Abstract
Physical inactivity is a major contributor to overweight/obesity and associated disorders including cardiovascular diseases (CVDs), diabetes, and insulin resistance (IR). Intensive lifestyle modification (ILM) is recommended as first-line treatment for obese individuals at risk for IR. Exercise is considered to be a critical component of ILM. This narrative review discusses the role of exercise in the management of IR in overweight/obesity.
PubMed and Google Scholar were searched for articles published between January 1990 and January 2019 that examined mechanisms behind the effects of exercise on IR states associated with overweight/obesity. Studies examining and/comparing effects of exercise mode, volume and/intensity on IR were also retrieved. Medical Subject Headings (MeSH) used were ‘metabolic diseases’ OR ‘chronic diseases’ AND ‘exercise’ and their related terms. Text words used in conjunction with the MeSH terms were ‘aerobic training/exercise’ OR ‘resistance training/exercise’ OR ‘high intensity interval training/exercise’, OR ‘low volume training/exercise’. Reference lists of retrieved articles were also searched for appropriate studies.
Aerobic exercise training (AET) and resistance exercise training (RET) appear to produce comparable effects on obesity-induced IR states. RET, however, appears to be associated with greater improvements in glucose disposal in skeletal muscle, which is usually the primary site for IR. This is partly attributed to greater increases in key proteins involved in the insulin signalling pathway including protein content of glucose transporter 4 (GLUT-4) following RET. A study on individuals with impaired glucose tolerance (IGT) showed that RET improved glucose disposal by 23%, primarily due to a 27% increase in non-oxidative glucose metabolism, suggesting that RET may delay the manifestation of diabetes in patients with IGT. Furthermore, studies reviewed here show that components of exercise including the mode, volume and intensity of exercise training are an integral element in exercise prescription and must be recommended in accordance with the desired outcome.
Introduction
Lack of exercise and sedentary behaviour (prolonged sitting) are major risk factors for insulin resistance.Citation1–3 Typically coupled with excessive intake of energy-dense foods, low-energy expenditure (physical inactivity) is associated with overweight/obesity.Citation4 In the development of obesity, macrophages infiltrate adipose tissue and alter its endocrine and metabolic functions to produce abnormal levels of adipokines and cytokines such as leptin and interleukin 6 (IL-6).Citation5, Citation6 This results in systemic inflammation, which is strongly associated with diminished response of liver, muscle and adipose tissue to cellular actions of insulin.Citation7 Liver, muscle and adipose tissue play a critical role in glucose homeostasis, thus a defect in insulin action in these tissues results in impaired glucose uptake and storage.Citation8 Consequences of impaired glucose homeostasis include dyslipidaemia, type 2 diabetes (diabetes) and cardiovascular diseases (CVDs).Citation9
Lifestyle modification including cessation of tobacco smoking, changes in diet and exercise are recommended as non-pharmacological therapeutic approaches for the management of obesity-associated diseases including cancer, diabetes and insulin resistance.Citation10 Below we discuss therapeutic effects of exercise on impaired insulin signalling, impaired glucose metabolism, systemic inflammation and endothelial dysfunction; and clinical states of insulin resistance in overweight/obesity.
Impaired insulin signalling
Impaired insulin signalling as shown in occurs at the level of insulin receptor substrate 1 (IRS-1), which leads to impaired glucose transport. Under normal physiological conditions, the insulin signalling pathway is initiated when insulin binds to its receptors located on the cell surface of insulin-sensitive tissue including skeletal muscle and adipose tissue. Activation of the insulin receptor leads to phosphorylation of IRS-1 which in turn activates the enzyme phosphatidylinositol 3-kinase (PI3 K).Citation11 Activation of PI3 K leads to the activation of protein kinase B/Akt, which is responsible for the phosphorylation of the substrate AS160 and activation of Rab proteins required for the translocation of vesicles containing glucose transporter 4 (GLUT4) to the cell membrane.Citation12 Once at the cell surface, GLUT4 shuffles glucose into the cell where it is stored or metabolised.
Figure 1. Schematic representation of the insulin signalling pathway showing defects in the signalling cascade in insulin resistance (Adapted from Fröjdö et al.).Citation13 IRS: insulin receptor substrate; Grb2: growth factor receptor-bound protein 2; SOS: Son of Sevenless; Erk/12: extracellular signal-regulated kinases 1/2; PIP3: phosphatidylinositol 3 kinase; PDK1: phosphoinositide-dependent kinase-1; PKC: protein kinase C; Akt: serine-threonine protein kinase; GSK3: glycogen synthase kinase 3; UDP: uridine diphosphate; GLUT4-glucose transported 4.
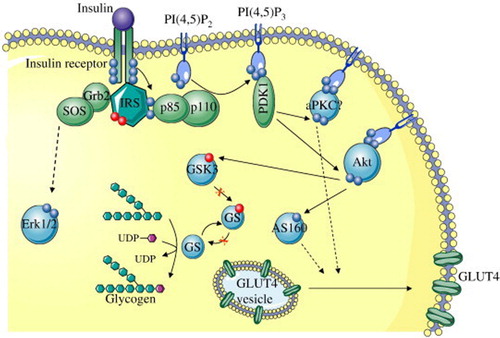
Thus when insulin signalling is impaired, hyperglycaemia ensures. Chronic hyperglycaemia has been linked to diabetes-related micro- and macrovascular complications including neuropathy, retinopathy and nephropathy.Citation14,Citation15
Impaired insulin signalling is caused by a variety of factors. In the skeletal muscle, it is attributed to the accumulation of fat in muscle fibres.Citation16 Increased skeletal muscle fat content, particularly inside the muscle fibre (intramyocellar fat-IMCL) is strongly correlated with impaired glucose transport even though IMCL contributes a smaller portion (∼1%) of the total fat content compared with fat accumulated outside the muscle cell (extramyocellar fat-EMCL).Citation17,Citation18 The mechanism behind accumulation of IMCL in the muscle is unclear. Some studies, however, have suggested that mitochondrial dysfunction contributes to increased IMCL fat content.Citation19–21
Skeletal muscle insulin resistance has been identified as a primary defect in insulin resistance and diabetes; the skeletal muscle therefore presents a critical target site for therapies aimed at controlling glycaemia.Citation22,Citation23 Therapeutic effects of exercise in the skeletal muscle are via acute and chronic adaptations. Acutely, exercise results in the translocation of GLUT 4, which results in improved glucose uptake by the muscle.Citation24 This exercise-induced GLUT 4 expression occurs via insulin-independent pathways including the calcium (Ca2+), 5’AMP-activated-kinase (AMPK) and nitric oxide synthase (NOS) kinases. Chronically, exercise results in increased mitochondrial content and muscle fibre transformation (from more glycolytic type IIb/IId/x to more oxidative type IIa fibres), which are associated with increased oxidative capacity.Citation25,Citation26 A high muscle oxidative capacity is associated with optimal glucose metabolism and has been found to be predictive of metabolic health including low fat mass and optimal insulin sensitivity.27 28
Impaired glucose metabolism
Impaired glucose metabolism (pre-diabetes) is a transition state between normal glucose homeostasis and diabetes. This metabolic state is characterised by impaired glucose tolerance (IGT) and impaired fasting glucose (IFG), which are attributed to skeletal muscle and hepatic insulin resistance, respectively.Citation29–31 Effects of exercise on human skeletal muscle are well elucidated and mechanisms have been highlighted in the preceding paragraph. Hepatocellular mechanisms, however, are unclear. Studies on animal models show that exercise reduces liver fat content, stimulates hepatic mitochondrial adaptations and increases 5’ adenosine monophosphate-activated protein kinase (AMPK) activity.Citation32 Increased AMPK activity inhibits transcription factors including hepatocyte nuclear factor 4 (HNF4) and CREB regulated transcription coactivator 2 (CRTC2) which maintain glucose homeostasis by inhibiting gluconeogenesis.Citation33,Citation34 Thus, exercise-induced AMPK activity may offer therapeutic benefit to patients with IFG who exhibit excessive rise in post-prandial glucose due to impaired hepatic glucose production.Citation35
Chronic systemic inflammation
The inflammatory process is a normal part of the body’s natural defence to infection or trauma and results in the expression of inflammatory mediators such as IL-6, TNF-α and CRP. Although this process is pivotal in immunological response, chronic inflammation (persistent elevated levels of inflammatory markers), as seen in total and abdominal obesity, is a major risk factor for chronic diseases including insulin resistance.Citation36 Pharmacological interventions such as the use of statins have been shown to decrease obesity-induced inflammation.Citation37, Citation38 Behavioural interventions including exercise (acute and chronic), however, are increasingly being shown to also have clinically significant benefits.Citation39,Citation40 It is important to note that, although exercise is associated with increased muscle-derived inflammatory cytokines acutely, these cytokines are of physiological benefit.Citation41 IL-6 secreted after an acute bout of exercise has been found to initiate the secretion of the anti-inflammatory cytokine (IL-10) from monocytes and lymphocytes.Citation41 Chronic (regular, long-term) exercise has been shown to have an inverse, independent dose–response relation with inflammation.Citation42 In a 12-month study, Marfella et al. reported that exercise decreased IL-6, CRP and TNF by 62%, 44% and 31%, respectively.Citation43 The mechanism by which exercise training mitigates inflammation has been suggested to be through the reduction of adipose tissue, which is the main secretory organ responsible for the production of inflammatory cytokines.Citation7,Citation43 Furthermore, exercise has been found to increase the expression of anti-inflammatory agents such IL-1 receptor antagonist (IL-1ra), IL-10 and adiponectin, which promote various physiological benefits including glucose metabolism and suppression of inflammatory markers such as TNF-α, which may reduce risk for insulin resistance.Citation44–46
Endothelial dysfunction
Obesity, particularly abdominal obesity, is a primary risk factor for impaired endothelial function, which results in abnormal regulation of vasoactive substances including nitric oxide (NO).Citation47 Nitric oxide is considered to be the most important endothelium-derived substance due to its antiatherogenic effects including the inhibition of inflammation and vascular smooth cell proliferation and migration. Endothelial dysfunction (ED), therefore, is strongly associated with chronically elevated levels of the pro-inflammatory markers including CRP.Citation48 Thus, ED is considered an initial step in the pathogenesis of insulin resistance and associated cardiovascular complications.Citation49 Research indicates that exercise training results in significant reduction in CRP and may produce cardioprotective effects. A recent meta-analysis of randomised controlled trials found that exercise was associated with a significant decrease in CRP (−0.66 mg/l (95% CI, −1.09 to −0.23 mg/l; −14% from baseline), suggesting that exercise could be a therapeutic alternative for the management of abnormal inflammation levels.Citation50
The effects of exercise on insulin resistance that are outlined in the preceding paragraphs are generally dependent on various components including the mode, volume and intensity of exercise training.Citation51 These components are integrated with patient health characteristics identified from the exercise pre-participation screening process, which involves assessing the patient’s level of physical activity and identifying any presence of signs or symptoms of cardiovascular and metabolic disease.Citation51
Exercise mode
The mode of exercise refers to the type of exercise. Aerobic exercise training (AET), which includes exercise types such as walking, jogging, bicycling and rowing, is the most commonly studied mode of exercise in patients with obesity and related metabolic disorders.Citation52 Long-duration AET has traditionally been prescribed to obese patients to aid in weight loss due to high energy demands associated with prolonged (> 60 minutes per day) exercise.Citation53 Recent studies, however, show that the effects of AET are amplified when it is combined with resistance exercise training (RET), which has previously not been recommended for obese patients.Citation54 In a 14-year study, Shiroma et al. reported that RET decreased the incidence of diabetes and cardiovascular disease by 30% and 17%, respectively.Citation54 Furthermore, the authors observed that people who performed RET reduced their risk of diabetes and CVDs by 30% and 17%, respectively.Citation54 This could be due to the fact that, unlike AET, RET maintains fat-free mass (FFM) and resting energy expenditure (REE), factors that are pivotal in weight loss and weight maintenance.Citation55 Resistance exercises included shoulder press, squats, lunges and deadlifts, which are performed with progressive resistance machines or bands and/or free weights.Citation56,Citation57 These exercises were performed at ∼70–85% one-repetition maximum (1RM), which has been reported to preserve or improve FFM and REE while decreasing body fat.Citation58
Exercise volume and intensity
Exercise volume and intensity refer to the amount and effort required for the exercise. Exercise intensity (moderate, vigorous or high) is characterised by objective and subjective measures such as percentage of heart rate reserve (HHR) and rate of perceived exertion (Borg’s 6–20 [RPE]), respectively.Citation59,Citation60 Exercise training at 40–60% of HRR or 12–13 RPE is considered moderate intensity training (MIT). Vigorous-intensity exercise is performed at 60–85% of HRR or RPE ≤ 14 while high-intensity training (HIT) is performed at near maximal to maximal effort, 90–100% HHR and 19–20 on the RPE scale.Citation51
The intensity of exercise determines the volume (amount) of exercise. The lower the intensity or load the higher the volume of exercise required to achieve therapeutic effect and vice versa.Citation61 High load (low volume) RET is traditionally considered to produce superior physiological adaptations including skeletal muscle hypertrophy when compared with low load (high volume) RET. A recent study by Morton et al. however, reported comparable increases in lean muscle mass in trained and untrained individuals following high and low load (high and low volume, respectively).Citation62 The authors speculated that the similar increases may have been due to the fact that the participants who performed low load (high volume) RET achieved a greater exercise volume since they were able to exercise until volitional failure, which allowed for maximal activation of their motor units and ultimately led to the similar increases in skeletal muscle adaptation seen in the high load (low volume) group, suggesting that the gains in muscle mass are not dependent on the load when the exercise is performed to volitional fatigue.Citation62
There is general consensus that exercise training produces therapeutic effects on health outcomes including improvements in insulin sensitivity and cardiorespiratory fitness. Nonetheless, levels of physical inactivity continue to rise.Citation63 Time commitment (30–60 minutes) required for high-volume moderate-intensity training (HVMIT) has been cited as the most common barrier by people who are physically inactive.Citation64 Low-volume high-intensity training (LVHIT), which requires approximately 10% of the time required for HVMIT, is therefore increasingly being studied as a time-efficient alternative.Citation65
High-intensity interval training (HIIT) and sprint interval training (SIT) are the most commonly studied forms of LVHIT. Literature evidence shows that HIIT and SIT, which are characterised by brief periods of high-intensity exercise separated by active or complete rest, produce similar and sometimes superior benefits in patients with cardiometabolic disorders despite major differences in time commitment.Citation66,Citation67 In a 12-week study, Gillen et al. reported that three sets of 20-second SIT three times per week produced comparable improvements in cardiorespiratory fitness, insulin sensitivity and mitochondrial content as 45 minutes of MIT despite the fivefold lower exercise volume.Citation68 Studies have suggested that training at high intensity is associated with greater cardiac output and stroke volume as well as activation of peripheral factors that enhance the extraction of oxygen from the blood to tissues, factors associated with enhanced cardiorespiratory fitness.Citation69
Conclusion
In conclusion, studies discussed in this narrative review show that exercise improves insulin resistance by improving insulin signalling and glucose metabolism. These improvements, however, are generally dependent on various components including the mode, volume and intensity of the exercise. The beneficial effects of exercise, which have been studied thoroughly, provide support for current physical activity guidelines that recommend 150 minutes of moderate-intensity or 75 minutes of vigorous-intensity aerobic physical activity per week, which are endorsed by diabetes organisations including the American Diabetes Association (ADA) and International Diabetes Federation (IDF). However, considering that ‘lack of time’ is the main contributor to the physical inactivity pandemic, public health guidelines ought to reflect time-efficient exercise training strategies for the prevention and management of insulin resistance and other metabolic disorders. Emerging research is increasingly showing that HIIT is a time-efficient and effective mode of exercise training that has potential to be an alternative strategy to encourage individuals to adopt and maintain a physically active lifestyle. However, comprehensive clinical trials are needed to advance knowledge on HIIT.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Tshidi Thaane http://orcid.org/0000-0002-2420-2905
Andrew J Mckune http://orcid.org/0000-0002-5479-1544
References
- Peterson MD, Al Snih S, Serra-Rexach JA, et al. Android adiposity and lack of moderate and vigorous physical activity are associated with insulin resistance and diabetes in aging adults. J Gerontol Ser A, Biomed Sci Med Sci. 2015;70(8):1009–17.
- Balducci S, D’Errico V, Haxhi J, et al. Level and correlates of physical activity and sedentary behavior in patients with type 2 diabetes: a cross-sectional analysis of the Italian diabetes and exercise study_2. PLOS One. 2017;12(3):e0173337.
- Dirks ML, Wall BT, van de Valk B, et al. One week of bed rest leads to substantial muscle atrophy and induces whole-body insulin resistance in the absence of skeletal muscle lipid accumulation. Diab. 2016;65(10):2862–75.
- Drenowatz C, Hill J, Peters J, et al. The association of change in physical activity and body weight in the regulation of total energy expenditure. Eur J Clin Nutr. 2017;71(3):377–82.
- Amano SU, Cohen JL, Vangala P, et al. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 2014;19(1):162–71.
- Daniele G, Mendoza RG, Winnier D, et al. The inflammatory status score including IL-6, TNF-α, osteopontin, fractalkine, MCP-1 and adiponectin underlies whole-body insulin resistance and hyperglycemia in type 2 diabetes mellitus. Acta Diabetol. 2014;51(1):123–31.
- Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–30.
- Reilly SM, Ahmadian M, Zamarron BF, et al. A subcutaneous adipose tissue–liver signalling axis controls hepatic gluconeogenesis. Nat Commun. 2015;6:6047.
- Patel TP, Rawal K, Bagchi AK, et al. Insulin resistance: an additional risk factor in the pathogenesis of cardiovascular disease in type 2 diabetes. Heart Fail Rev. 2016;21(1):11–23.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American association of clinical endocrinologists and American college of endocrinology on the comprehensive type 2 diabetes management algorithm-2019 executive summary. Endocr Pract. 2019;25(1):69–100.
- Alessi DR, James SR, Downes CP, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr Biol. 1997;7(4):261–9.
- Bruss MD, Arias EB, Lienhard GE, et al. Increased phosphorylation of akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diab. 2005;54(1):41–50.
- Fröjdö S, Vidal H, Pirola L. Alterations of insulin signaling in type 2 diabetes: a review of the current evidence from humans. Biochim Biophys Acta (BBA) – J Mol Basis Dis. 2009;1792(2):83–92.
- Yoon JW, Jun HS. Cellular and molecular pathogenic mechanisms of insulin-dependent diabetes mellitus. Ann Acad Sci. 2001;928(1):200–11.
- Kumar P, Swain MM, Pal A. Hyperglycemia-induced inflammation caused down-regulation of 8-oxog-DNA glycosylase levels in murine macrophages is mediated by oxidative-nitrosative stress-dependent pathways. Int J Biochem Cell Biol. 2016;73:82–98.
- Yu C, Chen Y, Cline GW, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277(52):50230–6.
- Lee JS, Pinnamaneni SK, Eo SJ, et al. Saturated, but not n-6 polyunsaturated, fatty acids induce insulin resistance: role of intramuscular accumulation of lipid metabolites. J Appl Physiol. 2006;100(5):1467–74.
- Bergman B, Hunerdosse D, Kerege A, et al. Localisation and composition of skeletal muscle diacylglycerol predicts insulin resistance in humans. Diabetologia. 2012;55(4):1140–50.
- Kelley DE, He J, Menshikova EV, et al. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diab. 2002;51(10):2944–50.
- Petersen KF, Befroy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Sci. 2003;300(5622):1140–2.
- Schrauwen P, Schrauwen-Hinderling V, Hoeks J, et al. Mitochondrial dysfunction and lipotoxicity. Biochim Biophys Acta Mol Cell Biol Lipids. 2010;1801(3):266–71.
- DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diab Care. 2009;32(suppl 2):S157–63.
- Massart J, Sjögren RJ, Lundell LS, et al. Altered mirna-29 expression in type 2 diabetes influences glucose and lipid metabolism in skeletal muscle. Diab. 2017:66(7):1807–18.
- Cartee GD. Mechanisms for greater insulin-stimulated glucose uptake in normal and insulin-resistant skeletal muscle after acute exercise. Am J Physiol Endocrinol Metab. 2015;309(12):E949–59.
- Porter C, Reidy PT, Bhattarai N, et al. Resistance exercise training alters mitochondrial function in human skeletal muscle. Med Sci Sports Exerc. 2015;47(9):1922–31.
- Prior SJ, Goldberg AP, Ortmeyer HK, et al. Increased skeletal muscle capillarization independently enhances insulin sensitivity in older adults after exercise training and detraining. Diab. 2015;64(10):3386–95.
- Bruce CR, Anderson MJ, Carey AL, et al. Muscle oxidative capacity is a better predictor of insulin sensitivity than lipid status. J Clin Endocrinol Metab. 2003;88(11):5444–51.
- Falegan OS, Vogel HJ, Hittel DS, et al. High aerobic capacity mitigates changes in the plasma metabolomic profile associated with aging. J Proteome Res. 2017;16(2):798–805.
- Unwin N, Shaw J, Zimmet P, et al. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabetic Med. 2002;19(9):708–23.
- Sjaarda LG, Bacha F, Lee S, et al. Oral disposition index in obese youth from normal to prediabetes to diabetes: relationship to clamp disposition index. J Pediatr. 2012;161(1):51–7.
- Magliano DJ, Zimmet P, Shaw JE. Classification of diabetes mellitus and other categories of glucose intolerance. International textbook of diabetes mellitus, Fourth Edition. 2015:1–16.
- Knudsen JG, Biensø RS, Hassing HA, et al. Exercise-induced regulation of key factors in substrate choice and gluconeogenesis in mouse liver. Mol Cell Biochem. 2015;403(1-2):209–17.
- Leclerc I, Lenzner C, Gourdon L, et al. Hepatocyte nuclear factor-4alpha involved in type 1 maturity-onset diabetes of the young is a novel target of amp-activated protein kinase. Diab. 2001;50(7):1515–21.
- Koo SH, Flechner L, Qi L, et al. The creb coactivator torc2 is a key regulator of fasting glucose metabolism. Nat. 2005;437(7062):1109–11.
- Alatrach M, Agyin C, Adams J, et al. Decreased basal hepatic glucose uptake in impaired fasting glucose. Diabetologia. 2017;60(7):1325–32.
- Swainson MG, Batterham AM, Tsakirides C, et al. Prediction of whole-body fat percentage and visceral adipose tissue mass from five anthropometric variables. PLOS One. 2017;12(5):e0177175.
- Sukhova GK, Williams JK, Libby P. Statins reduce inflammation in atheroma of nonhuman primates independent of effects on serum cholesterol. Arter Thromb Vasc Biol. 2002;22(9):1452–8.
- Gaudette É, Goldman DP, Messali A, et al. Do statins reduce the health and health care costs of obesity? PharmacoEconomics. 2015;33(7):723–34.
- Kasapis C, Thompson PD. 2005. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol, 2005;10:1563–9.
- Wedell-Neergaard AS, Lehrskov LL, Christensen RH, et al. Exercise-induced changes in visceral adipose tissue mass are regulated by IL-6 signaling: a randomized controlled trial. Cell Metab. 2018;29(4):844–855.
- Pedersen BK, Steensberg A, Schjerling P. Muscle-derived interleukin-6: possible biological effects. J Physiol. 2001;536(2):329–37.
- Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403.
- Marfella R, Esposito K, Siniscalchi M, et al. Effect of weight loss on cardiac synchronization and proinflammatory cytokines in premenopausal obese women. Diab Care. 2004;27(1):47–52.
- Goldman M, Velu T, Pretolani M. Interleukin-10. Biodrugs. 1997;7(1):6–14.
- Node K, Michishita R, Tsuruta T, et al. Effect of exercise therapy on monocyte and neutrophil counts in overweight women. Am J Med Sci. 2010;339(2):152–6.
- Oh S, Tanaka K, Warabi E, et al. Exercise reduces inflammation and oxidative stress in obesity-related liver diseases. Med Sci Sports Exerc. 2013;45(12):2214–22.
- Brook RD, Bard RL, Rubenfire M, et al. Usefulness of visceral obesity (waist/hip ratio) in predicting vascular endothelial function in healthy overweight adults. Am J Cardiol. 2001;88(11):1264–9.
- Nassis GP, Papantakou K, Skenderi K, et al. Aerobic exercise training improves insulin sensitivity without changes in body weight, body fat, adiponectin, and inflammatory markers in overweight and obese girls. Metab Clin Exp. 2005;54(11):1472–9.
- Landmesser U, Hornig B, Drexler H. Endothelial function: a critical determinant in atherosclerosis? Circ. 2004;109(21):2–27.
- Hayashino Y, Jackson JL, Hirata T, et al. Effects of exercise on c-reactive protein, inflammatory cytokine and adipokine in patients with type 2 diabetes: a meta-analysis of randomized controlled trials. Metab Clin Exp. 2014;63(3):431–40.
- Moore G, Durstine JL, Painter P. American College of Sports Medicine. ACSM's exercise management for persons with chronic diseases and disabilities. 4th ed. Human Kinetics; 2016.
- Siddiqui N, Nessa A, Hossain M. Regular physical exercise: way to healthy life. Mymensingh Med J. 2010;19(1):154–8.
- Donnelly JE, Blair SN, Jakicic JM, et al. Appropriate physical activity Intervention strategies for weight loss and prevention of weight Regain for Adults. Med Sci Sports Exerc. 2009;41(2):459–71.
- Shiroma EJ, Cook NR, Manson JE, et al. Strength training and the risk of type 2 diabetes and cardiovascular disease. Med Sci Sports Exerc. 2017;49(1):40–6.
- Hunter GR, Byrne NM, Sirikul B, et al. Resistance training conserves fat-free mass and resting energy expenditure following weight loss. Obes. 2008;16(5):1045–51.
- Sigal RJ, Kenny GP, Boulé NG, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147(6):357–69.
- Amador M, Meza CA, Montenegro CK, et al. Eight weeks of combined exercise training induced improvements in insulin sensitivity is associated with improvement in aerobic capacity, but not with improvement in strength. Int J Exerc Sci: Conference Proceedings; 2017.
- Walberg JL. Aerobic exercise and resistance weight-training during weight reduction. Sports Med. 1989;7(6):343–56.
- Borg GA. Psychophysical basis of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–81.
- Impellizzeri FM, Borg E, Coutts AJ, et al. Intersubjective comparisons are possible with an accurate use of the Borg's category ratio of scales. Int J Sport Physiol. 2011;6(1):2–7.
- Laursen PB, Jenkins DG. The scientific basis for high-intensity interval training. Sports Med. 2002;32(1):53–73.
- Morton RW, Oikawa SY, Wavell CG, et al. Neither load nor systemic hormones determine resistance training-mediated hypertrophy or strength gains in resistance-trained young men. J Appl Physiol. 2016;121(1):129–38.
- Ding D, Lawson KD, Kolbe-Alexander TL, et al. The economic burden of physical inactivity: a global analysis of major non-communicable diseases. Lancet. 2016;388(10051):1311–24.
- Borodulin K, Sipilä N, Rahkonen O, et al. Socio-demographic and behavioral variation in barriers to leisure-time physical activity. Scand J Public Health. 2016;44(1):62–9.
- Gibala MJ, Little JP, MacDonald MJ, et al. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Physiol. 2012;590(5):1077–84.
- Milanović Z, Sporiš G, Weston M. Effectiveness of high-intensity interval training (hit) and continuous endurance training for vo 2max improvements: a systematic review and meta-analysis of controlled trials. Sports Med. 2015;45(10):1469–81.
- Gillen JB, Percival ME, Skelly LE, et al. Three minutes of all-out intermittent exercise per week increases skeletal muscle oxidative capacity and improves cardiometabolic health. PLOS One. 2014;9(11):e111489.
- Gillen JB, Martin BJ, MacInnis MJ, et al. Twelve weeks of sprint interval training improves indices of cardiometabolic health similar to traditional endurance training despite a five-fold lower exercise volume and time commitment. PLOS One. 2016;11(4):e0154075.
- Astorino TA, Edmunds RM, Clark A, et al. High-intensity interval training increases cardiac output and VO2max. Med Sci Sports Exerc. 2017;49(2):265–73.
