ABSTRACT
Objectives
Acute undifferentiated leukemia (AUL) is a clinical rare leukemia with an overall poor prognosis. Currently, there are no well-established treatment guidelines for AUL, further exploration of optimal treatment options is now required.
Methods
We report an AUL patient who was complicated by a NRAS mutation and del5q was admitted to our hospital and we present the clinical features. In addition, we conducted a literature review.
Results
The “VA” scheme combines agents Venetoclax and Azacitidine that have synergistic therapeutic effect with a tolerable safety profile. There is no previous report of the “VA” scheme employed in AUL treatment. An AUL patient who was complicated by a NRAS mutation and del5q was admitted to our hospital. The “VA” scheme was administrated, and complete remission (CR) was achieved at the end of the first cycle. The patient then underwent HLA-identical sibling allogeneic hematopoietic stem cell transplantation.
Discussion
The “VA” scheme has been extensively used in AML treatment, but its application in AUL treatment has not yet been reported. This study is the first to report an AUL patient treated with the “VA” scheme and achieved CR. Our result preliminarily suggested the effectiveness and safety of the “VA” scheme in AUL treatment, but validation is required in more clinical samples. The “VA” scheme provides a new treatment option for AUL patients and deserves further clinical promotion.
Introduction
Among the acute leukemia cases, approximately 4% cannot be diagnosed as acute myeloid leukemia (AML) or acute lymphoid leukemia (ALL), and they are designated as acute leukemia of ambiguous lineage (ALAL) [Citation1]. According to the WHO classification, ALAL is classified into five subtypes: Acute Undifferentiated Leukemia (AUL); mixed phenotype acute leukemia (MPAL) with t(9; 22)(q34.1; q11.2) (or BCR-ABL1 rearrangement); MPAL with t(v;11q23.3) (or KMT2A rearrangement); MPAL, B/myeloid, NOS; MPAL, T/myeloid, NO [Citation2]. AUL does not express known lineage-specific markers and has a poor prognosis with a median overall survival (OS) time of only nine months, far lower than ALL and AML [Citation3, Citation4]. with no unified standard treatment. The ALAL treatment, including AUL, is the ALL treatment protocol during the remission induction period. Research revealed that allogeneic hematopoietic stem cell transplantation (allo-HSCT) following remission induction is an effective approach for long-term survival in AUL patients, superior to chemotherapy alone. The annual AUL incidence is 1.34 per million person-years [Citation3]; this small sample size requires further exploration for the optimal therapeutic scheme.
Venetoclax is a B-cell lymphoma-2 (BCL-2) inhibitor, while BCL-2 is an anti-apoptotic protein that plays a role in chemotherapy resistance and can inhibit mitochondria-mediated apoptosis. Azacitidine, a demethylating agent, can re-activate the tumor suppressor genes undergoing epigenetic silencing while suppressing RNA and DNA methylation, which restores cell proliferation to control levels and sensitivity toward apoptosis, exerting broad anti-leukemic effects [Citation5, Citation6]. Although venetoclax and azacitidine have synergistic effects with a tolerable safety profile [Citation7–9]. The ‘VA (Venetoclax + Azacitidine)’ scheme was not reported for use in AUL treatment. Accordingly, besides reviewing the related literature, we reported an AUL case successively treated by the ‘VA’ scheme in our hospital.
Case report
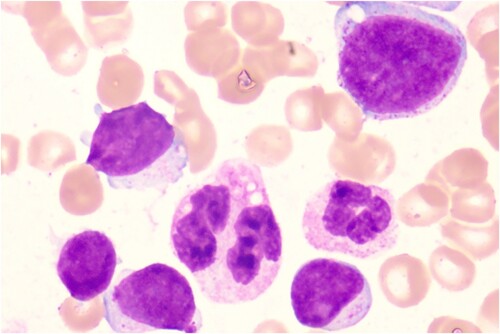
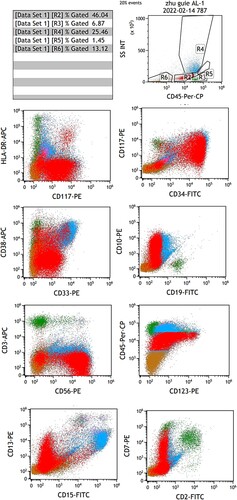
A 50-year-old female was admitted to our hospital due to fatigue. An admission routine blood test was performed after she became healthy and revealed a white blood cell (WBC) count of 11.24 × 109/L, hemoglobin (Hb) of 69 g/L, and platelet (PLT) of 29 × 109/L. Bone marrow aspiration and biopsy showed significant active proliferation of nucleated cells in bone marrow: 60.2% blasts and 9.8% primitive and naive monocytes (). Flow cytometry demonstrated 46.04% blasts expressing CD10/34/38/117/123, weakly expressing CD7/33/45, strongly expressing CD56, and not expressing HLA-DR, CD2/3/4/5/11b/11c/13/14/15/16/19/20/36/64/303, cMPO, and cCD3/79a (). Additionally, 25.46% of nucleated cells were skeletally mature granulocytes, which is highly suspicious for ALAL. Subsequently, AUL was considered. Chromosome analysis revealed a karyotype of 46, XX, del(5)(q21q35) [Citation10]. Mutation screening revealed an NRAS-exon3-41.17% (exon3.c.182A > G.p.Gln61Arg); the fusion gene test was negative. After obtaining the signed informed consent from the patient and their family, the first treatment cycle was performed: chemotherapy induction with the ‘VA’ scheme. Azacitidine (0.1 g) was given once daily on days 1–8, while venetoclax was administered as follows: 100 mg PO on day 1, 200 mg PO on day 2, 400 mg PO on days 3–11, and 100 mg PO on days 12–28. From day 12 onward, posaconazole was administrated as a preventive anti-fungal treatment due to the appearance of grade IV myelosuppression and fever. During the treatment process, the patient had a pulmonary and urinary Streptococcusmidis infection due to myelosuppression, which was then cured by anti-infection treatment. On day 17 of the VA treatment, a blood test showed a WBC count of 0.58 × 109/L, Hb of 66 g/L, PLT of 30 × 109/L, and neutrophil count of 0.35 × 109/L. Bone marrow aspiration and biopsy revealed a significant reduction in nucleated cell proliferation and blasts (17.5%) in the bone marrow, indicating good efficacy of the ‘VA’ scheme. Flow cytometry demonstrated a minimal residual disease (MRD) of 3.99%. On day 28 of the VA treatment, the blood test showed a WBC count of 1.33 × 109/L, Hb of 75 g/L, PLT of 262 × 109/L, and neutrophil count of 0.87 × 109/L. The bone marrow had further reduced nucleated cell proliferation and blasts (9.97%), and the flow cytometry demonstrated an MRD of 9.97; partial remission (PR) was achieved. On day 38 of the VA treatment, the blood test showed a WBC count of 4.12 × 109/L, Hb of 97 g/L, PLT of 292 × 109/L, and neutrophil count of 1.08 × 109/L. The nucleated cell proliferation in bone marrow was significantly reduced, with no observed blast cells; the flow cytometry demonstrated an MRD of 2.43%, and the chromosome analysis revealed a normal karyotype: 46, XX [Citation11]; CR was achieved. The second treatment cycle: azacitidine was given at 0.1 g once daily on days 1–8, while venetoclax was given at 100 mg PO on days 1–28. Meanwhile, posaconazole was administrated to elevate the blood drug concentration of venetoclax. HLA matching was performed during the treatment, and the HLA of the patient was identical to that of her sibling older sister. On the 38th day after the second treatment cycle, HLA-identical sibling allo-HSCT was performed. On day 9 of transplantation, her blood cell counts began to recover without any severe related complications, and CR was achieved in both bone marrow aspiration and routine blood tests per month after transplantation. Flow cytometry was negative for MRD, and full donor chimerism was reached. The patient is regularly followed up now.
Discussion
AUL and MPAL are two subtypes of ALAL that fail to differentiate toward a single hematopoietic lineage. AUL is clinically rare and does not express known lineage-specific markers but expresses CD34/38, TdT, HLA-DR, and less than two myeloid markers (CD13/33, CD117, or both) [Citation10, Citation12]. In the patient reported here, the myeloid marker expression was weakly positive, while peroxidase and monocyte differentiation markers (neuron-specific enolase [NSE], CD11c, CD14/64, and lysozyme) were not expressed, which was insufficient to diagnose AML. In addition, T-linage markers CyCD3 and sCD3 were negative, and B-linage marker CD19 was not expressed. Therefore, AUL was diagnosed according to the WHO classification.
AUL had no standard treatment; most research revealed that the ALL treatment protocol was superior to the AML treatment protocol for ALAL treatment, including AUL, concerning the remission rate and prognosis [Citation13–15]. Nonetheless, for a few ALAL cases with positive CD19 without other lymphoid-like features, AML treatment protocol was also recommended [Citation14]. Remission induction followed by allo-HSCT benefits more to patient survival than chemotherapy alone [Citation4, Citation10, Citation11, Citation16]. The therapeutic efficacy of ALL induction treatment for AUL was unsatisfactory, but the conclusion is not convincing due to the small sample size (n = 5) [Citation4]. Targeted AUL treatment, such as ivosidenib reported effective in AUL cases with IDH1 mutation (CR was eventually achieved) provides a new direction for AUL treatment [Citation17]. A demethylation-related gene, SET, was previously found in AUL patients; 5-azacitidine is a demethylating agent. A pediatric AUL case was reported to be irresponsive to large-dose chemotherapy but obtained clinical remission after administration with 5-azacitidine and ALL treatment protocol, and then haploid HSCT was used for consolidation treatment, obtaining good efficacy [Citation18]. which gives us much inspiration. The ‘VA’ scheme, a combination of Azacitidine and Venetoclax, has not been reported in treatment for AUL cases. However, there are reports in ALAL-NOS cases, and CR was achieved in all six reported patients receiving the ‘VA’ scheme [Citation19]. The present case was biased toward developing AML given the positive CD117 and weakly expressed CD33 at diagnosis, and thus, AML treatment protocol was administrated. Since AUL has a low response rate to conventional chemotherapy [Citation10, Citation17, Citation20], the ‘VA’ scheme was employed.
Venetoclax is an inhibitor of BCL-2, inhibiting the mitochondria-mediated apoptosis [Citation21]. Azacitidine is a demethylating agent capable of suppressing RNA and DNA methylation to induce DNA damage and decrease total protein synthesis; it can re-activate the tumor suppressor genes undergoing epigenetic silencing, thereby exhibiting broad anti-leukemic effects [Citation6]. Azacitidine and venetoclax have synergistic effects, which can reduce the amino acid uptake in leukemia stem cells (LSCs) and disrupt the tricarboxylic acid (TCA) cycle in LSCs to target and decrease the glutathionylation of succinate dehydrogenases, inhibiting electron transport chain (ETC) complex II and impeding oxidative phosphorylation. This allows for effective and selective targeting of the energy metabolism in LSCs and the eventual AML management. Venetoclax alone could not affect the oxidative phosphorylation level [Citation22]; it could directly activate T cells and enhance their cytotoxicity, while azacitidine administration could make AML cells more sensitive to their effect [Citation23]. Besides, venetoclax is easily resistant, and the recognized mechanism is overexpressing anti-apoptotic proteins belonging to the BCL-2 family (e.g. BCL-XL and MCL-1). Therefore, venetoclax should be used in chemotherapy together with other drugs. Azacitidine, which can down-regulate MCL-1, functions synergistically with venetoclax to potentiate chemotherapy efficacy [Citation8]. In a phase II trial, the overall response rate (ORR) of venetoclax alone was only 19% in AML patients who were refractory and at a high risk of relapse or unsuitable for intensive chemotherapy [Citation9]. In another phase III clinical trial, the CRi rate of azacitidine plus venetoclax was 66.4% in AML patients who had received no previous treatment and were ineligible for intensive chemotherapy, which was over two-fold higher than that in patients receiving azacitidine alone [Citation7]. Furthermore, a nonrandomized phase 1b clinical trial reported that in AML patients who were treatment-naive, older than 65 years old, and unsuitable for intensive chemotherapy, the combination of venetoclax with a hypomethylating agent (azacitidine or decitabine) contributed to a CR/CRi rate of 61% [Citation24]. Altogether, azacitidine combined with venetoclax is an excellent option for AML patients who are relapsed/refractory or not eligible for intensive chemotherapy.
Mutations in AUL commonly occur in signaling pathway genes [Citation4]. Mutations in the RAS gene, including NRAS, KRAS, and HRAS, are the most common in AML, NRAS mutation is found in 11% AML patients and critical to multiple cellular processes, such as proliferation, differentiation and apoptosis, via activating the RAS-RAF-MAPK and PI3 K signaling pathways [Citation25]. With respect to RAS mutation in hematologic diseases, targeted drugs need to be further developed [Citation26]. NRAS mutation has certain implications for AML onset, but its effect on prognosis remains elusive. NRAS-mutated AML patients tended to have a poor prognosis, short OS (overall survival) time, and low CR rate [Citation27], while pediatric patients were affected more. In contrast, NRAS mutation did not affect adult AML patient prognosis [Citation28]. Venetoclax (Ven) in combination with hypomethylating agents (HMAs) is now an effective treatment option for acute myeloid leukemia, and intensive chemotherapy with Venetoclax in patients with AML did not significantly improve the response rate of patients with RAS mutations, and combination with hypomethylating agents decreased the remission rate of such patients [Citation20, Citation21]. several reports have been published indicating that Ras mutations are often associated with venetoclax resistance (and even in some cases resistance to VA therapy). Ras mutations have been reported to mediate the upregulation of MCL1 in AML [Citation22, Citation23], and in addition RAS mutations are commonly associated with the induction of fatty acid oxidation and elimination of amino acid metabolism, leading to venetoclax resistance[Citation24]. Interestingly, despite the presence of the Ras mutation in this case, the patient responded well. The patient was diagnosed with a point mutation in the NRAS gene (exon3.c.182A > G.p.Gln61Arg), which happened in the common mutation site (Q61) of NRAS.
Cytogenetic abnormalities are found in 80–90% of AUL patients, mainly including deletion of chromosome 5q (del5q), trisomy of chromosome 13, and translocation t(9; 22)(q34; q11.2) [Citation29]. Clinical studies found that patients with del5q had a poor prognosis, with a median CR of 15 months and an OS of approximately 24.5 months [Citation29, Citation30]. Consistently, del5q was found in the patient reported here, and CR was achieved after one VA treatment cycle, showing distinct advantages of the ‘VA’ scheme.
Conclusion
The ‘VA’ scheme has been extensively used in AML treatment, but its application in AUL treatment has not yet been reported. This study is the first to report an AUL patient treated with the ‘VA’ scheme and achieved CR. Our result preliminarily suggested the effectiveness and safety of the ‘VA’ scheme in AUL treatment, but validation is required in more clinical samples. The ‘VA’ scheme provides a new treatment option for AUL patients and deserves further clinical promotion.
Authors’ contributions
Yu Cui was responsible for the literature review and writing the manuscript; Ruihua Mi, Chen Lin, and Lin Wang were responsible for providing patient data and collecting blood samples; Xudong Wei and Dongbei Li were involved in the revision of the manuscript. All authors read and agreed on the final version of the manuscript.
Consent for publication
The patient and their family signed the informed consent.
Ethical approval
The study was approved by the appropriate ethics review boards.
Acknowledgements
The authors thank the patient.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- van den Ancker W, Terwijn M, Westers TM, et al. Acute leukemias of ambiguous lineage: diagnostic consequences of the WHO2008 classification. Leukemia. 2010;24(7):1392–1396. doi:10.1038/leu.2010.119
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi:10.1182/blood-2016-03-643544
- Qasrawi A, Gomes V, Chacko CA, et al. Acute undifferentiated leukemia: data on incidence and outcomes from a large population-based database. Leukemia Res. 2020;89:106301. doi:10.1016/j.leukres.2020.106301
- Huang J, Zhou J, Xiao M, et al. The association of complex genetic background with the prognosis of acute leukemia with ambiguous lineage. Sci Rep. 2021;11(1):24290. doi:10.1038/s41598-021-03709-7
- Hollenbach PW, Nguyen AN, Brady H, et al. Triple combination of amantadine, ribavirin, and oseltamivir is highly active and synergistic against drug resistant influenza virus strains in vitro. PLoS One. 2010;5(2):e9332. doi:10.1371/journal.pone.0009332
- Stresemann C, Lyko F. Modes of action of the DNA methyltransferase inhibitors azacytidine and decitabine. Int J Cancer. 2008;123(1):8–13. doi:10.1002/ijc.23607
- DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617–629. doi:10.1056/NEJMoa2012971
- Wei AH, Strickland SA, Hou J, et al. Venetoclax combined with low-dose cytarabine for previously untreated patients with acute myeloid leukemia: results from a phase Ib/II study. J Clin Oncol. 2019;37(15):1277–1284. doi:10.1200/JCO.18.01600
- Konopleva M, Pollyea DA, Potluri J, et al. Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov. 2016;6(10):1106–1117. doi:10.1158/2159-8290.cd-16-0313
- Heesch S, Neumann M, Schwartz S, et al. Acute leukemias of ambiguous lineage in adults: molecular and clinical characterization. Ann Hematol. 2013;92(6):747–758. doi:10.1007/s00277-013-1694-4
- Duong VH, Begna KH, Kashanian S, et al. Favorable outcomes of acute leukemias of ambiguous lineage treated with hyperCVAD: a multi-center retrospective study. Ann Hematol. 2020;99(9):2119–2124. doi:10.1007/s00277-020-04179-z
- Weinberg OK, Hasserjian RP, Baraban E, et al. Clinical, immunophenotypic, and genomic findings of acute undifferentiated leukemia and comparison to acute myeloid leukemia with minimal differentiation: a study from the bone marrow pathology group. Modern Pathol. 2019;32(9):1373–1385. doi:10.1038/s41379-019-0263-3
- Gerr H, Zimmermann M, Schrappe M, et al. Acute leukaemias of ambiguous lineage in children: characterization, prognosis and therapy recommendations. Br J Haematol. 2010;149(1):84–92. doi:10.1111/j.1365-2141.2009.08058.x
- Hrusak O, de Haas V, Stancikova J, et al. International cooperative study identifies treatment strategy in childhood ambiguous lineage leukemia. Blood. 2018;132(3):264–276. doi:10.1182/blood-2017-12-821363
- Rubnitz JE, Onciu M, Pounds S, et al. Acute mixed lineage leukemia in children: the experience of St Jude Children’s Research Hospital. Blood. 2009;113(21):5083–5089. doi:10.1182/blood-2008-10-187351
- Liu QF, Fan ZP, Wu MQ, et al. Allo-HSCT for acute leukemia of ambiguous lineage in adults: the comparison between standard conditioning and intensified conditioning regimens. Ann Hematol. 2013;92(5):679–687. doi:10.1007/s00277-012-1662-4
- Patel SH, Vasu S, Guo L, et al. Molecular complete remission following ivosidenib in a patient with an acute undifferentiated leukemia. J Natl Comprehen Cancer Network. 2020;18(1):6–10. doi:10.6004/jnccn.2019.7368
- Polishchuk V, Khazal S, Berulava G, et al. 55-Azacitidine monotherapy followed by related haploidentical hematopoietic stem cell transplantation achieves durable remission in a pediatric patient with acute undifferentiated leukemia refractory to high-dose chemotherapy. Pediatr Blood Cancer. 2016;63(6):1111–1112. doi:10.1002/pbc.25948.
- Liu K, Li Y, Qiu S, et al. Efficacy of combination of venetoclax with azacitidine or chemotherapy in refractory/relapse acute leukemias of ambiguous lineage, not otherwise specified. Exp Hematol Oncol. 2021;10(1):46. doi:10.1186/s40164-021-00239-w
- Kurosawa S, Toya T, Kishida Y, et al. Outcome of patients with acute undifferentiated leukemia after allogeneic hematopoietic stem cell transplantation. Leuk Lymphoma. 2018;59(12):3006–3009. doi:10.1080/10428194.2018.1441410
- Chen X, Glytsou C, Zhou H, et al. Targeting mitochondrial structure sensitizes acute myeloid leukemia to venetoclax treatment. Cancer Discov. 2019;9(7):890–909. doi:10.1158/2159-8290.CD-19-0117
- Pollyea Stevens DA, Jones BM, Winters CL, et al. Venetoclax with azacitidine disrupts energy metabolism and targets leukemia stem cells in patients with acute myeloid leukemia. Nat Med. 2018;24(12):1859–1866. doi:10.1038/s41591-018-0233-1
- Lee JB, Khan DH, Hurren R, et al. Venetoclax enhances T cell-mediated antileukemic activity by increasing ROS production. Blood. 2021;138(3):234–245. doi:10.1182/blood.2020009081
- DiNardo CD, Pratz KW, Letai A, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19(2):216–228. doi:10.1016/S1470-2045(18)30010-X
- Kadia TM, Kantarjian H, Kornblau S, et al. Clinical and proteomic characterization of acute myeloid leukemia with mutated RAS. Cancer. 2012;118(22):5550–5559. doi:10.1002/cncr.27596
- Desikan SP, Ravandi F, Pemmaraju N, et al. A phase II study of azacitidine, venetoclax, and trametinib in relapsed or refractory acute myeloid leukemia harboring RAS pathway-activating mutations. Acta Haematol. 2022;145(5):529–536. doi:10.1159/000525566
- Park J, Park H, Byun JM, et al. Pan-RAF inhibitor LY3009120 is highly synergistic with low-dose cytarabine, but not azacitidine, in acute myeloid leukemia with RAS mutations. Oncol Lett. 2021;22(5):745. doi:10.3892/ol.2021.13006
- Liu X, Ye Q, Zhao XP, et al. RAS mutations in acute myeloid leukaemia patients: A review and meta-analysis. Clin Chim Acta. 2019;489:254–260. doi:10.1016/j.cca.2018.08.040
- Manola KN. Cytogenetic abnormalities in acute leukaemia of ambiguous lineage: an overview. Br J Haematol. 2013;163(1):24–39. doi:10.1111/bjh.12484
- Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209–2221. doi:10.1056/NEJMoa1516192