ABSTRACT
Objectives
The role of subcutaneous (SC) rituximab in the efficacy and safety to non-Hodgkin lymphoma (NHL) is not clear enough. The purpose of this study was to conduct a systematic review and meta-analysis, to assess the efficacy and safety of subcutaneous rituximab to NHL.
Method
A full-scale search was carried out based on the set search terms in PubMed, Web of Science, Embase and Cochrane CENTRAL until 12 October 2022 to identify relevant studies of subcutaneous rituximab for NHL. The efficacy and safety outcomes included complete response (CR) plus unconfirmed complete response (CRu), adverse events (AEs), grade ≥3 AEs, serious adverse events (SAEs), administration-related reactions (ARRs), adverse reaction rates.
Results
From a total of 758 studies, 9 trials were eligible. The CR/CRu of patients with NHL receiving SC rituximab was 57%, 55% for Diffuse large B-cell lymphoma (DLBCL) and 54% for Follicular lymphoma (FL). The meta-analysis performed on safety demonstrated that AEs of NHL patients with SC rituximab was 85%, grade ≥3 AEs was 38%, SAE was 27% and ARR was 33%. The result also showed that SC rituximab had a high risk of neutropenia and nausea.
Conclusion
For NHL patients, there is no significant difference in the efficacy between subcutaneous rituximab and conventional therapy, while subcutaneous injection can shorten exposure time in the hospital and reduce the risk of infection.
Introduction
Non-Hodgkin lymphoma (NHL) is a type of cancer that forms in the lymph system, which is the most common hematological malignancy, comprising 2.8% of worldwide cancer diagnoses [Citation1]. Diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL) constitute 55% of new NHL cases and are initially treated with rituximab-based chemoimmunotherapy.
Rituximab is a human/murine chimeric monoclonal antibody that specifically binds to CD20 protein present on the surface of B lymphocytes [Citation2]. Since intravenous (IV) rituximab first gained regulatory approval in 1997 from the US Food and Drug Administration (FDA), the use of IV rituximab has become standard in the management of patients suffering from various B-cell malignancies.
Clinical studies supporting these indications have shown that rituximab not only prolongs the time to disease progression but also extends overall survival [Citation3]. More than 60% DLBCL can be cured with R-CHOP and 80% of FL patients having an overall survival of >10 years with the rituximab treatment [Citation4,Citation5]. But Intravenous rituximab has one disadvantage that infusions typically administered over a period of 1.5–6 h to relieve the risk of infusion-related reactions (IRRs). IV infusions place a considerable burden on patients and healthcare systems [Citation6]. To simplify and shorten rituximab administration and improve resource utilization at the treatment facility, a subcutaneous formulation of rituximab has been developed. SC administration of rituximab takes 5–6 min only compared to IV infusion. So far, a number of clinical trials have been conducted to prove the efficacy and safety of subcutaneous rituximab.
The purpose of the present article aimed to confirm effectiveness and safety of rituximab SC in NHL patients in main indications and to provide an important and useful guide to inform treatment decisions.
Materials and methods
Search strategies for the identification studies
The literature of this study was published in English and systematically retrieved from four electronic databases, including PubMed, Web of Science, Cochrane Library and Embase before October 12, 2022. The key search words were: (‘non-Hodgkin lymphoma’ OR ‘diffuse large B-cell lymphoma’ OR ‘follicular lymphoma’) AND ‘Rituximab’ AND ‘subcutaneous’. The search strategy for each database (MeSH term) was detailed in Supplementary Material (S1).
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) prospective clinical studies (including randomized control trials and single-arm studies) and retrospective studies; (2) studies including patients confirmed with NHL, irrespective of the subtype; (3) studies involving patients treated with SC rituximab; (4) studies reporting either efficacy and/or safety end points, including complete response (CR) plus unconfirmed complete response (CRu), adverse events (AEs), grade ≥3 AEs, serious adverse events (SAEs), administration-related reactions (ARRs), adverse reaction rates.
The exclusion criteria were as follows: (1) sample size (enrolled patients with NHL) less than 10 patients; (2) reported outcomes from multiple populations or disease cohorts and (3) article type: conference abstract, review, comment, case report, experience that reported incomplete information, and cell or animal study.
Study selection and data extraction
The Endnote 20 software and manual checking have been used to remove duplicates. Two reviewers (STY and MXL) independently selected the remaining studies by reading the titles, abstracts, or full texts according to the eligibility criteria. And any difference of opinion was decided after discussion.
The following information from the included studies was extracted: (1) study information including publication year, first author, title and journal; (2) participants including sample size, age, sex, and disease diagnosis; (3) intervention and control including the type of therapy, dosage, frequency, and duration of treatment; (4)the evaluation items of efficacy and safety including complete response (CR) plus unconfirmed complete response (CRu), administration-related reactions (ARRs), serious adverse events (SAEs), grade ≥3 adverse events (AEs), adverse reaction rates Data extraction was done by a single unmasked reviewer (STY) and checked and validated independently by a second reviewer (MXL).
Quality assessment
The quality of scientific research and overall risk of bias were assessed using the Cochrane risk of bias tool (version 2.0) [Citation7] for randomized controlled trials (RCTs), the methodological index for non-randomized studies (MINORS) [Citation8] for non-randomized interventional studies.
Data analysis
Statistical analysis of the pooled CR/CRu, AE, grade ≥ 3 AE, SAE and ARR results of patients with NHL treated with SC rituximab was performed using STATA/SE version 15.1 (StataCorp LLC, College Station, TX, USA) [Citation9].
The effect size of all pooled results was represented by 95% CI with an upper limit and a lower limit. The Cochrane Q chi-square test and I2 statistic were used to examine the heterogeneity across studies. The fixed-effects model was used for pooled results with low heterogeneity (I2 ≤ 50%); otherwise, the random-effects model was used for analysis. The sensitivity analysis was performed by excluding each study one by one for the pooled results with high heterogeneity. Moreover, potential publication bias of included studies was examined using the Egger’s tests.
Results
Study selection and characteristics of the included studies
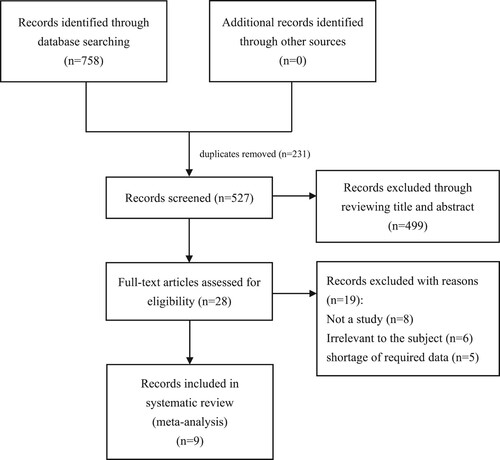
758 potentially relevant articles in total (PubMed: 161, Embase: 220, Cochrane Library: 36, Web of Science: 341) were initially identified, of which 231 duplicate publications were got rid of. Among the remaining 527 articles, 499 were excluded based on their titles and abstracts. Subsequently, 28 articles were further evaluated based on their full texts, and 18 articles were removed for the following reasons: not a study (n = 8), irrelevant to the subject (n = 5), and shortage of required data (n = 5). Eventually, nine researches were included in the subsequent quantitative analysis (). The details are summarized in .
Table 1. Characteristic of included studies.
Quality assessment
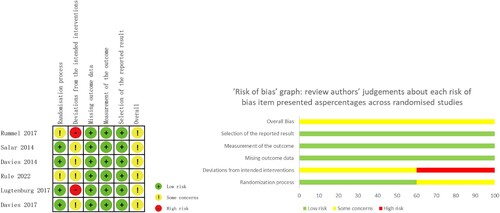
As shown in , three included non-randomized studies assessed using the MINORS index scored from 13 to 14 points, which were acceptable for the present meta-analysis. The results of six randomized controlled trials evaluated with Cochrane risk of bias tool were shown in .
Table 2. Quality assessment of included studies.
Efficacy
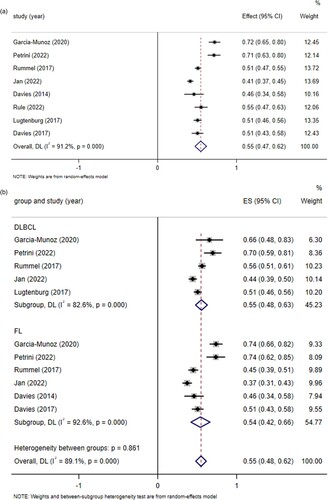
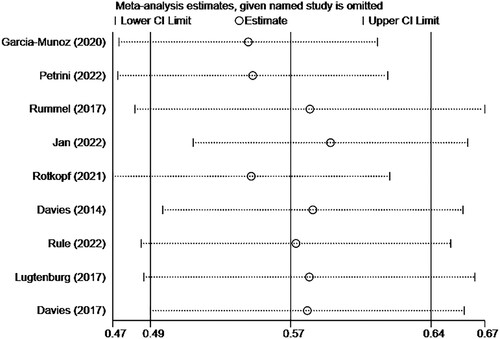
Eight clinical trials [Citation10–17], yielding 2209 patients with NHL and receiving subcutaneous injection of rituximab, provided CR/CRu data for prespecified clinical efficacy outcome, as shown in . The pooled CR/CRu was 55% (95%CI 0.47–0.62, I2 = 91.2%, P = 0.000). Through subgroup analysis of patients with clear NHL classification diagnosis who received subcutaneous injection of rituximab, the pooled CR/CRu was 55% (95%CI 0.48–0.62, I2 = 89.1%, P = 0.000), 55% (95%CI 0.48–0.63, I2 = 82.6%, P = 0.000) for DLBCL and 54% (95%CI 0.42–0.66, I2 = 92.6%, P = 0.000) for FL. The difference between groups was not statistically significant (P = 0.861 > 0.05), indicating that there was no significant difference in CR/CRu when subcutaneous injection of rituximab was used to treat DLBCL and FL. The pooled results of CR/CRu () in total turned out to be reliable through sensitivity analysis by the exclusion of each study one by one.
Safety
AEs, SAE and ARR were reported in all 9 studies [Citation10–18], and some common adverse events of concern were analyzed. Among the 2523 NHL patients, 1271 patients were definitely recorded with DLBCL, and 1161 were FL patients.
The results () showed that the pooled AEs of NHL patients with subcutaneous injection of rituximab was 86% (95%CI 0.80–0.91, I2 = 95.1%, P = 0.000). During the treatment, 38% (95%CI 0.27–0.49, I2 = 97.2%, P = 0.000) of patients had grade ≥ 3 AEs. 28% (95%CI 0.18–0.39, I2 = 97.4%, P = 0.000) of NHL patients had serious adverse events after subcutaneous injection of rituximab. The pooled ARR was 32% (95% CI 0.23–0.41, I2 = 97.0%, P = 0.000). I2 above is more than 50%, so random effect model is used for calculation. The results of AEs, grade ≥ 3 AEs SAE and ARR () turned out to be reliable through sensitivity analysis by the exclusion of each study one by one.
Figure 5. (A) Forest plot of AEs. (B) Forest plot of grade ≥ 3 AEs. (C) Forest plot of SAE. (D) Forest plot of ARR.
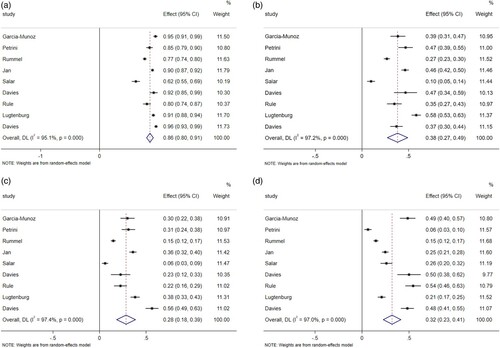
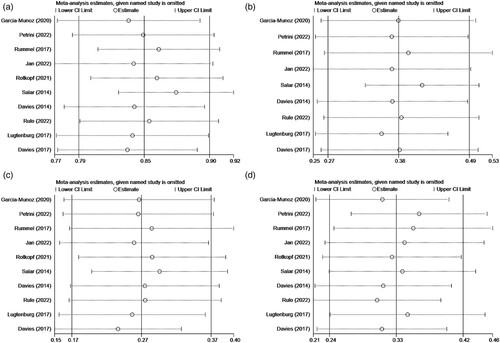
Figure 6. (A) Sensitivity analysis of AEs. (B) Sensitivity analysis of grade ≥ 3 AEs. (C) Sensitivity analysis of SAE. (D) Sensitivity analysis of ARR.
The most common AEs in NHL patients treated with SC rituximab are blood and lymphatic system disorders mainly including neutropenia, anemia, thrombocytopenia, leukopenia and febrile neutropenia, which is also the system disease with the highest incidence of grade ≥ 3 AEs, including neutropenia, leukopenia and febrile neutropenia. Gastrointestinal diseases AEs, such as nausea, constipation and vomiting, ranked next. Although these AEs had high incidence rates, the related especially serious cases rarely occurred. The main infections AEs were respiratory tract infection and urinary tract infection, and very few patients had SAEs with sepsis. Administration-related reactions are mainly asthenia, pyrexia, mucosal inflammation, injection site erythema and fatigue.
The subgroup analysis ((A)) showed that the pooled AEs was 88% (95%CI 0.84–0.93, I2 = 90.4%, P = 0.000), 90% (95%CI 0.87–0.92, I2 = 23.9%, P = 0.267) in DLBCL group and 87% (95%CI 0.80–0.95, I2 = 94.2%, P = 0.000) in FL group. There was no statistically significant heterogeneity between groups (P = 0.592), which indicates that there was no significant difference in the probability of AEs between DLBCL and FL when SC rituximab applied.
Figure 7. (A) Forest plot of subgroup analysis in AEs. (B) forest plot of subgroup analysis in grade ≥ 3 AEs. (C) forest plot of subgroup analysis in SAE. (D) forest plot of subgroup analysis in ARR.
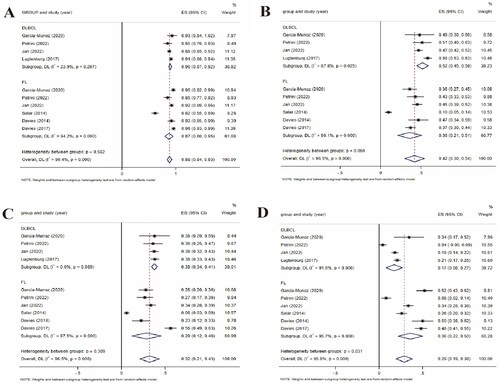
The subgroup analysis ((B)) showed that the pooled grade ≥ 3 AEs was 42% (95%CI 0.30–0.54, I2 = 96.5%, P = 0.000), 52% (95%CI 0.45–0.59, I2 = 67.8%, P = 0.025) in DLBCL group, and 36% (95%CI 0.21–0.51, I2 = 96.1%, P = 0.000) in FL group. There was no statistically significant heterogeneity between groups (P = 0.060), which indicates that there was no significant difference in the probability of grade ≥ 3 AEs between DLBCL and FL when SC rituximab applied.
The subgroup analysis ((C)) showed that the pooled grade SAEs was 32% (95%CI 0.21–0.43, I2 = 96.5%, P = 0.000), 38% (95%CI 0.34–0.41, I2 = 0%, P = 0.989) in DLBCL group, and 29% (95%CI 0.12–0.46, I2 = 97.5%, P = 0.000) in FL group. There was no statistically significant heterogeneity between groups (P = 0.309), which indicates that there was no significant difference in the probability of SAEs between DLBCL and FL when SC rituximab applied.
The subgroup analysis ((D)) showed that the pooled grade ARRs was 29% (95%CI 0.19–0.38, I2 = 95.8%, P = 0.000), 17% (95%CI 0.08–0.27, I2 = 91.6%, P = 0.000) in DLBCL group, and 36% (95%CI 0.22–0.50, I2 = 95.7%, P = 0.000) in FL group. The heterogeneity between the groups was statistically significant (P = 0.031), which indicates that the ARRs of FL was significantly higher than that of DLBCL when SC rituximab applied.
Publication bias
Egger’s tests were performed to recognize publication bias in this study. Assessment results of the pooled CR/CRu did not show significant publication bias among included studies with P = 0.172 for Egger’ test. Similarly, Egger’s test of AEs (P = 0.217), grade ≥ 3 AEs (P = 0.399) and SAEs (P = 0.129) did not find publication bias of safety outcome. Egger's test of ARRs (P = 0.013) means there was publication bias among included studies.
Discussion
As far as to our knowledge, the current study was novel in evaluating the efficacy and safety of subcutaneous rituximab in patients with NHL. With most of clinical studies being single-arm, the subcutaneous rituximab arm data on efficacy and safety were extracted and analyzed. Subcutaneous rituximab administration involves concentrating the rituximab by 12-fold to reduce the injection volume, and using recombinant human hyaluronidase as an osmotic enhancer, which enhances drug diffusion and absorption by temporarily hydrolyzing the hyaluronic acid fibers of the interstitial matrix to improve limited swelling and pain [Citation19]. Compared to IV rituximab, subcutaneous administration can simplify management, improve convenience, and reduce the incidence of serious ARRs [Citation20]. According to our research, 55% of NHL patients can achieve CR/CRu through subcutaneous rituximab injection.
As a current NCCN guideline recommended first-line treatment method for untreated DLBCL, R-CHOP can significantly improve the survival rate of many untreated DLBCL patients [Citation21], with CR of 63%–75% and PR of 7%–25% [Citation22,Citation23]. Furthermore, studies have shown that subcutaneous injection of rituximab is effective and safe for elderly patients with DLBCL [Citation23]. In a study comparing subcutaneous rituximab with intravenous rituximab as first-line treatment for follicular lymphoma [Citation17], 56.2% in the intravenous group and 50.6% in the subcutaneous group achieved CR/CRu. In studies of previously untreated and relapsed low-grade or follicular lymphoma patients treated with R-CHOP, the overall response rate was 95%, and the complete response rate was 55%. [Citation24]. A study reported that patients treated with R-CVP achieved a CR/CRu rate of 41% [Citation25]. It can be seen that there is almost no significant difference in efficacy between intravenous injection and subcutaneous injection.
From a safety perspective, the results of this study showed that while 86% of patients experienced AEs of varying degrees after receiving SC rituximab, only 38% of patients experienced ≥3-grade AEs, 28% experienced SAE, and 32% experienced ARR. Neutropenia was the most common AE and SAE. From past experience [Citation26], the use of rituximab does lead to a higher incidence of neutropenia, which increases the risk of infection and is usually associated with hypogammaglobulinemia and deeper B-cell suppression.
In terms of patient satisfaction, efficient subcutaneous injection not only makes patients more easily satisfied but also makes better use of medical resources. In a study [Citation10], patients’ satisfaction, measured by completing the RASQ questionnaire, showed that compared to IV rituximab, SC rituximab improves patients’ psychological status, impact on daily life, convenience, and overall satisfaction. In Yelvington’s study evaluating patient satisfaction in previously untreated DLBCL or FL patients, over 80% of patients preferred SC rituximab over IV rituximab. The most common reasons for this preference were shorter waiting time in the clinic (69%), more comfortable injection process (37%), and less emotional distress (29%) [Citation27]. In another study [Citation15], the average satisfaction with subcutaneous injection (89.6%) was higher than that with intravenous injection (77.4%), and 90.8% of patients claimed to prefer subcutaneous injection. Additionally, nurses expressed significantly higher satisfaction with SC rituximab regarding easier procedures, avoidance of infusion rush hour, and fewer task complications compared to intravenous injection [Citation13].
This study has some limitations. Firstly, there is high heterogeneity in most of the results, and many factors can lead to heterogeneity, such as differences among various treatment schedules, disease staging, age, and ECOG performance status. However, it is difficult to obtain complete data for subgroup analysis, and these factors are related to treatment decisions and prognosis, which further affect the results of efficacy and safety. Secondly, according to the results of Egger’s tests, there is publication bias in the data related to ARR, mainly because of the description of ARR in most experiments did not adopt a unified standard, and was subject to significant subjectivity. Thirdly, the estimated data is unreliable, and thus, the corresponding duration cannot be analyzed. Additionally, due to the inclusion of single-arm trials and lack of statistical analysis for the control group, the comparison between subcutaneous injection and other treatment methods is based on population data with baseline differences. Therefore, the results of this meta-analysis should be interpreted with caution.
For frail tumor patients, long-term treatment and exposure in hospital environments are undoubtedly a burden both on the health and safety of patients and the national medical security system. Subcutaneous rituximab can reduce the body burden caused by systemic treatment, while maintaining the safety and effectiveness of rituximab treatment, it can make valuable resources available for the treatment of other patients and save a lot of management costs for the medical system. For this reason, this study is still necessary, which can provide some theoretical support for the appropriate priority of subcutaneous injection therapy.
Conclusions
For weak tumor patients, long-term treatment and exposure in hospital environment are undoubtedly a burden on the health and safety of patients with low immunity and the national medical insurance system. Since subcutaneous injection can save more money and medical resources than intravenous injection, this study may improve the priority of subcutaneous injection in the treatment of NHL.
Author contributions
Si Tianyu and Ma Xiaolin conceptualized the study and curated the data. Si Tianyu analyzed and visualized the data. Ma Xiaolin wrote the draft. All authors contributed to the interpretation of the analysis and provided input. All authors read and approved the final manuscript.
Supplemental Material
Download Zip (3.8 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Thandra KC, Barsouk A, Saginala K, et al. Epidemiology of non-Hodgkin's lymphoma. Med Sci (Basel). 2021 Jan 30;9(1):5. doi:10.3390/medsci9010005
- Tedder TF, Engel P. CD20: a regulator of cell-cycle progression of B lymphocytes. Immunol Today. 1994 Sep;15(9):450–454. doi:10.1016/0167-5699(94)90276-3.
- Salles G, Barrett M, Foà R, et al. Rituximab in B-cell hematologic malignancies: a review of 20 years of clinical experience. Adv Ther. 2017 Oct;34(10):2232–2273. doi:10.1007/s12325-017-0612-x.
- Sehn LH, Salles G. Diffuse large B-cell lymphoma. N Engl J Med. 2021 Mar 4;384(9):842–858. doi:10.1056/NEJMra2027612.
- Carbone A, Roulland S, Gloghini A, et al. Follicular lymphoma. Nat Rev Dis Primers. 2019 Dec 12;5(1):83), doi:10.1038/s41572-019-0132-x.
- Davies A, Berge C, Boehnke A, et al. Subcutaneous rituximab for the treatment of B-cell hematologic malignancies: a review of the scientific rationale and clinical development. Adv Ther. 2017 Oct;34(10):2210–2231. doi:10.1007/s12325-017-0610-z.
- Higgins JP, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. Chichester: Wiley; 2019.
- Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003 Sep;73(9):712–716. doi:10.1046/j.1445-2197.2003.02748.x.
- Wang P X, Li H T, Liu J M. Meta-analysis of non-comparative binary outcomes and its solution by Stata. J Evid Based Med. 2012;12:52–56.
- García-Muñoz R, Quero C, Pérez-Persona E, et al. Safety of switching from intravenous to subcutaneous rituximab during first-line treatment of patients with non-Hodgkin lymphoma: the Spanish population of the MabRella study. Br J Haematol. 2020 Mar;188(5):661–673. doi:10.1111/bjh.16227.
- Petrini M, Gaidano G, Mengarelli A, et al. Safety and efficacy of subcutaneous rituximab in previously untreated patients with CD20+ diffuse large B-cell lymphoma or follicular lymphoma: results from an Italian phase IIIb study. Adv Hematol. 2022 Jan 27;2022:5581772. doi:10.1155/2022/5581772.
- Rummel M, Kim TM, Aversa F, et al. Preference for subcutaneous or intravenous administration of rituximab among patients with untreated CD20 + diffuse large B-cell lymphoma or follicular lymphoma: results from a prospective, randomized, open-label, crossover study (PrefMab). Ann Oncol. 2017 Apr 1;28(4):836–842. doi:10.1093/annonc/mdw685.
- Dürig J, Uhlig J, Gerhardt A, et al. Subcutaneous rituximab in patients with diffuse large B cell lymphoma and follicular lymphoma: final results of the non-interventional study MabSCale. Cancer Med. 2022 Aug 26. doi:10.1002/cam4.5160.
- Salar A, Avivi I, Bittner B, et al. Comparison of subcutaneous versus intravenous administration of rituximab as maintenance treatment for follicular lymphoma: results from a two-stage, phase IB study. J Clin Oncol. 2014 Jun 10;32(17):1782–1791. doi:10.1200/JCO.2013.52.2631.
- Rule S, Barreto WG, Briones J, et al. Efficacy and safety assessment of prolonged maintenance with subcutaneous rituximab in patients with relapsed or refractory indolent non-Hodgkin lymphoma: results of the phase III MabCute study. Haematologica. 2022 Feb 1;107(2):500–509. doi:10.3324/haematol.2020.274803.
- Lugtenburg P, Avivi I, Berenschot H, et al. Efficacy and safety of subcutaneous and intravenous rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in first-line diffuse large B-cell lymphoma: the randomized MabEase study. Haematologica. 2017 Nov;102(11):1913–1922. doi:10.3324/haematol.2017.173583.
- Davies A, Merli F, Mihaljević B, et al. Efficacy and safety of subcutaneous rituximab versus intravenous rituximab for first-line treatment of follicular lymphoma (SABRINA): a randomised, open-label, phase 3 trial. Lancet Haematol. 2017 Jun;4(6):e272–e282. doi:10.1016/S2352-3026(17)30078-9.
- Davies A, Merli F, Mihaljevic B, et al. Pharmacokinetics and safety of subcutaneous rituximab in follicular lymphoma (SABRINA): stage 1 analysis of a randomised phase 3 study. Lancet Oncol. 2014 Mar;15(3):343–352. doi:10.1016/S1470-2045(14)70005-1
- Frost GI. Recombinant human hyaluronidase (rHuPH20): an enabling platform for subcutaneous drug and fluid administration. Expert Opin Drug Deliv. 2007 Jul;4(4):427–440. doi:10.1517/17425247.4.4.427.
- Shpilberg O, Jackisch C. Subcutaneous administration of rituximab (MabThera) and trastuzumab (Herceptin) using hyaluronidase. Br J Cancer. 2013 Sep 17;109(6):1556–1561. doi:10.1038/bjc.2013.371.
- Hagemeister F. Rituximab for the treatment of non-Hodgkin's lymphoma and chronic lymphocytic leukaemia. Drugs. 2010 Feb 12;70(3):261–272. doi:10.2165/11532180-000000000-00000.
- Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002 Jan 24;346(4):235–242. doi:10.1056/NEJMoa011795.
- Cunningham D, Hawkes EA, Jack A, et al. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet. 2013 May 25;381(9880):1817–1826. doi:10.1016/S0140-6736(13)60313-X.
- Czuczman MS, Grillo-López AJ, White CA, et al. Treatment of patients with low-grade B-cell lymphoma with the combination of chimeric anti CD-20 monoclonal antibody and CHOP chemotherapy. J Clin Oncol. 1999;17:268–276. doi:10.1200/JCO.1999.17.1.268
- Marcus R, Imrie K, Belch A, et al. CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood. 2005 Feb 15;105(4):1417–1423. doi:10.1182/blood-2004-08-3175.
- Besada E. Rituximab og nøytropeni [Rituximab and neutropenia]. Tidsskr Nor Laegeforen. 2012 Oct 30;132(20):2259; author reply 2259. Norwegian. doi:10.4045/tidsskr.12.1147.
- Yelvington BJ. Subcutaneous Rituximab in follicular lymphoma, chronic lymphocytic leukemia, and diffuse large B-cell lymphoma. J Adv Pract Oncol. 2018 Jul-Aug;9(5):530–534.