced hemoglobin recovery in romiplostim-treated patients was also noted, highlighting romiplostim additional effects on hematopoietic stem cells [Citation14]. In a comprehensive review, twelve reports (consisting of six case series and six case reports) outlined the utilization of romiplostim in cases of prolonged thrombocytopenia following HSCT. These reports encompassed 17 patients with PIT and 32 patients with SFPR (Of note, 48 patients underwent allogenic HSCT). Among the 49 patients, an increase in platelet count leading to a positive response was documented in 40 individuals, yielding an overall response rate (ORR) of 82%. Among the respondents, 10 patients had PIT(ORR for PIT, 59%), while 30 patients had SFPR(ORR for SFPR, 94%). Worth mentioning is the fact that none of the reports detailed any instances of serious grade 3 or 4 adverse effects, thromboembolic events, or any indication of new bone marrow fibrosis [Citation7]. In a phase 1/2 multicenter, single-arm open trial, a total of 24 patients experiencing transfusion-dependent thrombocytopenia following allogeneic hematopoietic stem cell transplantation (HSCT) were examined. Out of these, 18 patients exhibited a positive response to romiplostim, achieved through a dosage of ≥5 μg/kg, leading to platelet counts exceeding 50 × 109/L. This encouraging outcome was achieved within a median duration of 45 days. Overall, the administration of romiplostim was well tolerated, with the likelihood of reported adverse events being directly related to the drug considered to be unlikely. Additionally, no instances of thromboembolic events or new bone marrow fibrosis were documented in the study [Citation13].
In this case series, we describe four cases with allogenic HSCT thrombocytopenia treated with romiplostim. All patients are below the age of 50 years. The indications in our cases were hematologic malignancies (AML = 2, DLBCL = 1) and severe aplastic anemia. Romiplostim was given in doses ranging from 4 to 10 μg/kg weekly (case 2 and 3 required the maximum dose for the favorable response). All patients achieved targeted platelet count response and the time to platelet count response is highly variable ranging from 4 to 132 days. No patient has developed any adverse effects, up to the date of writing this manuscript, including thromboembolism or new bone marrow fibrosis supporting the safety of romiplostim in this condition.
Conclusion
Prolonged thrombocytopenia following HSCT is a frequently encountered condition, characterized by complex and multifactorial etiology. This state is recognized as an unfavorable prognostic marker in patients who underwent HSCT. Despite platelet transfusion being a common practice, there is a lack of consensus guidelines for managing prolonged thrombocytopenia post-HSCT. In accordance with the current body of evidence, romiplostim emerges as a safe option for individuals with transfusion-dependent thrombocytopenia following HSCT. Remarkably, romiplostim demonstrates the capability to enhance platelet count without imposing significant adverse effects.
Consent
Written informed consent was obtained from our patients to allow the publication of information.
Ethics statement
The case series was approved by Hamad Medical Corporation Medical Research Center with reference number MRC-04-23-574.
Acknowledgements
Open Access funding provided by the Qatar National Library.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bento L, Canaro M, Bastida JM, et al. Thrombocytopenia and therapeutic strategies after allogeneic hematopoietic stem cell transplantation. J Clin Med. 2022;11:1364. doi:10.3390/jcm11051364
- Bruno B, Gooley T, Sullivan KM, et al. Secondary failure of platelet recovery after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2001;7:154–162. doi:10.1053/bbmt.2001.v7.pm11302549
- Kırcalı E, Cengiz Seval G, Öztürk C, et al. Eltrombopag for the treatment of poor graft function following haematopoietic cell transplantation: real-life data. Balkan Med J. 2023;40:51–56. doi:10.4274/balkanmedj.galenos.2022.2022-2-48
- Kharfan-Dabaja MA, Kumar A, Ayala E, et al. Standardizing definitions of hematopoietic recovery, graft rejection, graft failure, poor graft function, and donor chimerism in allogeneic hematopoietic cell transplantation: a report on behalf of the American society for transplantation and cellular therapy. Transplant Cell Ther. 2021;27:642–649. doi:10.1016/j.jtct.2021.04.007
- Huang L, Xu J, Zhang H, et al. Application and investigation of thrombopoiesis-stimulating agents in the treatment of thrombocytopenia. Ther Adv Hematol. 2023;14:20406207231152744. doi:10.1177/20406207231152746
- Samarkandi H, Al Nahedh M, Alfattani A, et al. Evaluation of eltrombopag in thrombocytopenia post hematopoietic cell transplantation: rertrospective observational trial. Hematol Oncol Stem Cell Ther. 2022;15:285–290.
- Mahat U, Rotz SJ, Hanna R. Use of thrombopoietin receptor agonists in prolonged thrombocytopenia after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2020;26:e65–e73. doi:10.1016/j.bbmt.2019.12.003
- Kuter DJ. The biology of thrombopoietin and thrombopoietin receptor agonists. Int J Hematol. 2013;98:10–23. doi:10.1007/s12185-013-1382-0
- Ghanima W, Cooper N, Rodeghiero F, et al. Thrombopoietin receptor agonists: ten years later. Haematologica. 2019;104:1112–1123. doi:10.3324/haematol.2018.212845
- Kuter DJ, Bussel JB, Newland A, et al. Long-term treatment with romiplostim in patients with chronic immune thrombocytopenia: safety and efficacy. Br J Haematol. 2013;161:411–423. doi:10.1111/bjh.12260
- Rodeghiero F. Is ITP a thrombophilic disorder? Am J Hematol. 2016;91:39–45. doi:10.1002/ajh.24234
- Scordo M, Gilbert LJ, Hanley DM, et al. Open-label pilot study of romiplostim for thrombocytopenia after autologous hematopoietic cell transplantation. Blood Adv. 2023; 7:1536–1544. doi:10.1182/bloodadvances.2022007838
- Peffault de Latour R, Chevret S, Ruggeri AL, et al. Romiplostim in patients undergoing hematopoietic stem cell transplantation: results of a phase 1/2 multicenter trial. Blood. 2020;135:227–229. doi:10.1182/blood.2019000358
- Capecchi M, Serpenti F, Giannotta J, et al. Off-label use of thrombopoietin receptor agonists: case series and review of the literature. Front Oncol. 2021;11:680411. doi:10.3389/fonc.2021.680411