ABSTRACT
Objective
To investigate efficacy and prognostic factors in the treatment of adult newly-diagnosed acute myeloid leukemia (AML) with or without allogeneic hematopoietic stem cell transplantation (Allo-HSCT).
Methods
We retrospectively analyzed 668 patients with newly-diagnosed AML (non-M3 type) in the Department of Hematology at Shanghai Changhai Hospital from January 2012 to December 2021. Based on different induction chemotherapy regimens, patients were categorized into an IA (idarubicin, IDA + cytarabine, Ara-C) (3 + 7, regimen) group (n = 303) and a DA (daunorubicin, DNR + cytarabine, Ara-C) (3 + 7, regimen) group (n = 365) with or without allo-HSCT. Minimal residual disease (MRD), complete response (CR), overall response rate (ORR), progression-free survival (PFS), overall survival (OS), and adverse effects (AE) were analyzed and compared. Characteristics significantly associated with overall or progression-free survival (OS or PFS) upon univariate analysis were subsequently included in a Cox proportional hazard model.
Results
This study used data from 668 AML patients. After induction therapy, the CR rate in the IA group was 70.63% and ORR was 79.87%, which were significantly higher than those in the DA group (with a CR rate of 56.99% and an ORR of 70.14%) (P = 0.0002 and P = 0.0035, respectively). There were no significant differences in drug safety between the two chemotherapy regimens used in IA and DA (P > 0.05). The recurrence rate was lower in patients with an MRD < 0.001 than in patients with an MRD ≥ 0.001. A continuous negative MRD during the period is significant because it is associated with prolonged OS and PFS of AML patients. Data from 100 patients in the two groups who underwent allo-HSCT were analyzed using univariate analysis and the Cox proportional hazards model. From the multivariate analysis, MRD was found to be the only independent predictor of OS (P = 0.042; HR 1; 95%CI 0.00–0.76).
Conclusion
In the treatment of adult AML patients, IA regimen is associated with a high CR rate and ORR rate and does not increase treatment-related toxicity. IA regimen prolongs OS and PFS in AML patients and reduces the likelihood of leukemia cells’ subsequent infiltration into the central nervous system. There is a high correlation between the level of MRD after treatment and the patient's bone marrow recurrence. To obtain superior treatment effects for patients undergoing allo-HSCT, the MRD should be reduced to less than 0.001 before pretreatment. A negative MRD before allo-HSCT can prolong OS in patients with AML. We examined the clinical characteristics and outcomes of AML patients in China, finding novel information on prognostic factors and primary treatment of AML that may be applicable in routine clinical practice.
Introduction
Acute myeloid leukemia (AML) is a heterogeneous hematologic malignancy involving progenitor cell clonal expansion in peripheral blood and bone marrow. The molecular and cytogenetic mechanisms of its occurrence and development are very complex. It is characterized by immature medulla, cell clumping, incomplete differentiation, and extensive proliferation [Citation1]. The etiology of AML is known to be related to ionizing radiation, chemical poisons, viral infections, regional environmental factors, and unhealthy lifestyle habits. AML is generally believed to be caused by genetics and environmental factors. The clinical symptoms of patients in the early stage of the disease lack typicality. As the disease develops, clinical manifestations include anemia, bleeding, infection, organ infiltration, and abnormal metabolism, resulting in poor prognosis [Citation2]. Therefore, optimizing treatment strategies for adult AML patients is crucial for improving the prognosis of AML patients.
With the development of clinical trials for AML, more treatment options have been developed to treat the disease, but each treatment option has advantages and disadvantages. However, anthracyclines combined with cytarabine comprise the classic regimen for AML treatment that is still used currently [Citation3]. Idarubicin (IDA) is a type of anthracycline, which can replace daunorubicin (DNR) in the treatment of AML and ALL [Citation4]. The IA chemotherapy regimen (consisting of IDA combined with Ara-C) has good efficacy in the treatment of newly-diagnosed AML patients. Currently, most of the evaluation of the efficacy of the IA regimen in AML patients comes from the retrospective summary of a small sample collected from a single medical center and the personal experience of clinicians [Citation5]. Given the above reasons, this study utilized a large sample of newly diagnosed AML patients and deeply analyzed the treatment response and long-term efficacy of IA and DA chemotherapy regimens in newly-diagnosed AML patients with or without allo-HSCT.
Patients and methods
Patients
This study retrospectively analyzed data (collected from January 2012 to December 2021) from 668 newly-diagnosed adult AML patients from the Department of Hematology at Changhai Hospital. The following inclusion criteria were employed: (1) All the enrolled patients met the diagnostic criteria for AML in the ‘Criteria for Diagnosis and Efficacy of Blood Diseases’ [Citation6]. Among them, 100 allo-HSCT patients (43 cases in the IA group and 57 cases in the DA group) had characteristics that matched the timing of transplantation and donor selection in the ‘Chinese Expert Consensus on Allogeneic Hematopoietic Stem Cell Transplantation for Hematological Diseases (Ⅲ)’ [Citation7]; (2) they were older than 18 years; (3) had an Eastern Cooperative Oncology Group (ECOG) physical performance status score of 0-2; (4) were assessed to be able to tolerate DA/IA chemotherapy; (5) had liver and kidney functions were normal and possessed cardiac function grades I to II; (6) and had complete clinical data. The following exclusion criteria were employed: (1) AML co-occurred with mental disorders, organic diseases, or severe autoimmune system diseases; (2) patients had other malignant tumors, cognitive dysfunction, or pregnancy and lactation; (3) or they had M3 AML, relapsed AML, or had previously received AML therapy. There was no statistical difference in general data between the IA and DA groups (P > 0.05) as depicted in .
Table 1. Patient population.
Data collection
Induction and consolidation treatment methods employed for the DA and IA groups: Before treatment, the two groups underwent relevant examinations, the patients’ physical conditions were assessed, and appropriate chemotherapy regimens were selected based on the patients’ economic ability. The DA group was treated with 45-60 mg/m2/d of DNR (Shenzhen Wanle Pharmaceutical Co., Ltd., National Medicine Zhunzi H44024361, specification: 20 mg) on days 1–3 and 100 mg/m2/d of Ara-C (Harbin Labotong Pharmaceutical Co., Ltd., Chinese medicine Zhunzi H23021805, specification: 0.1 g) on days 1-7. Group IA was treated with 10-12 mg/m2/d of IDA (Hisun Pfizer Pharmaceutical Co., Ltd., H20050145, specification: 5 mg) on days 1–3 and 100 mg/m2/d of Ara-C on days 1-7. After one course of treatment, if PR or blast cell decline was greater than 60%, the original regimen was repeated for one course. If CR was not achieved after the second course of treatment, the treatment was considered ineffective and other chemotherapy regimens were used. Patients who had a CR continued to use the original regimen for another course of treatment to consolidate the first course of treatment. After remission, all patients were treated with 4-6 g/m2/d of high-dose Ara-C (Harbin Labotong Pharmaceutical Co., Ltd., H23021805, specification: 0.1 g) on days 1-3, for 3–4 courses, to consolidate the treatment. Both groups completed a follow-up at 36 months after treatment.
MRD analysis and detection: Standard IA or DA regimens were used to induce remissions in patients. When CR was achieved in the bone marrow, to consolidate, one course of treatment of the original treatment regimen or high-dose cytarabine chemotherapy was used. After CR was achieved in their bone marrow, 100 patients underwent allogeneic hematopoietic stem cell transplantation (Allo-HSCT). MRD was detected using multiparameter flow cytometry (MFC) in 218 patients (comprising 92 in the IA group and 126 in the DA group). MFC monitoring of MRD is usually based on the analysis of LAIPs. After the patients had been treated until CR was achieved and the induction of remission, samples of the patients’ bone marrow were extracted for examination using flow cytometry. For patients undergoing regular consolidation chemotherapy, MRD levels were monitored every 1–3 months. MRD was considered positive when the detected MRD level was greater than 0.1%; otherwise, MRD was considered negative [Citation8].
Statistical analysis
Statistical analyses were performed using SPSS version 23 (SPSS Inc., Chicago, IL, USA). Overall survival (OS) was defined as the time from initial diagnosis until death from any cause, while progression-free survival (PFS) was defined as the time from diagnosis to disease recurrence, disease progression, or death. Categorical variables were presented as numbers and percentages, and they were compared using the Chi-squared test. Survival (both OS and PFS) was calculated using the Kaplan–Meier method. Subsequently, a confirmatory univariate Cox analysis was performed. The significance level was set at a threshold of P < 0.05. A multivariate proportional hazard model was created for characteristics exhibiting a trend toward statistical significance (P < 0.05) that were found to be associated with OS or PFS within both univariate approaches. A 2-tailed P-value less than 0.05 was considered statistically significant.
Results
Comparison of the therapeutic effect in two groups
After induction therapy in the IA group, the complete response (CR) rate was 70.63%, the partial remission (PR) rate was 9.24%, and the overall response rate (ORR) was 79.87%. After induction therapy in the DA group, the CR rate was 56.99%, the PR rate was 13.15%, and the ORR was 70.14%. The CR rate and ORR of patients in the IA group were higher than those in the DA group (P = 0.0002, P = 0.0035) as displayed in .
Table 2. Treatment response of IA and DA group.
Comparison of remission rates in the two groups
Grouped by cytogenetic risk, the CR rates among the three groups showed a decreasing trend from low, medium, to high risk, but the difference was not statistically significant (P > 0.05). When grouped by comprehensive prognostic risk, the CR rate and ORR of patients with good prognosis in the IA group were higher than those in the DA group, and there was no significant difference in ORR between the two groups in moderate and poor prognosis (P > 0.05) as displayed in .
Table 3. Response rate of IA and DA groups according to cytogenetic.
Comparison of treatment effects in the two groups of patients
Considering the age of the patients, with 60 years of age as the boundary, 261 patients in the IA group were <60 years old, and 340 patients in the DA group were <60 years old. The CR rate and ORR of patients who were <60 years old in the IA group were higher than those in the DA group who were <60 years old (P < 0.05). However, the CR rate and ORR of patients aged ≥60 years in both the IA and the DA groups were not statistically significant (P > 0.05) as shown in .
Table 4. Response rate of IA and DA groups according to age.
Comparison of efficacy in the two groups based on FAB classification
The efficacy of the M1M2 subgroup and the M4M5 subgroup was compared using the FAB classification. In the IA group, there were 107 patients with the M1 + M2 subtype and 181 patients with the M4 + M5 subtype. Additionally, there were 142 patients with the M1 + M2 subtype and 207 patients with the M4 + M5 subtype in the DA group. After induction therapy in the M1 + M2 subtype of patients, the CR rate and ORR of the IA group were higher than those of the DA group (P < 0.05). The CR rate of the M4 + M5 subtype of patients in the IA group was higher than that in the DA group (P < 0.05), but there was no significant change in ORR in the DA group (P > 0.05) as shown in .
Table 5. Response rate of IA and DA groups according to FAB classification.
Comparison of therapeutic effects in the two groups of patients after different induction remission courses
Using the number of treatment courses that patients had while taking a single course of treatment as the boundary, the CR rate after the first course of treatment in the IA group was higher than that in the DA group (P < 0.05). After the second course of treatment, there was no significant difference in the CR rate and ORR between the IA and DA groups (P > 0.05) as shown in .
Table 6. Response rate of IA and DA groups according to different induction remission courses.
Comparison of adverse effects in the two groups of patients
During induction therapy for remission in the IA and DA groups, the main adverse effects that were observed were bone marrow suppression, pulmonary infection, upper respiratory tract infection, fungal infection, and cardiotoxicity, but there were no statistically significant differences between the two groups (P > 0.05). However, the incidence of the infiltration of leukemia cells into the central nervous system in the IA group was lower than that in the DA group (P < 0.05) as shown in .
Table 7. Comparison of side effects of IA and DA groups.
Comparison of the prognosis of the two groups of patients
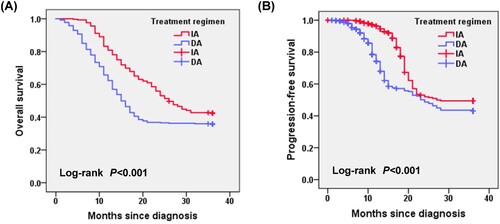
Patients in the IA group, when compared with those in the DA group, had a better OS (26.30 months vs. 15.68 months, P = 0.007) and PFS (19.16 months vs. 13.82 months, P = 0.028; A, B).
Comparison of OS and PFS between the two groups in patients with CR after the second course of treatment
The median OS of patients in the IA group who achieved CR after the second course of treatment was 25.50 months, and that of patients in the DA group was 16.38 months (P = 0.0119; A). The median PFS of patients in the IA group who achieved CR after the second course of treatment was 17.27 months and that of patients in the DA group was 10.78 months (P = 0.0128; B).
Comparison of MRD and prognosis of the two groups of AML patients
In the DA group, 126 patients achieved CR after induction, while 92 patients achieved CR after induction in the IA group. After induction therapy to achieve CR in the DA group, the average MRD-positive cell level was 1.35% (range: 0–3.78%), 80 patients (63.49%) were MRD-positive, and 46 patients (36.51%) were MRD-negative. After induction in the IA group, the average MRD-positive cell level of patients was 0.93% (range: 0–3.76%), 49 patients (53.26%) were MRD-positive, and 43 patients (46.74%) were MRD-negative. The 2 groups of patients received 2 courses of consolidation chemotherapy after CR before MRD evaluation. After consolidation therapy, the average MRD-positive cell level in the DA group was 0.62% (range: 0–3.69%), 59 patients (46.83%) were MRD-positive, and 67 patients (53.17%) were MRD-negative. After consolidation therapy, the average MRD-positive cell level among patients in the IA group was 0.31% (range: 0–3.65%), 36 patients (39.13%) were MRD positive, and 56 patients (60.87%) were MRD negative, as shown in .
Table 8. The number of MRD positive cases and clinical recurrences in the DA and IA groups.
In the IA and DA groups, regardless of gender, age, and the presence of refractory AML, the number of recurrences in patients with MRD < 0.001 was less than that in patients with MRD ≥ 0.001, but there was no significant difference in the recurrence rates of the two groups (P > 0.05) as shown in .
Table 9. The relationship between recurrence and MRD in the DA and IA groups.
Furthermore, a total of 43 patients relapsed in the DA group, including 2 patients who relapsed within 6 months, 20 patients who relapsed within 7–12 months, and 21 patients who relapsed after 12 months. The PFS of patients with MRD < 0.001 in the DA regimen group was 16.5 months, while the PFS of patients with MRD ≥ 0.001 was 6.3 months. However, in the IA group, there were 21 patients who experienced recurrence; among them, 10 patients experienced recurrence within 7–12 months, and 11 patients experienced recurrence after 12 months. The progression-free survival (PFS) of patients with MRD < 0.001 in the IA regimen group was 22.5 months, while the PFS of patients with MRD ≥ 0.001 was 14.0 months as shown in .
Table 10. The relationship between recurrence time and MRD in the DA and IA groups.
Comparison of prognosis after allogeneic hematopoietic stem cell transplantation between two groups of patients
Firstly, there were no significant statistical differences in gender, age, donor type, pre-transplantation conditioning regimen, and MRD between the IA and DA groups (P > 0.05) as shown in . A univariable log-rank test was applied to compare the prognostic value for achieving PFS and OS (the test level set P at 0.1 in univariate analysis). Findings from univariate analysis (P<0.1) indicated that the pre-transplantation conditioning regimen, donor type, and MRD predicted PFS and OS (). Characteristics found to be significantly associated with OS upon univariate analysis underwent subsequent confirmatory multivariate Cox regression analysis (). The MRD was found to predict OS (P = 0.042; HR 1; 95% CI 0.00-0.76).
Table 11. Comparison of general conditions of patients after allo-HSCT in the DA and IA groups.
Table 12. Univariate analysis of prognostic factors for overall survial and progression-free survival.
Table 13. Multivariate Cox regression analysis of prognostic factors for OS.
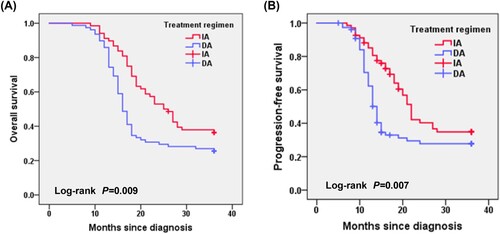
After transplantation, there were 71 patients with MRD < 0.01 in the two groups. All patients did not die or relapse during the follow-up period, so the median OS of the IA and DA groups could not be estimated. In the subgroup with MRD ≥ 0.01, the median OS of patients in the IA group was longer than in the DA group (13 months vs. 11 months, P = 0.043). The median PFS of patients in the IA group was also longer than in the DA group (10 months vs. 8 months, P = 0.012) as shown in .
Table 14. Comparison of OS and PFS after transplantation.
Discussion
AML is a malignant cancer of the blood system that originates in the bone marrow and can invade important organs throughout the body as the disease develops. The incidence of AML increases with age, and the average age of onset is 67 years. At present, although considerable progress has been made in the treatment of AML, some adults exhibit poor tolerance and reduced sensitivity to chemotherapy due to complicated organ dysfunction, resulting in limited choices of chemotherapy regimens for adult AML patients and a poor prognosis. Data reports show that compared with young patients, AML patients over 60 years old experience more adverse side effects from the use of chemotherapy drugs because they have more chromosomal abnormalities and gene mutations; as a result, their approximate survival time is only around one year [Citation9, Citation10]. Moreover, AML in adults aged ≥ 60 years is associated with inferior outcomes in comparison with younger patients [Citation11–13]. Allogeneic hematopoietic cell transplantation (HCT) is the only curative treatment option for many older patients with AML, but prognosis after transplant in this age group is limited [Citation14, Citation15]. Despite concerted efforts to improve treatment outcomes over the past few decades, the outcomes for AML patients remain unsatisfactory. For 40 years old newly-diagnosed AML patients, the 7 + 3 regimens (ie, cytarabine infused continuously for 7 days with three once-daily injections of an anthracycline) have been a standard for AML induction therapy [Citation16, Citation17]. After the administration of anthracycline for 3 days and cytarabine for 7 days (commonly referred to as the ‘3 + 7’ regimen), CR was achieved in 60%–80% of younger adults [Citation18, Citation19].
Idarubicin is a known antineoplastic drug, whose mechanism primarily consists of topo2 inhibition and its actions as a DNA intercalating agent that impairs cellular DNA replication and subsequent RNA synthesis [Citation20]. In this study, after induction therapy, the CR rate was 70.63% and the ORR was 79.87% in the IA group, which were significantly higher than those in the DA control group (the CR rate was 56.99% and the ORR was 70.14%) (P = 0.0002, P = 0.0035). In the randomized French Acute Leukemia Association (ALFA) 9801 trial, high-dose daunorubicin (80 mg/m2 /d for 3 days) or idarubicin (12 mg/m2 /d for 4 days [IDA4]) was compared with standard-dose idarubicin (12 mg/m2 /d for 3 days [IDA3]) for remission induction in older patients with AML (age 50–70 years). In the study, CR rates were found to be significantly different among the three treatment groups (IDA3, IDA4, and daunorubicin: 83%, 78%, and 70%, respectively; P = 0.04), but no significant differences were observed regarding relapse incidence, EFS, or OS [Citation21]. However, one clinical trial (NCT 01145846) from the Republic of Korea, which was a phase III trial comparing idarubicin (12 mg/m2 /d for 3 days) with high-dose daunorubicin (90 mg/m2 /d for 3 days), did not reveal significant differences in CR rates and survival between the two treatment groups [Citation22].
Through age-stratified studies, it was found that among patients under 60 years of age, the CR and PR rates in the IA group were higher than those in the DA group, indicating that the IA regimen may be more effective for young and middle-aged AML patients. The difference in effectiveness may be explained by the fact that elderly patients have worse immunity and poor tolerance of chemotherapy, so there is minimal difference in the efficacy of the two regimens in elderly AML patients. To explore the relationship between the induction remission rate and the course of treatment, this study examined the CR rate of the patients after induction of remission using the IA/DA regimen for different courses of treatment. The CR rate after the first course of treatment in the IA group was found to be significantly higher than that in the DA group. This result suggests that the CR rate associated with the first course of treatment in AML induction therapy has a significant impact on the long-term prognosis of patients. The IA regimen has the advantage of a higher CR rate after one course of treatment when compared with the DA regimen.
Although the two regimens have similar levels of severe adverse events, compared with the DA regimen, the IA regimen is likely to achieve better clinical efficacy in the treatment of AML. In a study, 299 patients (149 patients randomly assigned to cytarabine plus idarubicin (12 mg/m2 /d for 3 consecutive days) and 150 patients assigned to cytarabine plus high-dose daunorubicin (90 mg/m2 /d for 3 consecutive days) were analyzed. All patients received cytarabine (200 mg/m2 /d for 7 days). The frequency of severe adverse events was similar in the AI and AD treatment groups [Citation22]. In our study, the IA regimen was found to prolong the OS and PFS of AML patients and to reduce the subsequent infiltration of leukemia cells into the central nervous system. Because idarubicin is fat-soluble and it can penetrate the blood–brain barrier, it exerts an early clearance effect on leukemia cells in the central nervous system.
There is a high correlation between the level of measurable residual disease (MRD) after treatment and the patient's bone marrow recurrence. Detection of the MRD level can help clinicians to choose a more appropriate treatment plan to improve treatment outcomes. Our study identified MRD levels assessed using flow cytometry (FCM) to be a powerful predictor of outcomes for AML patients. In addition to predicting relapse, MRD levels are of great value in assessing OS and PFS. This study found that patients with MRD < 0.001 had lower relapse rates compared with patients with MRD ≥ 0.001 regardless of sex, age, and treatment-resistant status. For patients undergoing allo-HSCT to obtain better therapeutic effects, it was established that the MRD of the patients should be made less than 0.001 before pretreatment. Press et al. reported that the cumulative incidence of post-transplant leukemic relapse was significantly higher in the pre-transplant NGS MRD-positive (vs. MRD-negative) subjects (P = 0.014). After adjusting for the effect of TP53 mutation and transplant conditioning regimen, NGS MRD-positivity retained independent prognostic significance for leukemic relapse (P = 0.05). The pre-transplant NGS MRD-positive subjects also had a significantly shortened PFS (P = 0.038), and marginally shortened OS (P = 0.068). In patients with AML undergoing HSCT, the pre-transplant persistence of NGS-defined MRD imparts a significant, sensitive, strong, and independent increased risk for subsequent leukemic relapse and death [Citation23]. Dillon et al. analyzed pre-transplant blood and bone marrow samples using reverse-transcription polymerase chain reaction in 107 patients with NPM1-mutant AML. After a median follow-up of 4.9 years, patients with negative, low, and high levels of MRD had an estimated 2-year overall survival rate of 83%, 63%, and 13%, respectively (P < 0.0001) [Citation24]. The strength of this analysis lies in its large cohort. However, it is limited by its single-center and retrospective nature. Certain deviations need to be explored further in future studies.
In conclusion, this large retrospective study has revealed that compared with the use of a DA regimen, the use of an IA regimen in the treatment of adult newly-diagnosed AML is associated with a higher remission rate and lower treatment-related toxicity. The IA program can prolong the OS and PFS of AML patients and reduce the subsequent infiltration of leukemia cells into the central nervous system. The MRD level after treatment is highly correlated with recurrence in a patient's bone marrow. For patients undergoing allo-HSCT, to achieve a better treatment effect, it is recommended that the patient's MRD be negative before pretreatment. AML patients receiving the IA regimen benefit through improved OS and PFS following allo-HSCT.
Author contributions
J.D. and Y.S. carried out material preparation, participated in data collection and analysis, participated in the design of the study. Y.S. also drafted and revised the manuscript. Y.Ruan. contributed to the study conception and design. N.L., Q.Meng., and J.Yang. participated in statistical analysis. C.L. and L.C. conceived of the study, designed the study, coordinated the study, and helped draft the manuscript. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability
The original datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Willier S, Rothämel P, Hastreiter M, et al. CLEC12A and CD33 coexpression as a preferential target for pediatric AML combinatorial immunotherapy. Blood. 2021;137(8):1037–1049.
- Katagiri T, Ushiki T, Masuko M, et al. Successful 5-azacytidine treatment of myeloid sarcoma and leukemia cutis associated with myelodysplastic syndrome: a case report and literature review. Medicine (Baltimore). 2017;96(36):e7975.
- Harada K, Doki N, Hagino T, et al. Underweight status at diagnosis is associated with poorer outcomes in adult patients with acute myeloid leukemia: a retrospective study of JALSG AML 201. Ann Hematol. 2018;97(1):73–81.
- DiNardo CD, Lachowiez CA, Takahashi K, et al. Venetoclax combined with FLAG-IDA induction and consolidation in newly diagnosed and relapsed or refractory acute myeloid leukemia. J Clin Oncol: Official J Am Soc Clin Oncol. 2021;39(25):2768–2778.
- Xiang HL, Chen Y, Wang JW, et al. Enhancing cytotoxicity of daunorubicin on drug-resistant leukaemia cells with microparticle-mediated drug delivery system. J Microencapsul. 2019;36(3):291–304.
- Aldoss I, Yang D, Aribi A, et al. Efficacy of the combination of venetoclax and hypomethylating agents in relapsed/refractory acute myeloid leukemia. Haematologica. 2018;103(9):e404–e407. doi:10.3324/haematol.2018.188094
- Xu L, Chen H, Chen J, et al. Thrombospondin-2 promotes prostate cancer bone metastasis by the up-regulation of matrix metalloproteinase-2 through down-regulating miR-376c expression. J Hematol Oncol. 2017;10(1):33, doi:10.1186/s13045-017-0390-6
- Heuser M, Freeman SD, Ossenkoppele GJ, et al. Update on MRD in acute myeloid leukemia: a consensus document from the European LeukemiaNet MRD Working Party. Blood. 2021;138(26):2753–2767.
- Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447.
- Pogosova-Agadjanyan EL, Moseley A, Othus M, et al. AML risk stratification models utilizing ELN-2017 guidelines and additional prognostic factors: a SWOG report. Biomark Res. 2020;8:29.
- Mrózek K, Marcucci G, Nicolet D, Maharry KS, Becker H, Whitman SP, Metzeler KH, Schwind S, Wu YZ, Kohlschmidt J et al: Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alterations in adults with acute myeloid leukemia. J Clin Oncol: Official J Am Soc Clin Oncol. 2012, 30(36):4515-4523.
- Goldstone AH, Burnett AK, Wheatley K, et al. Attempts to improve treatment outcomes in acute myeloid leukemia (AML) in older patients: the results of the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98(5):1302–1311. doi:10.1182/blood.V98.5.1302
- Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica. 2012;97(12):1916–1924.
- Devine SM, Owzar K, Blum W, et al. Phase II study of allogeneic transplantation for older patients with acute myeloid leukemia in first complete remission using a reduced-intensity conditioning regimen: results from cancer and leukemia group B 100103 (alliance for clinical trials in oncology)/blood and marrow transplant clinical trial network 0502. J Clin Oncol: Official J Am Soc Clin Oncol. 2015;33(35):4167–4175. doi:10.1200/JCO.2015.62.7273
- Ustun C, Le-Rademacher J, Wang HL, et al. Allogeneic hematopoietic cell transplantation compared to chemotherapy consolidation in older acute myeloid leukemia (AML) patients 60-75 years in first complete remission (CR1): an alliance (A151509), SWOG, ECOG-ACRIN, and CIBMTR study. Leukemia. 2019;33(11):2599–2609.
- Rai KR, Holland JF, Glidewell OJ, et al. Treatment of acute myelocytic leukemia: a study by cancer and leukemia group B. Blood. 1981;58(6):1203–1212. doi:10.1182/blood.V58.6.1203.1203
- Yates JW, Wallace HJ, Jr., Ellison RR, et al. Cytosine arabinoside (NSC-63878) and daunorubicin (NSC-83142) therapy in acute nonlymphocytic leukemia. Cancer Chemother Rep. 1973;57(4):485–488.
- Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med 2015;373(12):1136–1152.
- Dombret H, Gardin C. An update of current treatments for adult acute myeloid leukemia. Blood. 2016;127(1):53–61.
- Hou HY, Lu WW, Wu KY, et al. Idarubicin is a broad-spectrum enterovirus replication inhibitor that selectively targets the virus internal ribosomal entry site. J Gen Virol 2016;97(5):1122–1133. doi:10.1099/jgv.0.000431
- Pautas C, Merabet F, Thomas X, et al. Randomized study of intensified anthracycline doses for induction and recombinant interleukin-2 for maintenance in patients with acute myeloid leukemia age 50 to 70 years: results of the ALFA-9801 study. J Clin Oncol: Official J Am Soc Clin Oncol. 2010;28(5):808–814. doi:10.1200/JCO.2009.23.2652
- Lee JH, Kim H, Joo YD, et al. Prospective randomized comparison of idarubicin and high-dose daunorubicin in induction chemotherapy for newly diagnosed acute myeloid leukemia. J Clin Oncol: Official J Am Soc Clin Oncol. 2017;35(24):2754–2763. doi:10.1200/JCO.2017.72.8618
- Press RD, Eickelberg G, Froman A, et al. Next-generation sequencing-defined minimal residual disease before stem cell transplantation predicts acute myeloid leukemia relapse. Am J Hematol 2019;94(8):902–912.
- Dillon R, Hills R, Freeman S, et al. Molecular MRD status and outcome after transplantation in NPM1-mutated AML. Blood. 2020;135(9):680–688. doi:10.1182/blood.2019002959