Abstract
Background
Diabetes mellitus (DM) affects millions of people worldwide, with associated morbidity and premature mortality exacerbated by all forms of smoking. The effects of smokeless tobacco, such as snuff and chewing tobacco, have not been well researched. The use of these products is on the increase and is an important public health issue.
Objectives
The objective is to assess the difference between snuff tobacco use and non-tobacco use with regard to all-cause mortality, diabetic nephropathy (DN), and diabetic retinopathy (DR) over a nine-year period.
Methods
The records of 1 241 patients were assessed at the diabetic clinic at Kalafong Provincial Tertiary Hospital. Patient data extracted from the database included demographic information, clinical observations, and laboratory data. All data regarding changes in tobacco use were extracted. Survival analyses were done using Kaplan–Meier analysis with log-rank tests to assess the relationship between snuff use and time to the development of diabetes complications and mortality. To adjust for confounders such as diabetic control and duration, as well as systolic hypertension, Cox proportional hazards modelling was done with the same outcome measures.
Results
Of the 1 241, 120 patients died, representing a natural all-cause mortality of 9.7%. There was no statistically significant difference between snuff users and non-snuff users with regard to all-cause mortality after adjustment for age, smoking, and diastolic blood pressure. The HR for snuff use was 1.116 (CI = 0.603–2.064) (p = 0.726) after adjustment for age (HR 1.042, CI 1.026–1.058, p < 0.001), smoking (HR 1.66, CI = 1.126–2.447, p = 0.01), and diastolic blood pressure (HR 1.014, CI 1.004–1.025, p = 0.007).
Conclusion
This study could not demonstrate any additional risk to all-cause mortality, diabetic nephropathy, or diabetic retinopathy due to the use of snuff in diabetic patients.
Introduction
Diabetes mellitus (DM) is a global health concern that impacts millions of people and can lead to significant morbidity and premature mortality. Type 2 diabetes (T2D) is the most common form of diabetes, accounting for 90–95% of cases, and is strongly influenced by environmental, nutritional, and lifestyle factors. One such lifestyle factor that has been linked to diabetes is smoking, which has been shown to increase morbidity and mortality in patients with diabetes. Unfortunately, the prevalence of smoking in Africa is high, with notable regional and gender differences.Citation1 Smokeless tobacco (SLT) use makes up about 25% of global tobacco consumption, with some individuals using more than one type of tobacco concurrently.Citation2,Citation3 Aggarwal et al.Citation4 and Luo et al.Citation5 have shown that the risk of developing T2D after smoking cessation is the same as for non-smokers after 10 years. Smoking is, therefore, one of the most important reversible factors that could be implemented on all levels of medical care to improve morbidity and mortality.Citation6
The negative effects of smoking on human health are well documented,Citation7–10 but the effects of SLT use have not been well researched. The limited evidence published from studies in this regard is contradictory and was done predominantly on male subjects.Citation11 SLT is a category of tobacco products that can be used through the mouth or nose without being burned. These products are available in various forms and preparations, and are used all over the world. The use of SLT is becoming an increasingly important public health concern due to its rising popularity. Some examples of SLT products include traditional chewing tobacco and snuff, as well as Snus, which originated in Sweden. In South Africa, the predominant form of SLT used is snuff, which is a dried form of powdered tobacco.Citation12,Citation13
Although the perception may be that SLT might be a safer option than smoking cigarettes, it contains the same or even more nicotine than cigarettes and is just as, if not more, addictive depending on its content.Citation14 From the literature, it appears that SLT consumption could have incremental adverse effects in patients with diabetes, resulting in increased morbidity and premature mortality.Citation15,Citation16 The objective of this paper is to evaluate the incidence of diabetes complications in a cohort of diabetic patients to see if snuff tobacco use contributes to all-cause mortality, diabetic nephropathy (DN), and diabetic retinopathy (DR).
Methods
This was a retrospective cohort study of diabetic patients who received treatment over a nine-year period, from January 2009 to December 2017, at the Kalafong Provincial Tertiary Hospital (KPTH) diabetes clinic. KPTH is a tertiary care facility in the western part of Tshwane.
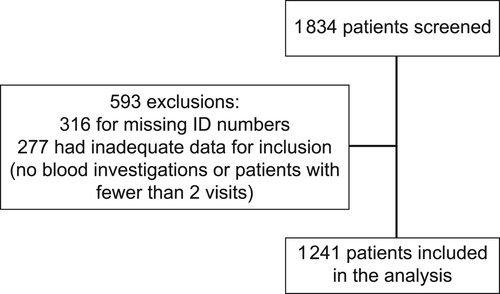
Patient records were extracted from the electronic clinical record system in use at the diabetes clinic. All patients following up at the clinic were eligible for inclusion in this study if they were older than 18 years of age when entering the clinic, diagnosed with T1D or T2D, and were followed up at the clinic for at least one year or a minimum of two clinic visits. Patients were excluded from the study if significant data were missing with regard to smoking or snuff use. Patients were also excluded if no national identification number was recorded on the electronic system; this included non-SA citizens who were not permanent residents. The reason for excluding patients without an identification number is that the patients’ survival status could not be determined without the national identification number from the Department of Home Affairs.
All clinical information on patients attending the KPTH diabetic clinic is captured on an electronic record-keeping system (MS Access; Microsoft Corp, Redmond, WA, USA) since 2009. Relevant data were extracted from this record-keeping system for use in the study. Clinical data, data related to tobacco use, and outcome measures were extracted; this included all variables that could potentially confound the relationship between tobacco use and the outcome variables. Patient data extracted from the database included demographic information, clinical observations, and laboratory data.
Three subsets of tobacco users were assessed: smokers, never-smokers, and SLT (snuff) users. The smokers were further categorised into currently smoking and previously smoking. Previous smokers were then further subdivided into those who had stopped smoking for more than one year, and those who had stopped smoking for less than one year. Snuff use was classified as snuff users and not snuff users. Individual tobacco exposure for both smokers and snuff users was not quantified otherwise.
All deaths were verified, and unnatural mortalities were differentiated from natural mortalities using an information service provider for the Department of Home Affairs of South Africa (identity.org). The exact cause of death could not be verified where patients did not die in KPTH. All deaths were verified for patients who died during the period of the study from January 2009 to December 2017.
The sample size for this study was calculated, which required at least 790 patients with at least 79 patients who were using snuff. All data analyses were done using IBM SPSS version 28.0.1.0 (142) 2021 (IBM Corp, Armonk, NY, USA). All demographic and descriptive data are described in terms appropriate for the type and distribution of data. Survival analyses were done using Kaplan–Meier analysis with time to death, time to development of DR in the first eye, time to eGFR less than 60, time to increase albuminuria moderately (urine albumin to creatinine ratio between 3 and 30 mg/mmol), and time to severe albuminuria (urine albumin to creatinine ratio more than 30 mg/mmol) as outcome measures. Log-rank tests were done to compare time-to-event data between snuff users and patients not using snuff. To adjust for confounders, Cox proportional hazards modelling was done with the same outcome measures. For this analysis, snuff category and multiple potential confounding variables were included as predictor variables in the modelling. The variables considered for inclusion in the Cox proportional hazards modelling were selected from a univariate analysis of each potential confounding variable. Confounding variables with a p-value of equal or less than 0.15 were included in the multivariate modelling. All eligible confounding variables and snuff status were entered in the multivariate models, whereafter the models were simplified to the most parsimonious model by excluding variables, one by one, that were significantly correlated or had the lowest contribution to the model.
The study was approved by the Faculty of Health Sciences Research Ethics Committee of the University of Pretoria (70/2018). Permission was also obtained from the CEO of Kalafong Provincial Tertiary Hospital (custodian of the data) for the use of patient information. The information obtained from patient records in this study was anonymised to ensure patient confidentiality.
Results
A total of 1 241 patients were included in the study, of whom 120 patients died of natural causes during the nine years of follow-up. This represents an all-cause mortality of 9.7%. Two mortalities were excluded from the final analysis because one death was unnatural, and in the other case, the name of the deceased as per the national ID number did not match the patient’s name on the clinical records ().
The mean age of the study subjects was 51.46 years, and the majority were female. Of the 1 241 patients analysed, 913 (73.6%) patients had never smoked, 135 (10.9%) patients were still smoking, and 190 (15.3%) patients had stopped smoking. Of those who had stopped smoking, 164 had stopped for more than one year and 26 for less than a year. ()
Table 1: Demographic, clinical and laboratory variables at baseline
presents data on the sample that are divided into snuff users and those who did not use snuff. A total of 101 of the 1 241 patients included in the study used snuff at baseline. Of these, 94 (93%) never smoked, 5 (5%) stopped smoking more than one year ago, 1 (1%) stopped smoking less than 1 year ago, and 1 (1%) were still smoking.
Table 2: Variables divided by snuff users and never used snuff
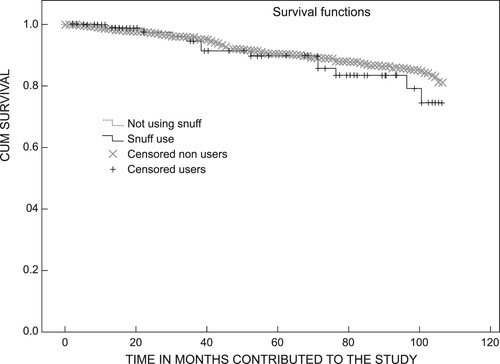
Snuff users had a statistically non-significant poorer mean survival rate than that of non-snuff users (95.9, CI 90.4–101.4 vs. 101, CI 97.9–104.4) months (on univariate analysis [log-rank p = 0.346]) ().
Figure 2: Kaplan–Meyer graph for all-cause mortality between snuff users and non-snuff users without adjustment.
In a multivariate Cox proportional hazards model, there was no statistically significant difference between snuff users and non-snuff users with regard to all-cause mortality after adjustment for age, smoking, and diastolic blood pressure. The HR for snuff use was 1.116 (CI = 0.603–2.064, p= 0.726) after adjustment for age (HR 1.042, CI 1.026–1.058, p < 0.001), smoking (HR 1.66, CI = 1.126–2.447, p = 0.01) and diastolic blood pressure (HR 1.014, CI 1.004–1.025, p = 0.007)] in the most parsimonious Cox proportional hazards model.
In the univariate Kaplan–Meyer analysis the mean time to reduction in eGFR to less than 60 ml/min for snuff users (4.75, CI 3.77–5.73 years) was statistically significantly shorter than for non-snuff users (6.62, CI 6.33–6.9 years) (log-rank p < 0.001).
The Cox proportional hazards model comparing snuff users vs. non-snuff users for time to development of an eGFR < 60 (CKD epi without cystatin), found an HR of 1.217 (CI 0.935–1.586, p = 0.144) after adjustment for the following confounding variables in the most parsimonious Cox proportional hazards model: age (HR 1.047, CI 1.041–1.054, p < 0.001), HbA1c (HR 1.035, CI 1.007–1.063, p = 0.013) and systolic blood pressure (HR 1.006, CI 1.002–1.009, p = 0.001). In the univariate analysis, the duration of diabetes showed a statistically significant effect on the time to development of eGFR < 60. However, it was excluded from the multivariate model because of a strong correlation with age. Prior or current smoking did not contribute to the risk in the model.
In the Kaplan–Meyer analysis comparing snuff users vs. non-snuff users for time to development of moderately increased albuminuria (formerly microalbuminuria) (urine albumin: creatinine between 3 and 30 mg/mmol), the mean time to moderate albuminuria for snuff users (2.9, CI 2.21–3.61 years) was statistically non-significantly shorter than for non-snuff users (3.66, CI 3.43–3.89 years) (log-rank p < 0.074). In the multivariate analysis, there was no statistically significant difference between snuff users and non-snuff users in the mean time to development of moderate albuminuria with an HR of 1.163 (CI = 0.917–1.475, p = 0.213). This was after adjustment for confounding variables in the most parsimonious Cox proportional hazards regression model: HbA1c (HR 1.046, CI 1.023–1.069, p < 0.001), systolic blood pressure (HR 1.010, CI 1.007–1.013, p < 0.001), and duration of diabetes (HR 1.023, CI 1.016–1.031, p < 0.001). Age correlated with the duration of diabetes and was therefore excluded from the model. Smoking did not contribute significantly and was excluded in the most parsimonious model.
In the Kaplan–Meyer analysis, the mean time to severe albuminuria (> 30 mg/mmol in urine albumin to creatinine ratio) (formerly macroalbuminuria) for snuff users (6.52, CI 5.8–7.2 years) was statistically non-significantly less than for non-snuff users (6.83, CI 6.62–7.24 years) (log-rank p < 0.491). After adjustment for confounding in the multivariate analysis, there was no statistically significant difference between snuff users and non-snuff users in the time to development of severe albuminuria (HR 1.099, CI 0.764–1.582, p = 0.610). The confounders adjusted for in the most parsimonious Cox proportional hazards model were HbA1c (HR 1.069, CI 1.035–1.105, p < 0.001), systolic blood pressure (HR 1.016, CI 1.012–1.020, p < 0.001), and duration of diabetes (HR 1.036, CI 1.025–1.048, p < 0.001). Smoking did not contribute significantly to the most parsimonious model.
In the Kaplan–Meyer analysis, the mean time to the detection of retinopathy in the first eye for snuff users (7.51, CI 6.98–8.05 years) was statistically non-significantly longer than for non-snuff users (6.98, CI: 6.8–7.2 years) (log-rank p < 0.136). In the multivariate analysis, there was no statistically significant difference between snuff users and non-snuff users in the time to development of retinopathy in the first eye with an HR of 0.857 (CI 0.657–1.117, p = 0.253). This was after adjustment for the following confounding variables in the most parsimonious Cox proportional hazards model: systolic blood pressure (HR 0.995, CI 0.992–0.998, p = 0.001) and duration of diabetes (HR 0.947, CI 0.938–0.956, p < 0.001). Both HbA1c and smoking (current or prior) did not contribute to the most parsimonious model.
Discussion
In this retrospective cohort study of 1 241 patients followed up over a median of a six-year period, snuff usage was associated with an increased all-cause mortality before adjustment for age and diastolic blood pressure. After adjustment for other major confounding variables, i.e. age, current or prior smoking, and systolic blood pressure, snuff usage did not relate to a statistically significant difference in all-cause survival. This is consistent with data from studies done in Europe, particularly in Sweden where Snus, a cleaner, regulated product is used, which found no association with mortality in the general population.Citation17 However, the adverse effects of SLT (including snuff use) in the general population vary from region to region and are influenced by the type of product and population. An increased all-cause mortality was demonstrated in studies from Asia, the Middle East, and Africa (AMEA). In contrast, studies from the USA showed inconsistent effects on all-cause mortality.Citation17 A multitude of different SLT products are used in the AMEA region, and studies report an association with head and neck cancers, a reduction in disability-adjusted life years and an increase in the annual death rate.Citation3
DN and DR are significant complications accounting for a substantial burden of disease in diabetic patients. Research has shown that tobacco usage may contribute to increased rates and severity of these diabetic complications.Citation4 Our cohort demonstrated no increase in DN (decrease in GFR or increase in moderate or severe albuminuria) or DR in snuff users after adjustment for other risk factors. There are no data available in the literature on diabetic cohorts using SLT and any association with DN or DR. A small South Asian study showed an association of SLT use and diabetic foot ulcers, with a higher incidence of DR and DN in patients with foot ulcers.Citation18 A study conducted in Europe found no significant increase in morbidity associated with the use of Snus, an SLT product, in a cohort of individuals from the general population. However, the negative impact of cigarette smoking on the onset and progression of diabetic nephropathy (DN) is well-established in diabetic patients.Citation19 The effect of smoking on DR is inconclusive, with studies demonstrating conflicting results.Citation4
Snuff usage in our cohort was not associated with increased all-cause mortality or a negative effect on DN and DR. This raises an interesting argument, as it is proposed by some authorities that SLT may potentially act as an agent to facilitate smoking cessation.Citation20 SLT is associated with significant adverse effects, but there is enough evidence to support the principle of less harm than cigarette smoking.Citation20 This may not be applicable generally, because the heterogeneity in product quality, composition, and route of administration is likely to influence substantially the effect of SLT in different geographical locations.
Limitations of the study include the fact that snuff usage was not quantifiable. There was also no objective way to confirm current tobacco use status other than patient self-reporting. Patients were also classified as snuff users or smokers based on baseline reporting; the study did not take into account that patients could have stopped smoking or used snuff during the follow-up period.
Conclusion
Snuff usage in our diabetic cohort did not appear to be harmful in terms of all-cause mortality, DR, and DN after adjustment for other comorbidities.
Author contributions
RMA is the primary researcher, collected data, wrote, edited, and approved the final article. DvZ supervised the research, provided the database, and edited and approved the final article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Noubiap JJ, Nansseu JR, Endomba FT, et al. Active smoking among people with diabetes mellitus or hypertension in Africa: a systematic review and meta-analysis. Sci Rep. 2019;9:588. https://doi.org/10.1038/s41598-018-37858-z
- Singh PK, Yadav A, Lal P, et al. Dual burden of smoked and smokeless tobacco Use in India, 2009-2017: A repeated cross-sectional analysis based on global adult tobacco survey. Nicotine Tob Res. 2020;22(12):2196–202. https://doi.org/10.1093/ntr/ntaa033
- Mehrotra R, Kaushik N, Kaushik R. Why smokeless tobacco control needs to be strengthened? Cancer Control. 2020;27:1–3. https://doi.org/10.1177/1073274820914659
- Aggarwal S, Khandelwal D, Dutta D, et al. Diabetes and smoking: The burden of evidence. In: Rodriguez-Saldana J, editor. The diabetes textbook. Cham.: Springer; 2019. p. 611–6. https://doi.org/10.1007/978-3-030-11815-0_40
- Luo J, Rossouw J, Tong E, et al. Smoking and diabetes: does the increased risk ever Go away? Am J Epidemiol 2013;178(6):937–45. https://doi.org/10.1093/aje/kwt071
- de Siqueira Galil AG, Cupertino AP, Banhatoa EFC, et al. Factors associated with tobacco use among patients with multiple chronic conditions. Int J Cardiol 2016;221:1004–7. https://doi.org/10.1016/j.ijcard.2016.07.041
- Maddatu J, Anderson-Baucum E, Evans-Molina C. Smoking and the risk of type diabetes. Transl Res. 2017;184:101–7. https://doi.org/10.1016/j.trsl.2017.02.004
- Chang SA. Smoking and type 2 diabetes. Diabetes and Metabolism Journal. 2012;36:399–403. https://doi.org/10.4093/dmj.2012.36.6.399
- Zhu P, Pan X-F, Sheng L, et al. Cigarette smoking, diabetes, and diabetes complications: call for urgent action. Curr Diab Rep. 2017;17(9). https://doi.org/10.1007/s11892-017-0903-2
- Eliasson B. Cigarette smoking and diabetes. Prog Cardiovasc Dis. 2003;45(5):405–13. https://doi.org/10.1016/S0033-0620(03)80004-X
- Janzon E, Hedblad B. Swedish snuff and incidence of cardiovascular disease, a population-based cohort study. BMC Cardiovasc Disord. 2009;9:21–8. https://doi.org/10.1186/1471-2261-9-21
- Siddiqi K, Shah S, Abbas SM, et al. Global burden of disease due to smokeless tobacco consumption in adults: analysis of data from 113 countries. BMC Med. 2015;13:194–2016. https://doi.org/10.1186/s12916-015-0424-2
- Tam J, Day HR, BL R, et al. A systemic review of transitions between cigarette and smokeless tobacco product use in the United States. BMC Public Health. 2015;15:258–70. https://doi.org/10.1186/s12889-015-1594-8
- Sabayanagam C, Shankar A. The association between active smoking, smokeless tobacco, second-hand smoke exposure and insufficient sleep. Sleep Med 2011;12:7–11. https://doi.org/10.1016/j.sleep.2010.09.002
- Kumar R, Kant S, Chandra A, et al. Tobacco use and nicotine dependence among patients with diabetes and hypertension in ballabgarh, India. Monaldi Arch Chest Dis. 2021;92(1). https://doi.org/10.4081/monaldi.2021.1799
- Gupta AK, Mehrotra R. Alarmingly high levels of nicotine and carcinogenic nitrosamines in smokeless tobacco products sold worldwide. Nicotine Tob Res. 2021;23(3):621–2. https://doi.org/10.1093/ntr/ntaa184
- Hajat C, Stein E, Ramstrom L, et al. The health impact of smokeless tobacco products: a systematic review. Harm Reduct J. 2021;18:123. https://doi.org/10.1186/s12954-021-00557-6
- Chellan G, Srikumar S, Varma AK, et al. Foot care practice – the key to prevent diabetic foot ulcers in India. Foot (Edinb). 2012;22(4):298–302. https://doi.org/10.1016/j.foot.2012.08.007
- Tonstad S. Cigarette smoking, smoking cessation, and diabetes. Diabetes Res Clin Pract 2009;85(1):4–13. https://doi.org/10.1016/j.diabres.2009.04.013
- Fisher MT, Tan-Torres SM, Gaworski CL, et al. Smokeless tobacco mortality risks: an analysis of two contemporary nationally representative longitudinal mortality studies. Harm Reduct J. 2019;16:27. https://doi.org/10.1186/s12954-019-0294-6