ABSTRACT
The first line of malaria vector control to date mainly relies on the use of long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS). For integrated vector management, targeting the vector larvae with biological larvicides such as Bacillus thuringiensis israelensis (Bti) can be an effective additional mainstay. This study presents data from the second intervention year of a large-scale trial on biological larviciding with Bti that was carried out in 127 rural villages and a semi-urban town in Burkina Faso. Here we present the reductions in malaria mosquitoes that were achieved by continuing the initial interventions for an additional year, important to assess sustainability and repeatability of the results from the first intervention year. Larviciding was performed applying two different larviciding choices ((a) treatment of all environmental breeding sites, and (b) selective treatment of those that were most productive for Anopheles larvae indicated by remote sensing based risk maps). Adult Anopheles spp. mosquito abundance was reduced by 77.4% (full treatment) and 63.5% (guided treatment) compared to the baseline year. The results showed that malaria vector abundance can be dramatically reduced using biological larviciding and that this effect can be achieved and maintained over several consecutive transmission seasons.
Responsible Editor Stig Wall, Umeå University, Sweden
Background
Impregnated bed nets and indoor residual spraying are the mainstays of malaria vector control, and they have strongly contributed to the considerable results achieved in malaria reduction worldwide [Citation1]. However, the development of resistance to pyrethroids is decelerating and sometimes reversing current gains in malaria control [Citation2,Citation3]. Equally, shifts in vector biting behavior from night biting to early evening or early morning biting have been observed, evading bed net barriers [Citation4–Citation7]. An alternative approach to circumnavigate these limitations is to target the larval stages of mosquitoes in their breeding sites where they are concentrated, bounded and easily accessible. This larval source management comprises the elimination, transformation and treatment of larval breeding sites. Compared to early undertakings in the era of Dichlorodiphenyltrichloroethane (DDT), today´s vector control can make use of environmentally sound larvicides that cause no harm for humans or animals, including other insects. Field trials with the biological larvicide Bacillus thuringiensis israelensis (Bti) have shown the efficacy of reducing larvae and vector populations [Citation8–Citation11].
To research the feasibility and the impact of biological larviciding against malaria in a rural area of sub-Saharan Africa, we implemented a large-scale field trial, covering 127 rural villages and a semi-urban town in North-Western Burkina Faso. The study´s impact evaluation comprised several indicators on the epidemiological pathway, from mosquito larvae to human malaria infections [Citation12]. This manuscript presents the mosquito reductions that were achieved during the second intervention year of the trial; the reductions of the first intervention year were presented elsewhere [Citation11]. Performing the same larviciding interventions during an additional year, allowed us to show the repeatability of results achieved during the first intervention year, laying the foundation to better assess those interventions for their use in routine malaria control.
Methods
The study was conducted in the Kossi region in North-Western Burkina Faso and covered all 127 rural villages of the Nouna health district and the semi-urban town of Nouna itself. The region is subject to year-round malaria transmission with a distinct maximum during the rainy season, which extends from beginning of July through beginning of October. The principal malaria vectors are Anopheles gambiae sensu lato, making up more than 90% of the population, followed by A. funestus and A. nili [Citation5].
The study setup comprised three arms with different larviciding choices: exhaustive treatment of all breeding sites (full treatment), guided treatment of only the breeding sites with the highest larval densities determined by remote sensing-based risk maps, and an untreated control group (described in detail elsewhere [Citation12]). As in the first treatment year 2014, in 2015 larviciding was performed with Bti VectoBac® WG, AM65-52 strain (Valent BioSciences Corporation, IL, USA) during and after the rainy season from July throughout October. The 2015 season focused on indoor mosquitoes because our earlier results indicated greater mosquito abundance indoors (34.0% more on average). Mosquito captures were carried out using Center for Disease Control light traps (Model 512, John W. Hock Company, Gainesville, Florida) in 36 villages and seven town quarters of the district capital. To cover all geographical areas, mosquito sampling rounds (called ‘batches’) were performed over two-week periods. At least 10 sample rounds per village took place during each annual rainy season.
Statistical analysis was performed using Stata/IC 14.2 for Windows (StataCorp LLC, 4905 Lakeway Drive, College Station, TX 77845, USA). A Poisson regression was performed to model the number of female Anopheles spp. mosquito counts per trap per night using the following categorical variables: batch, treatment choice, year, and the interaction term (difference-in-difference estimates) between treatment choice and year. Standard errors allowed for intragroup correlation at the village level.
Results
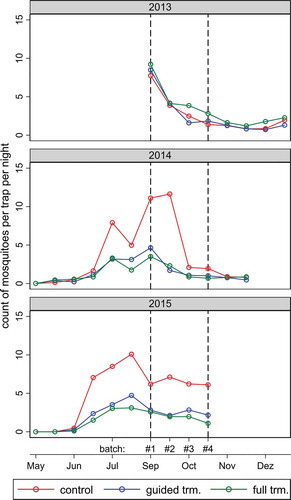
Mosquito abundance varied over time (). Results from the baseline year (2013, no Bti treatment) indicate that mosquito abundance exhibited a similar pattern over time in each geographical area corresponding to a specific treatment arm. In 2013, mosquito abundance was comparable in all three treatment zones but slightly elevated in the full treatment area. As expected, mosquito abundance was reduced in treatment areas during treatment years (2014 and 2015). In the control areas during the rainy season (July-October), the natural mosquito abundance in 2015 was on average 24.3% higher compared to 2014, and in 68.8% of the villages the mosquito counts were higher in 2015 compared to 2014. As a result, the same reduction rates resulted in higher absolute numbers of Anopheles that were captured per trap per night. The September and October mosquito counts per night per trap in the control villages were higher in 2015 with on average 6.4 ± 2.0 female Anopheles compared to with 3.8 ± 2.5 in the baseline year. In 2015, spatial variability was highest among control villages with a standard deviation ranging from ± 0.56 to ± 3.42 mosquitoes per night per trap. Unsurprisingly, the variations were lower in treated areas (guided treatment: ± 0.34 to ± 1.26; full treatment: ± 0.18 to ± 1.20) because the lower number of mosquitoes captured reduced the variability.
Figure 1. Average numbers of female Anopheles mosquitoes per trap per night captured indoors during successive sampling rounds of the three study years. The colors correspond to the average values in geographical areas receiving different Bti treatments in 2014 and 2015 (2013 was the baseline year). The vertical dotted lines indicate the common sampling period over the 3 years. Trm = treatment.
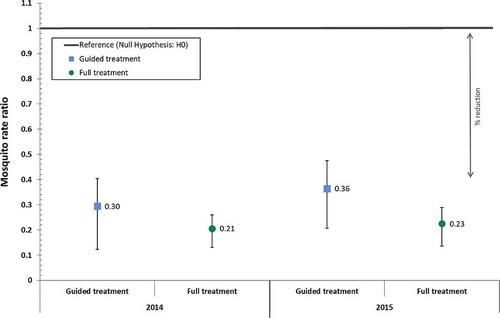
Statistical analysis of data during the common sampling period (, Table S1) shows that larviciding with Bti reduced the Anopheles densities at indoor capture posts in 2015 by 77.4% (95% CI: 68.4% – 83.8%) in the full treatment arm and by 63.5% (95% CI: 47.8% – 74.5%) in the guided treatment arm. These reductions were slightly lower but comparable to those achieved at indoor capture posts in the previous year (full: 79.4% [95% CI: 71.9% – 84.9%]; guided: 70.5% [95% CI: 53.3% – 81.3%]).
Figure 2. Difference-in-difference estimates during the common sampling period obtained with a Poisson regression model comparing the intervention years with the baseline year and indicating the reduction in the count of indoor female Anopheles mosquitoes per night per trap achieved through guided or full Bti treatment. The reference line represents the rate ratio value of 1 under the null hypothesis. (p-values were <0.001 for all entries).
Discussion
We showed that malaria vector abundance in the second intervention year was largely reduced, and that attained rate ratios were comparable to those of the preceding intervention year 2014 for both treatment choices [Citation11]. The estimated reduction with the difference-in-difference approach took into account the natural mosquito increase, which was observed in the control areas. The second intervention year featured higher natural Anopheles spp. abundance. This indicates, that similar reduction rates in adult vector mosquitoes are likely to be achievable over extended periods of time, even through years with naturally higher mosquito infestations. These adult vector reductions were realized at moderate yearly per capita intervention costs of US$ 1.05 for the full treatment and US$ 0.77 for the guided treatment, despite the rural nature of the study villages. The spatial variability of mosquito reductions among villages indicates that the assessment of the efficacy of larviciding interventions needs to be based on a mixed calculation of a larger area. The reductions achieved in 2015 and in the previous year are comparable to findings from Kenya [Citation13] where reductions of 85.9% have been observed during indoor resting collections. Other studies found reductions of more than 80% in the Entomological Inoculation Rates, while reductions at resting stations were lower [Citation8].
Although we limited the mosquito collections to indoor sample points, our results underline that the impact of biological larviciding on Anopheles vector populations is reproducible and was almost identical between our two intervention years. Having this data available from a large-scale trial over the period of two years, is valuable and might help to better estimate the entomological impact and sustainability of such interventions in a rural African environment over longer periods of time, which is important for assessing their usefulness as a routine measure.
Author contributions
PD, RS, NB and IT developed the conception and the design of the study. VL, TB, VW, and PD analyzed the data. TB and VW contributed statistical and computational tools. PD and VL wrote the paper. SO collected the field data and analyzed the mosquito samples. RS, NB, TB, VW, AS and IT contributed in writing of the paper. All authors read and approved the final manuscript.
Ethics and consent
The study was approved by the ethics committees of the University of Heidelberg under the certificate number S-438/2013 and additionally presented to and granted by the national ethics board of Burkina Faso in Ouagadougou and the local ethics committee at the research site in Nouna. We collected aggregated collective informed consent for the spraying activities for each village. The population was gathered by the local village chiefs and the project, its goals and involved activities were explained in local language. Afterwards, public discussions were held with the opportunity to ask questions or express concern. Community sensitization and information were performed during the two intervention years and additionally via the local radio station.
Paper context
Biological larviciding is a method of controlling mosquito larvae in their breeding sites. Although the approach has been shown effective in several trials in urban settings, evidence of the feasibility and effectiveness of a large-scale intervention in a rural African environment had not yet been provided. With this trial we tried to address this lack of evidence, and additionally, to evaluate if remote sensing derived risk maps can further improve efficient use of the larvicide.
Supplemental Material
Download (13.7 KB)Acknowledgments
We are deeply thankful to the communities for their support and willingness to participate in this research. We are also grateful to the field and laboratory staff at the research facility in Nouna for their valuable work and commitment to make the project successful and evolving.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- WHO | World malaria report. WHO; 2018 [cited 2019 Mar 19]. Available from: http://www.who.int/malaria/publications/world-malaria-report-2018/en/
- Strode C, Donegan S, Garner P, et al. The impact of pyrethroid resistance on the efficacy of insecticide-Treated bed nets against African Anopheline mosquitoes: systematic review and meta-analysis. PLoS Med. 2014;11:e1001619.
- Namountougou M, Simard F, Baldet T, et al. Multiple insecticide resistance in Anopheles gambiae s.l. populations from Burkina Faso, West Africa. PloS One. 2012;7:e48412.
- Moiroux N, Gomez MB, Pennetier C, et al. Changes in Anopheles funestus biting behavior following universal coverage of long-lasting insecticidal nets in Benin. J Infect Dis. 2012;jis565:1622–1629.
- Dambach P, Schleicher M, Korir P, et al. Nightly biting cycles of Anopheles species in rural Northwestern Burkina Faso. J Med Entomol. 2018;55:1027–5.
- Killeen GF, Govella NJ, Lwetoijera DW, et al. Most outdoor malaria transmission by behaviourally-resistant Anopheles arabiensis is mediated by mosquitoes that have previously been inside houses. Malar J. 2016;15. DOI:10.1186/s12936-016-1280-z
- Thomsen EK, Koimbu G, Pulford J, et al. Mosquito behavior change after distribution of bednets results in decreased protection against malaria exposure. J Infect Dis. 2017;215:790–797.
- Tusting LS, Thwing J, Sinclair D, et al. Mosquito larval source management for controlling malaria. Cochrane Database Syst Rev. 2013;8:CD008923.
- Dambach P, Louis VR, Kaiser A, et al. Efficacy of Bacillus thuringiensis var. israelensis against malaria mosquitoes in northwestern Burkina Faso. Parasit Vectors. 2014;7:371.
- Fillinger U, Knols BGJ, Becker N. Efficacy and efficiency of new Bacillus thuringiensis var. israelensis and Bacillus sphaericus formulations against Afrotropical anophelines in Western Kenya. Trop Med Int Health. 2003;8:37–47.
- Dambach P, Baernighausen T, Traoré I, et al. Reduction of malaria vector mosquitoes in a large-scale intervention trial in rural Burkina Faso using Bti based larval source management. Malar J. 2019;18:311.
- Dambach P, Traoré I, Becker N, et al. EMIRA: ecologic malaria reduction for Africa – innovative tools for integrated malaria control. Glob Health Action. 2014;7:25908.
- Fillinger U, Ndenga B, Githeko A, et al. Integrated malaria vector control with microbial larvicides and insecticide-treated nets in western Kenya: a controlled trial. BullWorld Health Organ. 2009;87:655–665. oissonregression estimating the number of female Anopheles mosquito counts per trap per night with random effect for village level.
