ABSTRACT
Background: Perinatal mortality in Uganda remains high at 38 deaths/1,000 births, an estimate greater than the every newborn action plan (ENAP) target of ≤24/1,000 births by 2030. To improve perinatal survival, there is a need to understand the persisting risk factors for death.
Objective: We determined the incidence, risk factors, and causes of perinatal death in Lira district, Northern Uganda.
Methods: This was a community-based prospective cohort study among pregnant women in Lira district, Northern Uganda. Female community volunteers identified pregnant women in each household who were recruited at ≥28 weeks of gestation and followed until 50 days postpartum. Information on perinatal survival was gathered from participants within 24 hours after childbirth and at 7 days postpartum. The cause of death was ascertained using verbal autopsies. We used generalized estimating equations of the Poisson family to determine the risk factors for perinatal death.
Results: Of the 1,877 women enrolled, the majority were ≤30 years old (79.8%), married or cohabiting (91.3%), and had attained only a primary education (77.7%). There were 81 perinatal deaths among them, giving a perinatal mortality rate of 43/1,000 births [95% confidence interval (95% CI: 35, 53)], of these 37 were stillbirths (20 deaths/1,000 total births) and 44 were early neonatal deaths (23 deaths/1,000 live births). Birth asphyxia, respiratory failure, infections and intra-partum events were the major probable contributors to perinatal death. The risk factors for perinatal death were nulliparity at enrolment (adjusted IRR 2.7, [95% CI: 1.3, 5.6]) and maternal age >30 years (adjusted IRR 2.5, [95% CI: 1.1, 5.8]).
Conclusion: The incidence of perinatal death in this region was higher than had previously been reported in Uganda. Risk factors for perinatal mortality were nulliparity and maternal age >30 years. Pregnant women in this region need improved access to care during pregnancy and childbirth.
Responsible Editor Stig Wall, Umeå University, Sweden
Background
Perinatal mortality is a major public health concern worldwide with estimated 2 million stillbirths and 1.8 million early neonatal deaths reported in 2019 [Citation1]. Most of these deaths (98%) occur in low- and middle-income countries (LMIC), with sub-Saharan Africa and South Asia being the most affected [Citation2]. A recent meta-analysis of data from sub-Saharan Africa reported an overall perinatal mortality rate of 34.7 per 1,000 births [Citation3]. This is higher than the global estimate of 26.7 deaths per 1,000 births [Citation1] and doubles the perinatal mortality rate of 13.6 per 1,000 births reported in Georgia, a country with the highest perinatal mortality in Europe [Citation4].
The effects of perinatal death are so devastating for mothers and their families, it is associated with long-lasting economic, psychological and social consequences [Citation5–7]. Several studies have drawn attention to the risk factors for perinatal death [Citation8–14]. However, most of these studies were hospital-based or in an urban/peri-urban setting, excluding home births. Moreover, the incidence of perinatal death in rural areas is higher than in peri/urban areas [Citation10,Citation15,Citation16]. Furthermore, data suggest a contextual variation in the magnitude and factors associated with perinatal death, both within and between countries [Citation3,Citation17].
The Every Newborn Action Plan (ENAP) aims to reduce stillbirths to <10 per 1,000 total births and early neonatal deaths to no more than 10 per 1,000 live births by 2035 [Citation18]. Although this target has been met in 94 high-income countries, the majority of the African countries, including Uganda, need to half the rates to reach the target [Citation19]. While the estimated global perinatal deaths have reduced by about 20% over the last 4 years [Citation1,Citation20], Uganda’s perinatal mortality of 38 deaths per 1,000 births has stagnated over the last 5 years [Citation21,Citation22]. This is above the regional estimates for Eastern Africa (34.5 per 1,000 births) and Western Africa (35.7/1,000 births) [Citation3]. Hence, there is an urgent need for reduction in perinatal mortality in this region. The realization of a perinatal mortality reduction, however, is a complex issue that does not only involve improvement of the accessibility and quality health care [Citation3] but also on improving nutrition, sanitation and education [Citation23,Citation24].
For the reduction of perinatal mortality, a reliable perinatal registration data is essential [Citation10]. However, in rural settings like Northern Uganda, childbirth also regularly takes place outside health facilities and therefore are not reflected in the vital statistics [Citation25,Citation26]. Hence, the incidence, causes and risk factors for perinatal death remain unknown. Therefore, this study aimed to determine the incidence, risk factors, and causes of perinatal death at a community level in order to provide context-specific information for planning interventions to reduce perinatal mortality.
Methods
Study design
This was a prospective cohort study among pregnant women recruited at ≥28 weeks and followed up for the first 50 days of life. It was nested in the Survival Pluss trial (NCT0260505369), a cluster-randomized community-based trial. The more detailed description of this study can be found in a previous publication [Citation27]. All pregnant women in the study were identified by community volunteers and followed up until 1 week after birth.
Study setting
The study was conducted in Lira, a district in Northern Uganda, between January 2018 and March 2019. Lira district has 3 administrative counties, 13 sub-counties, 89 parishes and 751 villages. The district had a population of 410,000 in 2014 [Citation28], served by 31 healthcare facilities, including one referral hospital, 3 healthcare centres with operation rooms, 17 healthcare centres with maternity ward but no surgical facilities, and 10 healthcare centres with only out-patient services (dispensary). The main economic activity in the region is subsistence farming.
The study was carried out in 3 sub-counties, Aromo, Agweng and Ogur. These sub-counties were chosen based on the poor maternal and perinatal indicators and the location in a rural and hard-to-reach area of the district.
Study participants
Participants were identified by 250 community female volunteers who had received training on ethical conduct and record keeping. They contacted the research team via mobile communication whenever they identified a pregnant woman in their communities. A research assistant, accompanied by the community volunteer, then visited the pregnant woman at home; she was included if she was found to be at least 28 weeks pregnant, resident in the study area and willing to participate in the study. The gestational age was determined based on the last normal menstrual period. Participants were recruited irrespective of antenatal care utilization. Follow-up phone calls and visits were made to ensure that the mothers were visited within 24 hours after childbirth and 7 days postpartum. During recruitment, pregnant women who were likely to leave the study area before the end of 6 months and those with psychiatric illness were excluded.
Sample size
For this study, the required sample size of 1,812 was estimated using Fleiss statistical methods for rates and proportions [Citation29]. It was assumed that 35% of the pregnant women who experienced a perinatal death had no formal education and 28% with perinatal death had a secondary education [Citation30]. This estimation factored in 0.05 alpha, 80% power and 10% non-response.
Data collection
A team of 42 trained research assistants, fluent in the local language Lango collected the data using a standardized questionnaire in face-to-face interviews conducted at the participant’s home. The standardized questionnaire, with structured questions on demographic, socio-economic and current pregnancy was administered at recruitment. The sections on birth and perinatal survival status were administered within 24 hours after childbirth and at 7 days postpartum.
Research assistants (university graduates) underwent a one-week intensive training on data collection procedures and tools before deployment in the field. This training was facilitated by doctors, nurses and a data analyst. The research assistants that were in two groups lived within the community in Aromo and Agweng sub-counties. They were given mobile cell phones and motorcycles to make timely follow-up visits. Data were checked on a daily basis by the coordinators and supervisors for completeness and consistency before submission.
A verbal autopsy was carried out in cases of perinatal death, using standard verbal autopsy questionnaires developed by the WHO [Citation31]. A verbal autopsy was taken within 2 weeks if relatives felt able and willing to provide information. Deaths were grouped according to timing; if it occurred during the antepartum period, intrapartum period or in the early neonatal period. The cause of death was assigned by two paediatricians after independently reviewing the collected data. A consensus was reached on the verbal autopsy data collected to determine the probable cause of death, which were later assigned by one of the authors to the new International Classification of Death – Perinatal Mortality (ICD-PM) grouping [Citation32]. All data collection tools were translated in Lango, pretested, and adjusted as necessary.
Study variables
The primary study outcome for this study was perinatal death. According to the WHO definition, a stillbirth was a baby born with a gestation of 28 weeks or more and an early neonatal death was a live-born baby that died within the first 7 days of life. Perinatal death was used as an umbrella term for both stillbirth and early neonatal death [Citation33]. The birth weight of stillborn infants was not used to determine gestational age, as this was culturally unacceptable in the study area. Therefore, gestational age was determined based on the last normal menstrual period. The perinatal mortality rate was defined on the WHO definition as the number of perinatal deaths per 1,000 births included in the study (≥28 weeks) [Citation18]. The stillbirth rate was defined as the number of stillbirths per 1,000 total births [Citation18]. The early neonatal death rate was the probability that a child born alive within the study period died during the first 7 days after birth, expressed per 1,000 live births [Citation34]. Twin pregnancies were counted as one (last twin), irrespective of the number who died.
An asset-based wealth index was used to estimate the economic status of the participant’s household, using the principal component analysis. The ‘wealth index’ was computed using the first principal component and based on the availability of nine different household assets. The wealth index was later reduced into three groups; lowest 40%, middle 40% and top 20%. Maternal age was categorized as <20 years, between 20 and 30, and >30 years. Marital status was regrouped as married or unmarried. Maternal education was categorized as no education, only primary education, and secondary education or higher education. Parity (at enrolment) was defined as the number of deliveries a woman had had and grouped as Para 0 (nullipara), Para 1–4 (multipara) and Para 5+ (Grand multipara). Antenatal care utilization was collected as ‘yes/no’ at enrolment. Obesity was defined as a body mass index (BMI) >30 kg/m2. Participants’ place of residence was included to assess for the difference in perinatal mortality between the two administrative divisions (sub-counties). A variable representing the intervention or the control arm of the parent trial was included in the analysis as a potential confounder.
Statistical analysis
Data were collected using an android-based mobile application (Open Data Kit: https://opendatakit.org) and analysed using STATA version 14.0 (StataCorp; College Station, TX, USA). Categorical variables were summarized as proportions and continuous variables as means with their standard deviations as appropriate.
We used generalized estimating equations of the Poisson family, with a log link, taking into account clustering, and assuming an exchangeable correlation to calculate the risk ratios estimating the magnitude of any associations between exposure variables and perinatal death. Based on scientific literature and biological plausibility, we included the following factors into our model: maternal age, marital status [Citation10,Citation11], maternal education, wealth index, antenatal care attendance [Citation9], parity [Citation11,Citation13,Citation14], intervention of the community trial [Citation12] and maternal obesity [Citation15,Citation35]. We also included the place of residence to assess whether perinatal death varied in the two sub-counties represented in our study. All the variables in the model were assessed for collinearity, which was considered present if the variables had a variance inflation factor (VIF) of >10. In situations of collinearity, we retained the variable with the greater biological plausibility. Multivariable regression analysis was used to take into account potential confounding.
Results
Incidence of perinatal death
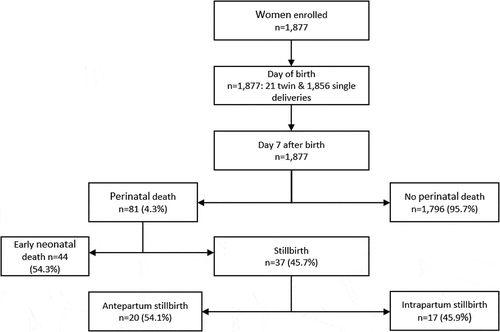
There were 81 perinatal deaths, resulting in perinatal mortality rate of 43 per 1,000 pregnancies (95% CI: 35, 53), constituted by 37 stillbirths (20 per/1,000 total births) and 44 early neonatal deaths (23 per/1,000 live births). From the 37 stillbirths, there were 20 (54.1%) antepartum and 17 (45.9%) intrapartum deaths (). Of the 81 perinatal deaths, two-thirds, 53 (65.4%), were health facility births.
Risk factors for perinatal death
shows the risk factors associated with perinatal death. Nulliparous women had more than twice the risk of experiencing a perinatal death compared to multiparous women (incidence risk ratio IRR: 2.7 [95% CI 1.3, 5.6]). Women over the age of 30 years were more likely to experience a perinatal death than those between 20 and 30 years of age (adjusted IRR: 2.5 [95% CI: 1.1, 5.8]). The risk of perinatal death was higher among women who lived in Agweng sub-county compared to those in Aromo (adjusted IRR: 1.7 [95% CI: 1.1, 2.6]). Other factors, including maternal education, wealth index, obesity, marital status, ANC at enrolment and arm allocation in the parent trial were not associated with perinatal death.
Table 1. Characteristics of pregnant women in Lira, Uganda, by perinatal death and crude incidence risk ratio (IRR) and adjusted IRRs of perinatal death
From the 1,877 participants that were followed up within 24 hours after birth, there were 21 twin births and 1,856 single births (). Mean maternal age (±SD) was 25 ± 7 years and the mean duration of education was 4 ± 2 years (range 0 to 18 years). The average number of children a participant had had was 3 (range 0 to 12), details are in .
Probable causes of perinatal death
The main probable causes of stillbirths were acute intrapartum events 14 (82.4%) and infections 7 (35.0%). The main probable contributors to early neonatal death were birth asphyxia 16 (36.4%) and respiratory failure 16 (36.4%) ().
Table 2. Probable cause of the 81 perinatal deaths among 1,877 women followed up from at least 28 weeks of gestation to 7 days postpartum in Lira, Northern Uganda, 2018–2019
Discussion
This study found a perinatal mortality rate of 43 per 1,000 births, constituted by a stillbirth rate of 20 per 1,000 total births and early neonatal mortality rate of 23 per 1,000 live births. The risk of experiencing a perinatal death was greater among nulliparous women and women over the age of 30 years and those living in Agweng. Birth asphyxia, respiratory failure, infections and intra-partum events were the most probable contributors to perinatal death.
Perinatal mortality of 43 deaths per 1,000 births and 95% confidence interval of 35, 53 is slightly higher than the previously reported rates of 38 and 41 deaths per 1,000 births noted in 2016 and 2011, respectively [Citation13,Citation21]. This is above the estimated rates of 29, 39 and 29 deaths per 1,000 births for Kenya (2015), Tanzania (2015) and Rwanda (2014), respectively [Citation36–38]. The higher rate could be due to limited access to quality maternal–child healthcare services. From the evidence, Agweng sub-county registered more mortality than Aromo sub-county yet both are adjacent and in a rural setting. The proximity of some health facilities with maternity services made it easier for pregnant women from Aromo to access better health care services than those in Agweng. In the last national demographic health survey, the region was reported to have poor maternal health indicators such as a larger proportion of pregnant women giving birth outside health facilities compared to the national average, 33 vs. 24% [Citation21,Citation26]. This emphasizes that accessibility to maternal and neonatal health care is a key challenge in the northern region which needs to be addressed to reduce perinatal mortality.
The most probable causes of perinatal death were infection, acute intrapartum events, birth asphyxia and respiratory failure. These can be related to complications of prematurity, labour and delivery, where emergency obstetric and newborn care could have managed the complications [Citation12,Citation39]. The findings are consistent with earlier community-based studies in Uganda, Rwanda, Nepal and Ghana [Citation13,Citation40–43], where neonatal asphyxia, infections and prematurity were the major causes of perinatal death. Further studies are required to establish whether sub-standard care factors are contributing to these deaths so that interventions are well focused towards preventing the deaths.
Nulliparous women (at recruitment) have a higher risk of perinatal death compared to multiparous women. Nulliparous women are prone to labour and delivery complications such as obstruction [Citation12], that increase the risk for neonatal asphyxia and perinatal death [Citation44]. Often, childbirth is initially tried at home, often ending up in complications without timely access to the emergency obstetric care services [Citation45]. Our findings are consistent with results from previous studies conducted in Bangladesh, Uganda and Burkina Faso [Citation11,Citation13,Citation14], where nulliparous women had a higher risk of perinatal mortality relative to multiparous women. Similarly, women over the age 30 years at baseline were twice as likely to experience perinatal death compared to those between 20 and 30 years, as in previous studies conducted in Uganda, Nigeria and Ethiopia [Citation13,Citation15,Citation16,Citation43]. A possible explanation for our findings might be that an older maternal age increases the risk of pregnancy complications, such as placenta previa, gestational diabetes, malpositioning/malpresentation and antepartum haemorrhage, which are proximate factors for perinatal death [Citation46]. Pregnant women who are nulliparous and those over 30 years of age could benefit from risk awareness creation and mobilization for timely seeking of the health care services. Home visitation, counselling and mobilization for care during pregnancy were demonstrated to contribute to reduced perinatal death [Citation47].
Whereas earlier studies have linked socioeconomic status to perinatal death [Citation9–11,Citation23], our findings are similar to other studies that found no significant association between perinatal death and maternal education status [Citation13,Citation24,Citation30,Citation48], household wealth index [Citation13,Citation30] and marital status [Citation13,Citation30]. This could be due to the rural setting of the study, and that access to quality maternity care services is more important than other factors. The poverty level in the region is higher than the national average (32.5 versus 21.4%), and therefore the participants overall are poor, resulting in no difference. Although earlier studies suggest that obesity is a predictor of perinatal mortality [Citation15,Citation35], our results showed no such association, but this may be due to the low prevalence of obesity in the study region [Citation49].
Strengths and limitations
One strength of this study is that it is prospective and community-based, including ‘all’ women in the clusters. Moreover, the study recorded a complete follow-up. This means that the results are likely to reflect a fairly accurate estimate of perinatal mortality. There are also some limitations: gestation age was estimated basing on the last normal menstrual period (LNMP), which has its uncertainties. Post-mortem diagnostics are absent in the study area; therefore, the probable causes of death were based on ‘verbal autopsy’, which is subject to the recall of events before, during and after childbirth which may be incomplete and unreliable. The parent trial could have increased the health facility deliveries and therefore reduced the number of perinatal deaths (the Hawthorne effect) [Citation50].
Conclusion
The incidence of perinatal death in the northern region was higher than previously reported in Uganda. The risk of perinatal death was greater among nulliparous women and women over the age of 30 years. Birth asphyxia, respiratory failure, infections and intra-partum events were the major probable contributors to perinatal death. The findings of this study suggest that pregnant women in the region need improved access to care during pregnancy and childbirth. Further studies are required to understand whether the sub-standard care factors are contributing to perinatal deaths so that interventions are well targeted.
Authors’ contributions
A.A.O.A., V.N., G.N., N.N., J.K., T.T., D.M. and J.K.T. conceived, designed, supervised the study, analysed the data, and wrote the first draft of the manuscript. J.B.T., A.N., M.W.M., V.A. and B.O. were instrumental in the design and supervision of the study and in drafting of the manuscript. All authors critically reviewed the manuscript read and approved the final version to be published.
Ethics and consent
We obtained ethical approval for the study from Makerere University School of Medicine Higher Degrees Research Ethics Committee (REC REF 2017-171), the Uganda National Council for Science and Technology (HS356ES). The randomized community-based trial was registered with ClinicalTrial.gov and the Regional Committees for Medical and Health Research Ethics, Norway (2017/2079/REK vest). Permission was obtained from the Uganda Ministry of Health and Lira District Local Government. Written informed consent was obtained from all participants after a detailed explanation of the study and purpose. During the training of research assistants, the emphasis was placed on issues of confidentiality, respect for participants and the right of the participants to withdraw their participation from the study at any time without any penalty. We used identification numbers instead of names to conceal the identity of participants. We obtained permission to conduct the study from the community leaders.
Paper context
To achieve the ‘every newborn action plan’ (ENAP) target of reducing stillbirth and early neonatal death, there is a need to provide specific data locally to guide interventions. The findings show that perinatal mortality in Northern Uganda is higher than previously reported. The risk of experiencing a perinatal death was greater among nulliparous women and women >30 years of age. Pregnant women in this region need improved access to care during pregnancy and childbirth.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We thank all the women who participated in this study and research assistants, who gave their invaluable time during data collection. The help of Vivian Zalwango, Samuel Daniel Kagongwe and Christine Akumu is fully appreciated. We appreciate the Lira district health department, the sub-county, parish and village leaders for their assistance in this study. We are grateful to William Oyang and Nelson Okello, who reviewed the verbal autopsy forms and assigned the likely cause of death. This manuscript was edited by BioMedES UK (www.biomedes.biz).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- United Nations Inter-agency Group for Child Mortality Estimation (UN IGME). A neglected tragedy: the global burden of stillbirths. New York: United Nations Children’s Fund; 2020.
- Lawn JE, Yakoob MY, Haws RA, et al. 3.2 million stillbirths: epidemiology and overview of the evidence review. BMC Pregnancy Childbirth. 2009;9:S2–S. PubMed PMID: PMC2679408.
- Akombi BJ, Renzaho AM. Perinatal mortality in sub-Saharan Africa: a meta-analysis of demographic and health surveys. Ann Glob Health. 2019;85. DOI:https://doi.org/10.5334/aogh.2348.
- Manjavidze T, Rylander C, Skjeldestad FE, et al. Incidence and causes of perinatal mortality in Georgia. J Epidemiol Glob Health. 2019;9:163–8.
- Heazell AEP, Siassakos D, Blencowe H, et al. Stillbirths: economic and psychosocial consequences. Lancet. 2016;387:604–616.
- Gausia K, Moran AC, Ali M, et al. Psychological and social consequences among mothers suffering from perinatal loss: perspective from a low income country. BMC Public Health. 2011;11:451.
- Arach AAO, Nakasujja N, Nankabirwa V, et al. Perinatal death triples the prevalence of postpartum depression among women in Northern Uganda: a community-based cross-sectional study. PloS One. 2020;15:e0240409.
- Getiye Y, Fantahun M. Factors associated with perinatal mortality among public health deliveries in Addis Ababa, Ethiopia, an unmatched case control study. BMC Pregnancy Childbirth. 2017;17:245. PubMed PMID: 28747161; PubMed Central PMCID: PMC5530490.
- Kc A, Nelin V, Wrammert J, et al. Risk factors for antepartum stillbirth: a case-control study in Nepal. BMC Pregnancy Childbirth. 2015;15:146.
- Lawn JE, Blencowe H, Waiswa P, et al. Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet. 2016;387:587–603.
- Sikder SS, Labrique AB, Shamim AA, et al. Risk factors for reported obstetric complications and near misses in rural northwest Bangladesh: analysis from a prospective cohort study. BMC Pregnancy Childbirth. 2014;14:347.
- Kabakyenga JK, Östergren P-O, Turyakira E, et al. Individual and health facility factors and the risk for obstructed labour and its adverse outcomes in south-western Uganda. BMC Pregnancy Childbirth. 2011;11:73.
- Nankabirwa V, Tumwine JK, Tylleskär T, et al. Perinatal mortality in eastern Uganda: a community based prospective cohort study. PloS One. 2011;6:e19674.
- Diallo AH, Meda N, Zabsonré E, et al. Perinatal mortality in rural Burkina Faso: a prospective community-based cohort study. BMC Pregnancy Childbirth. 2010;10:45.
- Ezeh OK, Uche-Nwachi EO, Abada UD, et al. Community-and proximate-level factors associated with perinatal mortality in Nigeria: evidence from a nationwide household survey. BMC Public Health. 2019;19:811.
- Woldeamanuel BT, Gelebo KK. Statistical analysis of socioeconomic and demographic correlates of perinatal mortality in Tigray region, Ethiopia: a cross sectional study. BMC Public Health. 2019;19:1301. PubMed PMID: 31619210; PubMed Central PMCID: PMC6796483.
- Saleem S, Tikmani SS, McClure EM, et al. Trends and determinants of stillbirth in developing countries: results from the Global Network’s Population-Based Birth Registry. Reprod Health. 2018;15:100.
- World Health Organization, UNICEF. Every newborn: an action plan to end preventable death. Geneva: WHO; 2014.
- WHO, UNICEF. 2018 progress report: reaching every newborn national 2020 milestones. Geneva: Health MNCaA; 2018 May 2. https://www.healthynewbornnetwork.org/resource/every-newborn-progress-report-2018/.
- Akseer N, Lawn JE, Keenan W, et al. Ending preventable newborn deaths in a generation. Int J Gynecol Obstet. 2015;131:S43–S8.
- Uganda Bureau of Statistics (UBOS), ICF. Uganda demographic and health surveys 2016: key indicators report. Kampala, Uganda: UBOS, and Rockville, Maryland, USA: UBOS and ICF; 2017.
- Uganda Bureau of Statistics (UBOS), ICF. Uganda demographic and health survey 2011. Kampala, Uganda and Rockville, Maryland: UBOS and ICF; 2012.
- Ghimire PR, Agho KE, Renzaho AMN, et al. Factors associated with perinatal mortality in Nepal: evidence from Nepal demographic and health survey 2001–2016. BMC Pregnancy Childbirth. 2019;19:88. PubMed PMID: 30866847; PubMed Central PMCID: PMC6417106.
- Kibria GMA, Burrowes V, Choudhury A, et al. Determinants of early neonatal mortality in Afghanistan: an analysis of the demographic and health survey 2015. Global Health. 2018;14:47. PubMed PMID: 29743085; PubMed Central PMCID: PMC5944060.
- Jitta J, Kyaddondo D. Situation analysis of newborn health in Uganda. Kampala, Uganda: Ministry of Health, The Republic of Uganda; 2008.
- Mukunya D, Tumwine JK, Ndeezi G, et al. Inequity in utilization of health care facilities during childbirth: a community-based survey in post-conflict Northern Uganda. J Public Health (Berl.). 2019:1–9. https://doi.org/https://doi.org/10.1007/s10389-019-01114-z.
- Nankabirwa V, Tumwine JK, Tylleskär T. SURVIVAL PLUSS: increasing capacity for mama-baby survival in post-conflict Uganda and South Sudan ClinicalTrials.gov. 2015 [updated 2019 Apr 2]. https://clinicaltrials.gov/ct2/show/NCT02605369
- Uganda Bureau of Statistics (UBOS). The national population and housing census 2014 – area specific profile series, Kampala, Uganda; UBOS; 2017.
- Kevin SM, Minn SM. Sample size for a cross-sectional, cohort, or clinical trial studies. 2007. Available from Nov 17 2020 in https://www.openepi.com.
- Engmann C, Walega P, Aborigo RA, et al. Stillbirths and early neonatal mortality in rural Northern Ghana. Trop Med Int Health. 2012;17:272–282. Epub 2011/12/20. PubMed PMID: 22175764.
- World Health Organization. The 2014 WHO verbal autopsy instrument. 2014 [cited 2018 Mar 5]. https://www.who.int/healthinfo/statistics/verbalautopsystandards/en/index1.html
- World Health Organization. The WHO application of ICD-10 to deaths during the perinatal period: ICD-PM Geneva, Switzerland. 2016 [updated Aug 2016]; [ 77]. https://www.who.int/reproductivehealth/publications/monitoring/icd-10-perinatal-deaths/en/
- World Health Organization. Maternal health; maternal child adolescent health. 2017 [cited 2017 Jul 28]. http://www.who.int/maternal_child_adolescent/topics/maternal/maternal_perinatal/en/.
- World Health Organization. Global reference list of 100 core health indicators: Geneva, Switzerland: WHO . 2015.Available from Sept 20 2020 in http://apps.who.int/iris/bitstream/10665/173589/1/WHO_HIS_HSI_2015.3_eng.pdf.
- Hossain MB, Mistry SK, Mohsin M, et al. Trends and determinants of perinatal mortality in Bangladesh. PloS One. 2019;14:e0221503.
- Ministry of Health, Community Development, Gender, Elderly and Children (MoHCDGEC) [Tanzania Mainland], Ministry of Health (MoH) [Zanzibar], National Bureau of Statistics (NBS), Office of the Chief Government Statistician (OCGS), and ICF. Tanzania Demographic and Health Survey and Malaria Indicator Survey (TDHS-MIS) 2015–16. Dar es Salaam, Tanzania, and Rockville, Maryland, USA: MoHCDGEC, MoH, NBS, OCGS, and ICF; 2016.
- National Institute of Statistics of Rwanda (NISR) [Rwanda], Ministry of Health (MOH) [Rwanda], and ICF International. Rwanda Demographic and Health Survey 2014–15. Rockville, Maryland, USA: NISR, MOH, and ICF International; 2015.
- Kenya National Bureau of Statistics, Ministry of Health [Kenya], National AIDS Control Council [Kenya], Kenya Medical Research Institute, National Council for Population and Development [Kenya], and ICF International. Kenya Demographic and Health Survey 2014. Rockville, MD, USA: Kenya National Bureau of Statistics, Ministry of Health [Kenya], National AIDS Control Council [Kenya], Kenya Medical Research Institute, National Council for Population and Development [Kenya], and ICF International; 2015.
- Waiswa P, Kallander K, Peterson S, et al. Using the three delays model to understand why newborn babies die in eastern Uganda. Trop Med Int Health. 2010;15:964–972.
- Khanal S, Dawson P, Houston R. Verbal autopsy to ascertain causes of neonatal deaths in a community setting: a study from Morang, Nepal. J Nepal Med Assoc. 2011;51. DOI:https://doi.org/10.31729/jnma.34.
- Liu L, Li M, Cummings S, et al. Deriving causes of child mortality by re–analyzing national verbal autopsy data applying a standardized computer algorithm in Uganda, Rwanda and Ghana. J Glob Health. 2015;5. DOI:https://doi.org/10.7189/jogh.05.010414
- Grant E, Munube D, Lumala P, et al. Neonatal deaths and umbilical cord care practices in Luweero district, Uganda. Paediatr Child Health. 2014;19:333.
- Roro EM, Sisay MM, Sibley LM. Determinants of perinatal mortality among cohorts of pregnant women in three districts of North Showa zone, Oromia Region, Ethiopia: community based nested case control study. BMC Public Health. 2018;18:888.
- Ghimire PR, Agho KE, Akombi BJ, et al. Perinatal mortality in South Asia: systematic review of observational studies. Int J Environ Res Public Health. 2018;15:1428.
- Kyomuhendo GB. Low use of rural maternity services in Uganda: impact of women’s status, traditional beliefs and limited resources. Reprod Health Matters. 2003;11:16–26.
- Carolan M. Maternal age≥ 45 years and maternal and perinatal outcomes: a review of the evidence. Midwifery. 2013;29:479–489.
- Gogia S, Sachdev HS. Home visits by community health workers to prevent neonatal deaths in developing countries: a systematic review. Bull World Health Organ. 2010;88:658–666.
- Nkwo PO, Lawani LO, Ezugwu EC, et al. Correlates of poor perinatal outcomes in non-hospital births in the context of weak health system: the Nigerian experience. BMC Pregnancy Childbirth. 2014;14:341.
- Yaya S, Ghose B. Trend in overweight and obesity among women of reproductive age in Uganda: 1995–2016. Obes Sci Pract. 2019;5:312–323.
- McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol. 2014;67:267–277.