ABSTRACT
Objectives
T-cell acute lymphoblastic leukemia/lymphoblastic lymphoma (T-ALL/LBL) are highly malignant and aggressive hematologic tumors for which there is no standard first-line treatment. Chidamide, a novel histone deacetylase inhibitor, shows great promise. We assessed the efficacy and safety of an irradiation-containing conditioning regimen for allogeneic hematopoietic stem cell transplantation (allo-HSCT) and post-transplantation chidamide maintenance in patients with T-ALL/LBL.
Methods
We retrospectively analyzed the clinical data of six patients with T-ALL/LBL who underwent allo-HSCT with a radiotherapy-containing pretreatment regimen and post-transplant chidamide maintenance therapy. The endpoints were relapse, graft-versus-host disease (GVHD), transplant-related mortality (TRM), progression-free survival (PFS), overall survival (OS), and adverse events (AEs).
Results
All of the patients had uneventful post-transplant hematopoietic reconstitution, and all achieved complete molecular remission within 30 days. All six patients survived, and two relapsed with a median relapse time of 828.5 (170–1335) days. The 1-year OS rate was 100%, the 2-year PFS rate was 66.7%, and the TRM rate was 0%. After transplantation, two patients developed grade I-II acute GVHD (2/6); grade III-IV acute and chronic GVHD were not observed. The most common AEs following chidamide administration were hematological AEs, which occurred to varying degrees in all patients; liver function abnormalities occurred in two patients (grade 2), and symptoms of malaise occurred in one patient (grade 1).
Conclusion
Chidamide maintenance therapy after T-ALL/LBL transplantation is safe, but the efficacy needs to be further investigated.
1. Introduction
Both T-cell acute lymphoblastic leukemia (T-ALL) and T-cell lymphoblastic lymphoma (T-LBL) originate from T-lymphocyte precursor cells; they differ only in the extent of bone marrow blast cell involvement [Citation1] and manifest the same clinical symptoms during their different stages. T-ALL and T-LBL are classified as the same entity, T-ALL/LBL, in accordance with the 2016 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia [Citation2]. T-ALL/LBL comprise a group of highly heterogeneous hematolymphoid malignancies.
There has been progress in T-ALL/LBL treatment over the past few decades. The complete remission (CR) rate in adult patients can reach 90% or higher; however, long-term survival is still unsatisfactory [Citation3–5]. Currently, allogeneic hematopoietic stem cell transplantation (allo-HSCT) is considered the only potentially curative therapy [Citation6–8]. Irradiation-containing conditioning regimens such as total body irradiation (TBI) and total marrow irradiation/total marrow and lymphoid irradiation (TMI/TMLI) may improve overall survival (OS) [Citation9,Citation10]. However, maintenance therapy after transplantation remains an urgent issue owing to the risk of relapse. Since its introduction in 2014, chidamide, a histone deacetylate inhibitor (HDACi), has achieved satisfactory results for relapsed/refractory peripheral T-cell lymphoma [Citation11]. Chidamide also has an antitumor effect on T-ALL/LBL in preclinical research [Citation12,Citation13]. Here, we conducted a retrospective study on the efficacy and safety of an irradiation-containing conditioning regimen for allo-HSCT and post-transplantation chidamide maintenance therapy in patients with T-ALL/LBL.
2. Materials and methods
2.1. Clinical data
The clinical data of patients with T-ALL/LBL who received an irradiation-containing conditioning regimen for allo-HSCT and chidamide maintenance after transplantation were collected. T-ALL/LBL was diagnosed according to the 2016 revision of the WHO classification of myeloid neoplasms and acute leukemia [Citation14], and risk stratification was based on the study by Gökbuget and Goldstone [Citation15,Citation16]. Patients were classified as having high-risk disease if any of the following criteria were met: age ≥35 years; leukocytes >100 × 109/L; ETP-ALL or mature T-ALL immunophenotype; and poor therapeutic response (time to CR >4 weeks or persistent minimal residual disease [MRD] greater than 1 × 10−4). Patients were considered to be at standard risk if none of these factors were present.
2.2. Therapeutic regimen
2.2.1. Initial induction therapy and transplantation regimen
The induction regimens were the VDCLP, hyper-CVAD, and P-CIOD (modified VDCLP) regimens; the CAG regimen was used as salvage therapy. All patients received an irradiation-containing myeloablative conditioning regimen. All patients underwent haplotype allo-HSCT from related donors, and all hematopoietic stem cells were collected from G-CSF-mobilized donor peripheral blood. ATG (rabbit-derived antithymocyte globulin, 7.5 mg/kg/d −5d to −2d), cyclosporine A (1.25 mg/kg per day), short-course methotrexate (15 mg/m2 +1d; 10 mg/m2 +3d, +6d, +11d), and mycophenolate mofetil (0.5 g bid) were used to prevent graft-versus-host disease (GVHD).
2.2.2. Prevention and treatment of central nervous system leukemia (CNSL)
Conventional triple intrathecal chemotherapy, including methotrexate (10 mg), cytarabine (30 mg), and dexamethasone (5 mg), was used to prevent CNSL. After transplantation, six courses of lumbar puncture intrathecal chemotherapy injections were conventionally administered to prevent CNSL. A combination of intrathecal chemotherapy and whole-brain and spinal cord radiation therapy was used to treat patients with CNSL.
2.2.3. Post-transplantation maintenance therapy
The patients received chidamide maintenance therapy as early as possible within 3 months after transplantation to prevent relapse, with an initial dose of 5 mg and a maximum dose of 10 mg (gradually increasing the dosage according to patient tolerance). Chidamide maintenance therapy was delivered twice a week for 2 years after transplantation. The patients underwent regular follow-ups for symptoms, complete blood count, liver and renal functions, and other parameters. If a patient showed intolerance and/or significant adverse reactions, the dosage was adjusted and medication was administered based on the guidelines.
2.3. Efficacy and safety evaluation
Patient follow-up was performed according to the guidelines [Citation17]. Relapses included hematological, molecular, and extramedullary relapse. Molecular relapse was defined as MRD positivity. The MRD level was monitored using flow cytometry, and MRD negativity was defined as <10−4. Based on the criteria for hematopoietic reconstitution after transplantation, neutrophil engraftment was defined as a neutrophil count ≥0.5 × 109/L for 3 consecutive days. Platelet engraftment was defined as a blood platelet count of ≥20 × 109/L for 7 consecutive days without platelet transfusions. GVHD was evaluated based on expert consensus [Citation18]. OS was defined as the duration from HSCT to death for any reason or the last follow-up. Progression-free survival (PFS) was defined as the duration from transplantation to disease relapse, death from the disease, or the last follow-up. Transplantation-related mortality (TRM) was defined as death arising from non-disease progression or relapse after transplantation. Chidamide-related adverse reactions were evaluated using clinical features, laboratory examinations, medical imaging data, and electrocardiography. Adverse events (AEs) were classified and graded according to the Common Terminology Criteria for Adverse Events v4.0 of the National Cancer Institute.
2.4. Statistical processing and follow-up
IBM SPSS Statistics 26.0 (statistical software) was used for statistical analysis and processing. The Kaplan–Meier approach was used for survival analysis. Various modes, such as case reference, outpatient follow-up, and telephone follow-up, were used for the follow-up and observation of patients, and the follow-up endpoint was 1 July 2023.
3. Results
3.1. Patient characteristics
The baseline patient characteristics are presented in . The median age was 29.5 years (range: 18–54 years), and all patients were men. All patients were classified as having high-risk disease. The median number of pre-transplantation chemotherapy cycles was 4.5 (range: 3–6). The disease statuses before transplantation were as follows: five patients achieved CR1 (two: MRD positivity; one: complication of CNSL), and one patient achieved CR2 (MRD positivity) ().
Table 1. Baseline characteristics of patients.
Table 2. Pre-transplant and transplant-related medical conditions of the patients.
3.2. Transplantation outcomes
For pre-transplantation radiotherapy, one patient underwent TBI with 10 Gy; two patients underwent TMLI with 200 cGy bid on days 1–3 with a total dose of 12 Gy; and three patients underwent partial irradiation (mediastinal and lymph node lesions) with 200 cGy bid on days 1–3 with a total dose of 12 Gy. For pre-transplantation chemotherapy, five patients received the myeloablative regimen with irradiation or busulfan (3.2 mg/kg/d −8d to −6d) combined with cyclophosphamide (1.8 g/m2 −5d to −4d); one patient (case 3) received the regimen based on busulfan (3.2 mg/kg/d −8d to −6d), melphalan (120 mg/m2 −3d), and fludarabine (30 mg/m2 −7d to −4d). The median number of mononuclear cells transfused was 8.06 × 108/kg (4.43-10.95 × 108/kg), and the median number of CD34+ cells was 6.47 × 106/kg (3.8-12.06 × 106/kg). Allogeneic hematopoietic stem cells were successfully implanted in all patients, showing complete chimerism and successful hematopoietic reconstitution. The median time to neutrophil engraftment was 11 days (range: 9–14 days), and the median time to platelet engraftment was 13 days (range: 9–16 days). Disease evaluation conducted within 30 days of transplantation showed that all patients achieved CR and were MRD-negative ().
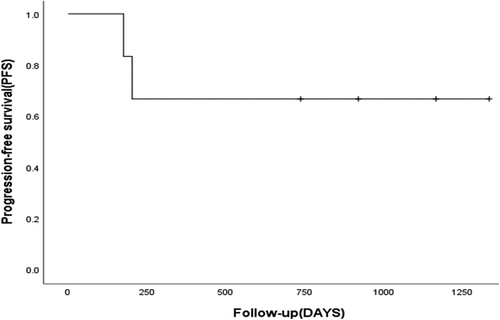
3.3. Survival analysis
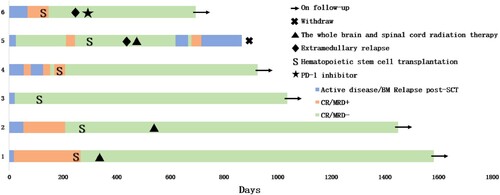
The median follow-up after transplantation was 828.5 days (range, 565–1335 days). The median survival was 828.5 days (range: 565–1335 days), and the 1-year OS rate was 100%. The median time to relapse was 828.5 days (range: 170–1335 days), and the 2-year PFS rate was 66.7% (). The TRM was 0%. Four patients achieved continuous complete remission (CCR). Patient 1 had CNSL before transplantation, which was still present after transplantation, so he underwent lumbar puncture intrathecal treatment for CNSL 4 months after transplantation combined with whole-brain and whole-spinal cord radiotherapy at 200 cGy 10 times. He maintained CCR of the bone marrow and cerebrospinal fluid during follow-up. Patient 2 had repeated elevated CSF total protein and nucleated cells during routine lumbar puncture after transplantation; therefore, he underwent whole-brain and whole-spinal cord radiotherapy at a dose of 180 cGy 12 times after transplantation. Patient 5 developed extramedullary CNSL 203 days after transplantation, re-tested negative for CNSL after four intrathecal lumbar puncture injections, and then underwent whole-brain and whole-spinal cord radiotherapy at a dose of 200 cGy 10 times. He experienced complete intramedullary relapse on day 323 after transplantation and achieved complete molecular remission after one course of CAG salvage therapy. However, the patient had another molecular relapse half a month after treatment, and then a myeloid relapse occurred approximately 1 month after treatment. He discontinued treatment after failing to achieve remission after one course of the CAG regimen. Patient 6 had an extramedullary relapse of the cervical lymph nodes 170 days after transplantation and was treated with one course of camrelizumab (a PD-1 inhibitor). He achieved CR (PET-CT negative) and sustained CCR. The patients’ post-transplantation conditions and survival outcomes are shown in and .
Table 3. Patients with post-transplant conditions.
3.4. Graft-versus-host disease
Two patients developed grade I-II acute GVHD, mainly manifesting as stage 1 skin and GI tract involvement. No cases of chronic GVHD were reported. The transplantation outcomes are presented in .
3.5. Adverse events and safety
During conditioning radiotherapy, some patients experienced side effects such as nausea, vomiting, sore mouth and throat, and myelosuppression, but none of the patients developed ARS. Long-term complications were observed in one patient after 1 year, including overflow incontinence and erectile dysfunction; no long-term complications of radiotherapy were observed in the other patients. The major chidamide-related AEs were adverse hematological reactions. All patients developed adverse hematological reactions, mainly thrombocytopenia (grades 2–3), neutropenia (grades 1–4), and anemia (grade 3). Two patients developed nausea and vomiting (grade 1), two patients had abnormal liver function (grade 2), and one patient developed fatigue (grade 1). No serious AEs such as cardiovascular symptoms were observed (). All adverse reactions could be eliminated through dose reduction or suspension and symptomatic treatment.
Table 4. Adverse effects of Chidamide maintenance therapy.
4. Discussion
Therapeutic strategies for T-ALL/LBL are relatively limited. T-ALL/LBL progresses quickly, with strong invasion and an extremely high risk of relapse in a short duration of time. Additionally, the prognosis of patients who relapse is very poor, with a 5-year survival rate <10% [Citation5]. Both consolidation and maintenance therapies are crucial for preventing relapse. For patients who achieve CR after induction therapy, performing allo-HSCT as early as possible is considered the most efficient and feasible cornerstone therapy [Citation17]. However, an optimal conditioning regimen is yet to be established. In accordance with the research findings of the European Group for Blood and Marrow Transplantation (EBMT), young adult patients with T-ALL undergoing myeloablative allo-HSCT may benefit from a TBI-based regimen [Citation10] and most patients can achieve deeper remission. A multicenter retrospective study conducted by the EBMT demonstrated that a TBI-based conditioning regimen was associated with higher OS and leukemia-free survival and lower recurrence incidence. Patients benefited from a TBI-based conditioning regimen, regardless of their MRD status [Citation19]. Other studies have also demonstrated that the TBI regimen is associated with improved overall survival (HR = 0.57) [Citation20]. Radiotherapy regimens that prioritize precision, such as TMI/TMLI, may offer significant advantages [Citation21] because of technique optimization and toxicity reduction. In this study, a radiotherapy-based conditioning regimen improved patient prognosis. The study showed a fair 2-year PFS and no significant short-term radiotoxicity or long-term radiotherapy complications; however, the sample size was small. Regardless, our results suggest that a radiotherapy-based conditioning regimen could benefit patients with T-ALL. However, further research is required to determine the optimal type and dosage of RT. This direction is worth exploring in larger studies.
Relapse remains the leading cause of death following transplantation. Consequently, maintenance therapy after transplantation is essential for reducing the risk of T-ALL/LBL relapse. The ‘ideal’ drugs for maintenance therapy after transplantation should have the following characteristics: efficacy in treating primary disease, low toxicity, early administration after transplantation, induction of a strong graft-versus-leukemia (GVL) effect, and reduction in the occurrence of GVHD. Currently, post-transplantation maintenance therapy for T-ALL/LBL includes prophylactic donor lymphocyte infusion and demethylation drugs [Citation6], but the existing therapies are limited; therefore, performing clinical trials and identifying molecular targeted therapeutic strategies are important. T-ALL/LBL is a heterogeneous disease induced by polygenic abnormalities with a pathogenesis closely associated with potential therapeutic targets [Citation22]. Clinical trials that are already underway for T-ALL/LBL are testing NOTCH1 inhibitors, PI3 K/AKT/mTOR inhibitors, and BCL-2 inhibitors; however, their therapeutic effects need to be further explored due to toxicity and side effects. Immunotherapy is also overcoming difficulties in treating T-ALL/LBL [Citation23]. Epigenetic regulatory mechanisms have received considerable attention in research on hematological malignancies. Epigenetic regulation consists of DNA modification, histone modification, chromatin remodeling, and non-coding RNA [Citation24]; hence, the research field will be wide and explore a full range of possibilities. Epigenetic therapy can be used throughout tumor development. Therefore, epigenetic regulation is important in the pathogenesis of T-ALL/LBL, which is currently a popular research topic.
HDACi are a new class of epigenetic anticancer drugs that affect the level of histone acetylation, as well as multiple biological characteristics related to tumor progression, thereby achieving an antitumor effect. Furthermore, HDACi can regulate cellular immunity, increase the sensitivity of leukemia cells to chemotherapy, and provide antitumor effects, making them attractive for combination therapy [Citation25]. HDACi can decrease the levels of proinflammatory factors but retain the function of cytotoxic T lymphocytes and enhance the function of donor regulatory T cells and natural killer cells, thus decreasing the development of GVHD without reducing GVL effects [Citation26]. Chidamide is an HDACi that was independently researched and developed in China and has been approved by the Food and Drug Administration for clinical research in the United States. Chidamide can inhibit related HDACs to increase the acetylation level of histones to induce chromatin remodeling, reverse acetylation disorder, affect the expression of signaling pathway genes, reactivate the immunosuppressive tumor microenvironment, inhibit the proliferation of tumor cells, and induce apoptosis of tumor cells [Citation13,Citation27,Citation28].
Some studies have examined chidamide in T-ALL/LBL. Yu et al. used chidamide in combination with conventional chemotherapy to treat patients with relapsed/refractory T-ALL/LBL [Citation12]. Compared to historical cases treated with chemotherapy alone, they found that the addition of chidamide safely increased the CR and overall response rates of patients with relapsed/refractory T-ALL/LBL and provided more opportunities for bridging to allo-HSCT. In addition, five patients with NOTCH1 and RAS/PTEN mutations were treated with chidamide in the first 6 months after completion of chemotherapy and during maintenance therapy, five of whom did not relapse [Citation29]. A prospective study found that chidamide as maintenance after chemotherapy or HSCT in pediatric patients with T-ALL yielded OS and EFS rates of 94.1% and 95.2%, respectively, indicating good efficacy and safety of chidamide in relapse prevention [Citation30]. Therefore, chidamide has potential as a novel anticancer medicine, marking a new dawn in the treatment of adult patients with T-ALL/LBL. Currently, several clinical trials and studies are examining chidamide in combination with different antitumor drugs for leukemia and lymphoma, with promising prospects for potential applications. Therefore, this study considered chidamide as maintenance therapy after transplantation to decrease T-ALL/LBL relapse in adult patients. The AEs were tolerable, safety was within the controllable range, and as an oral preparation, chidamide showed good patient compliance and acceptability.
This study has limitations owing to the small number of cases and lack of a control group; therefore, the long-term efficacy of chidamide needs to be further explored. A domestic multicenter phase II clinical study of chidamide as post-transplant maintenance therapy in T-ALL/LBL is underway (ChiCTR2300069772), and we look forward to the release of its results.
5. Conclusion
Irradiation-containing conditioning regimens and maintenance therapy with chidamide are safe for patients T-ALL/LBL, but the efficacy needs to be confirmed in further large-scale prospective clinical trials.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analyzed during this study are included in this article.
References
- Bassan R, Maino E, Cortelazzo S. Lymphoblastic lymphoma: an updated review on biology, diagnosis, and treatment. Eur J Haematol. 2016;96(5):447–460. doi:10.1111/ejh.12722
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi:10.1182/blood-2016-03-643544
- Eroglu C, Pala C, Kaynar L, et al. Comparison of total body irradiation plus cyclophosphamide with busulfan plus cyclophosphamide as conditioning regimens in patients with acute lymphoblastic leukemia undergoing allogeneic hematopoietic stem cell transplant. Leukemia Lymphoma. 2013;54:2474–2479. doi:10.3109/10428194.2013.779691
- Abaza YM, Kantarjian H, Faderl S, et al. Hyper-CVAD plus nelarabine in newly diagnosed adult T-cell acute lymphoblastic leukemia and T-lymphoblastic lymphoma. Am J Hematol. 2018;93:91–99. doi:10.1002/ajh.24947
- Samra B, Alotaibi AS, Short NJ, et al. Outcome of adults with relapsed/refractory T-cell acute lymphoblastic leukemia or lymphoblastic lymphoma. Am J Hematol. 2020;95(9):E245–E247. doi:10.1002/ajh.25896
- Baek DW, Lee JM, Kim J, et al. Therapeutic strategies, including allogeneic stem cell transplantation, to overcome relapsed/refractory adult T-cell acute lymphoblastic leukemia. Expert Rev Hematol. 2021;14:765–775. doi:10.1080/17474086.2021.1960817
- Yasuda S, Najima Y, Konishi T, et al. Outcome of allogeneic hematopoietic stem cell transplantation for T-cell lymphoblastic leukemia/lymphoma: A single-center study. Leukemia RES. 2021;108:106627. doi:10.1016/j.leukres.2021.106627
- Hu M, Wang H, Wang L, Yang M, Lou Y, Jin J. Outcome of adult T-lymphoblastic lymphoma depends on ALL-type chemotherapy, prognostic factors, and performance of allogeneic hematopoietic stem cell transplantation. Medicine (Baltimore). 2018;97:e11374. doi:10.1097/MD.0000000000011374
- Famoso JM, Grow JL, Laughlin B, et al. The impact of Low-dose cranial boost on the long-term outcomes of adult patients with high-risk acute lymphoblastic leukemia undergoing total body irradiation and allogeneic hematopoietic stem cell transplantation. Pract Radiat Oncol. 2019;9:e283–e289. doi:10.1016/j.prro.2018.12.005
- Cahu X, Labopin M, Giebel S, et al. Impact of conditioning with TBI in adult patients with T-cell ALL who receive a myeloablative allogeneic stem cell transplantation: a report from the acute leukemia working party of EBMT. Bone Marrow Transpl. 2016;51:351–357. doi:10.1038/bmt.2015.278
- Shi Y, Jia B, Xu W, et al. Chidamide in relapsed or refractory peripheral T cell lymphoma: a multicenter real-world study in China. J Hematol Oncol. 2017;10(1):69. doi:10.1186/s13045-017-0439-6
- Guan W, Jing Y, Dou L, et al. Chidamide in combination with chemotherapy in refractory and relapsed T lymphoblastic lymphoma/leukemia. Leukemia Lymphoma. 2020;61:855–861. doi:10.1080/10428194.2019.1691195
- Xi M, Guo S, Bayin C, et al. Chidamide inhibits the NOTCH1-MYC signaling axis in T-cell acute lymphoblastic leukemia. Front Med. 2022;16:442–458. doi:10.1007/s11684-021-0877-y
- Haferlach T, Kern W, Schnittger S, et al. Modern diagnostics in acute leukemias. Crit Rev Oncol Hemat. 2005;56:223–234. doi:10.1016/j.critrevonc.2004.04.008
- Gokbuget N, Hoelzer D. Treatment of adult acute lymphoblastic leukemia. Semin Hematol. 2009;46:64–75. doi:10.1053/j.seminhematol.2008.09.003
- Goldstone AH, Richards SM, Lazarus HM, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final Results of the International ALL Trial (MRC UKALL XII/ECOG E2993). Blood. 2008;111:1827-1833.
- Brown PA, Shah B, Advani A, et al. Acute lymphoblastic leukemia, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:1079–1109. doi:10.6004/jnccn.2021.0042
- Schoemans HM, Lee SJ, Ferrara JL, et al. EBMT-NIH-CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone Marrow Transpl. 2018;53:1401–1415. doi:10.1038/s41409-018-0204-7
- Pavlu J, Labopin M, Niittyvuopio R, et al. Measurable residual disease at myeloablative allogeneic transplantation in adults with acute lymphoblastic leukemia: a retrospective registry study on 2780 patients from the acute leukemia working party of the EBMT. J Hematol Oncol. 2019;12:108. doi:10.1186/s13045-019-0790-x
- Hamilton BK, Rybicki L, Abounader D, et al. Allogeneic hematopoietic cell transplantation for adult T cell acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2017;23:1117–1121. doi:10.1016/j.bbmt.2017.04.003
- Wong J, Filippi AR, Scorsetti M, et al. Total marrow and total lymphoid irradiation in bone marrow transplantation for acute leukaemia. Lancet Oncol. 2020;21:e477–e487. doi:10.1016/S1470-2045(20)30342-9
- Belver L, Ferrando A. The genetics and mechanisms of T cell acute lymphoblastic leukaemia. Nat Rev Cancer. 2016;16:494–507. doi:10.1038/nrc.2016.63
- Cordo’ V, van der Zwet J, Cante-Barrett K, Pieters R, Meijerink J. T-cell acute lymphoblastic leukemia: A roadmap to targeted therapies. Blood Cancer Discov. 2021;2:19-31. doi:10.1158/2643-3230.BCD-20-0093
- Zhao A, Zhou H, Yang J, et al. Epigenetic regulation in hematopoiesis and its implications in the targeted therapy of hematologic malignancies. Signal Transduct Target Ther. 2023;8:71. doi:10.1038/s41392-023-01342-6
- Cheung LC, Cruickshank MN, Hughes AM, et al. Romidepsin enhances the efficacy of cytarabine in vivo, revealing histone deacetylase inhibition as a promising therapeutic strategy for KMT2A-rearranged infant acute lymphoblastic leukemia. Haematologica. 2019;104:e300–e303. doi:10.3324/haematol.2018.192906
- Choi S, Reddy P. HDAC inhibition and graft versus host disease. Mol Med. 2011;17:404–416. doi:10.2119/molmed.2011.00007
- Sanchez-Martin M, Ferrando A. The NOTCH1-MYC highway toward T-cell acute lymphoblastic leukemia. Blood. 2017;129:1124–1133. doi:10.1182/blood-2016-09-692582
- Chi Z, Gao H, Liu H, et al. Chidamide induces necroptosis via regulation of c-FLIPL expression in Jurkat and HUT-78 cells. Mol Med Rep. 2020;21:936–944.
- Chen F, Pang D, Guo H, et al. Clinicopathological characteristics and mutational profiling of adult T-cell lymphoblastic lymphoma in a Chinese population. Cancer Manag Res. 2020;12:3003–3012. doi:10.2147/CMAR.S242903
- Li X, Han X, Huang K, et al. Chidamide as maintenance after chemotherapy or hematopoietic stem cell transplantation in 27 children with T-cell lymphoblastic leukemia: A real-world prospective study. Front Med-Lausanne. 2023;10.