ABSTRACT
The present study deals with chemical leaching tests conducted on Batn El-Ghoul kaolin clay deposit, south Jordan. Mineral identification and characterization studies were conducted using X-ray diffraction. The chemical analyses were performed by X-ray fluorescence. The chemical leaching study with sodium dithionite (Na2S2O4) as leachant was accomplished with the fraction below 44 μm, after classification by screening. The main objective of this study was to evaluate the conditions of chemical leaching for removal of the colouring materials and to improve the brightness. The leaching tests improved the brightness from 56% to 86%. The corresponding iron-oxide removal was in the order of 94%. Removal of 94% iron gives a product containing 0.48% Fe2O3, which is fit for industrial applications such as fine ceramics and as a filler materials in paper, plastic and rubber industries.
1. Introduction
Kaolin is a finely granulated rock, usually white and chemically inert. Due to its favourable properties such as natural whiteness, fine particle size, plasticity, non-abrasiveness and chemical stability, it is widely used in many industrial fields [Citation1,Citation2]. As a key element in the paper industry, kaolin is also used as a raw material in the production of paints, ceramic articles, rubber, plastics, medicine, fiberglass, catalysts, fertilizers and other products. Kaolin minerals consist of a mixture of hydrous silicates and aluminium and silicon hydroxides as the main component. The important and commonly used kaolin is kaolinite. Kaolinite is chemically [Al2Si2O5 (OH)4], having an ideal composition of SiO2 – 46.54%, Al2O3 – 39.5 % and H2O – 13.96% [Citation3,Citation4].
The main impurities associated with commercial kaolins are quartz, feldspar, muscovite, biotite, titanium oxides and iron oxides or hydroxides, such as goethite, hematite and magnetite [Citation5,Citation6]. The presence and proportions of these impurities depend on origin and depositional environment of the mineral [Citation3].
The presence of iron oxides in kaolin has a deleterious effect on the colour of the clay, which declines in brightness with increasing iron content [Citation2–4, Citation7–9]. This limits its use as a raw material or constituent in many industrial applications, e.g. paper, rubber, pigments, refractories, pottery, porcelains and foundry moulds, white-ware, wall tiles, facing brick and white cement.
The iron removal processes can be categorized as physical, chemical or a combination of both such as sieving, magnetic separation [Citation8,Citation9], EDTA [Citation10], selective flocculation [Citation11], carbohydrates [Citation12], leaching with various chemicals like oxalic acid and other organic acids [Citation13–23], organic acids in the presence of a fermented medium [Citation24], lixiviant containing microbially produced oxalic and hydrochloric acid [Citation25], electron paramagnetic resonance [Citation26], a more eco-friendly and effective method of ferric iron removal using organic acids is evaluated in comparison with magnetic separation processes followed by chemical leaching [Citation14] and so on, removed some iron bearing phases to lower the iron content of the clay. The reductive leaching of Fe from kaolins with sodium hydrosulfite (Na2S2O4), alternatively known as sodium dithionite, is particularly efficient and is currently employed by the kaolin industry, in some cases giving very low iron concentrations (below 0.3% Fe2O3) and very high brightness values (above 94%) required for high specification kaolin products [Citation27–29].
The main goal of the present study was to investigate the possibility of using chemical leaching with sodium dithionite (Na2S2O4) of the kaolin samples of a high iron content from Batn El-Ghoul deposit, for the removal of iron, and consequently improve the brightness degree that is compatible with the application of kaolin in fine ceramics and filler clays.
2. Materials and experimental methods
2.1. Materials
For this study, five representative samples (30 kg each) of raw kaolin were collected from Batn El-Ghoul deposit, South Jordan. In the laboratory, the samples were subjected to disintegration by crushing in order to separate the optimum grain sizes. Each sample was mixed several times to form a homogeneous sample. Each of these composite samples was divided into sub-samples using the coining and quartering method for mineralogical and chemical analyses. These samples were dispersed with sodium hexametaphosphate and water with a pulp with 30% solids keeping a 7.5 pH pulp, approximately and stirring at 500 rpm using mechanical shaking, and then it classified in fractions +63 μm, −63 + 44 μm and −44 μm using a vibrating wet sieving (US standard system). The fraction with a particle size of less than 44 μm (which forms on average about 94 wt. % of the original sample) [Citation30,Citation31] was dried and disaggregated samples of 100 g were used in the test of chemical leaching. The average of grain size of the kaolin samples shows that over 75% by weight is finer than 44 µm (averages of five samples).
2.2. Mineralogical and chemical characterization
Chemical analyses for the major oxides (wt. %) and some minor elements of feed sample (−44 µm) and the refined treated concentrate were performed using a Bruker S4 wavelength X-ray dispersive fluorescence spectrometer with a Rh X-ray tube at the Natural Resources Authority, Amman, Jordan. The X-ray diffraction (XRD) of the feed sample less than 44 µm (before and after treatment) was obtained by the powder method, the Bruker-AXS D4, Cu-Kα radiation (40 kV and 40 mA). The goniometer velocity was 0.02 (2θ) per 1 s in the interval between 2° and 80° (2θ). The powder data were analysed with the Stoe WinXPOW software package.
Brightness, or whiteness, is one of the most outstanding qualities of kaolin and is of prime importance in its major uses in paper, paint and plastics. Brightness is a measurement of light reflectance by standard method, based on the Zeiss Elrepho (MgCO3 as a reference material).
2.3. Chemical leaching
The chemical leaching processes were performed on the kaolin of a high iron content, which has resulted in its characterization as commercially unusable (low grade kaolin) at Batn El-Ghoul.
For pigment-grade kaolin, brightness should be +80% ISO [Citation32] and beneficiation to achieve this level is one of the highest value additions of kaolin. To eliminate the mineralogical impurities from the kaolin and improve the brightness of the Batn El-Ghoul kaolin, the fraction (−44 μm) for each sample was subjected to chemical treatment with sodium dithionite (Na2S2O4) at a concentration of 0.3 g/100 g (3 g/1 kg) of kaolin with a pH value of 3 (diluted sulphuric acid was used to adjust the pH value). The tests were carried out with chemical leaching of pulp with 30% using controlled stirring (100 rpm) for a period of up to 2 h agitation to remove the iron impurities. As already known, titanium compounds are very difficult to dissolve by acid leaching and mainly iron contaminants get dissolved in this process [Citation33]. After drying, the brightness was determined from treated concentrate using the procedure described above.
3. Results and discussion
The XRD is the main technique used to identify mineralogical kaolin.
The XRD was used to determine the mineralogical characteristics of kaolin samples before and after leaching.
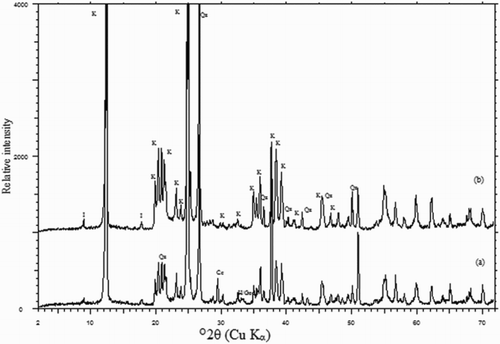
Peaks representing Fe2O3 are evident in the pattern obtained for untreated samples ((a)), demonstrating that the iron occurs as its oxides and does not substitute in the kaolin structure. The absence of iron oxides is obvious in the pattern obtained after leaching ((b)), which also indicates that the mineralogical characteristics of the kaolin have not been significantly affected.
The presence of a relatively significant amount of colouring materials (iron and titanium oxides) of the kaolin feed fraction (<44 µm) separated from the investigated original samples of Batn El-Ghoul deposit hindered their application in fine ceramics and in paper industry [Citation30]. They are mainly formed of kaolinite mineral (75 wt. % in average) together with quartz and free iron-oxide-bearing minerals (hematite-goethite) as a non-clay mineral [Citation30,Citation31]. Microscopic analysis of Batn El-Ghoul kaolin indicates that they contain white to yellowish brown grains and show different degrees of ferrugination [Citation31]. XRD analysis confirms that hematite or goethite rapidly increases in the kaolin concentrate fraction (<44 µm). This observation is supported by the increasing yellowish brown colour of the concentrate fraction [Citation30,Citation31].
For this reason, some trials were conducted to refine these kaolin feed fraction (<44 µm) by reducing their Fe2O3 contents using the chemical leaching with sodium hydrosulphite (dithionite) on the Batn El-Ghoul kaolin.
The feed of kaolin samples (<44 µm) were subjected to chemical analysis by X-ray fluorescence (XRF) as a complementary technique to XRD. The results of the chemical analysis of the kaolin feeds (<44 µm) used in the investigation before and after leaching is presented in .
Table 1. The chemical results of kaolin samples before and after leaching.
Quantitative measurements by XRF provided confirmation of this by showing that Al and Si were present in the leaching solution at negligible levels. Thus, it was concluded that the proposed leaching process has a little effect on the structural integrity of the Batn El-Ghoul kaolin [Citation30,Citation31].
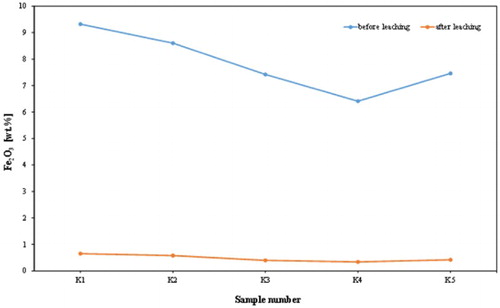
The results of the experiments to determine the influence of leaching process are given in and are illustrated in and . It is evident that on average – 94% of the Fe2O3 are removed by leaching with dithonite (Na2S2O4).
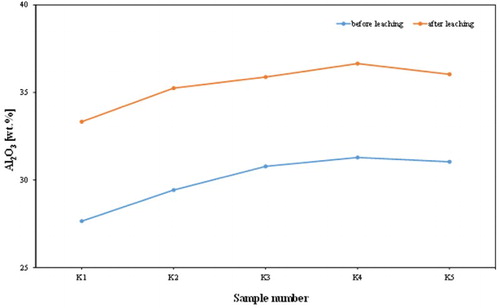
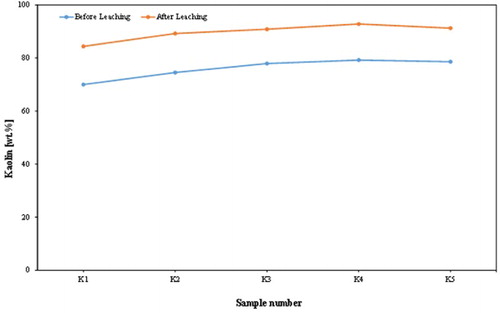
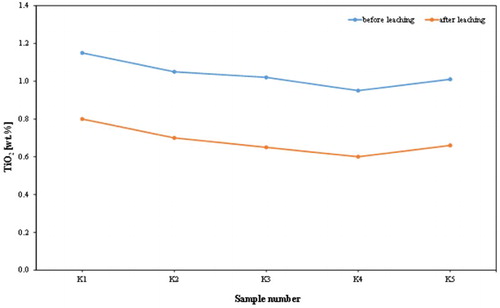
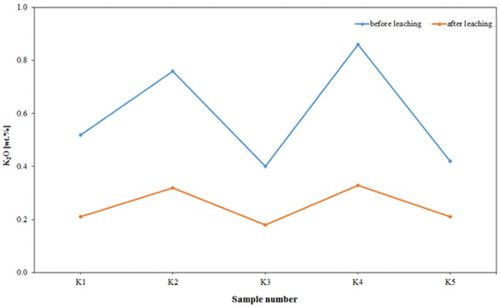
The chemical assay () shows that the alumina contents of the analysed samples increased from an average of 30.04 wt. % to an average of 35.43 wt. %; this leads to a significant increase in kaolin content (). Of course, the silica contents increased with a mean of 47.90–50.27%. This was accompanied by a dramatic reduction in iron content from an average of 7.84–0.48 wt. %, and a decrease in TiO2 content from an average of 1.04–0.68 wt. %. Also, the chemical composition of the products revealed that a high percentage reduction in iron was recorded, varying approximately between 93% and 95%. K2O content decreased from an average of 0.59–0.25 wt. %, indicating a significant removal of mica or iron bound in the lattice of illite or muscovite. and show the dramatic reduction of TiO2 and K2O, respectively, by leaching process in the examined samples from Batn El-Ghoul kaolin. shows a typical chemical analysis of leached Jordan kaolin (Batn El-Ghoul kaolin) compared to pure kaolinite and leached commercial kaolin. The chemical results ( and ) showed that the leached Batn El-Ghoul kaolin has a higher content of SiO2 (50.3%) and lower content of Al2O3 (35.4%) compared with the kaolinite kaolin.
Table 2. Comparative analysis of leached Jordanian kaolin with a refined commercial kaolin and with theoretical kaolinite.
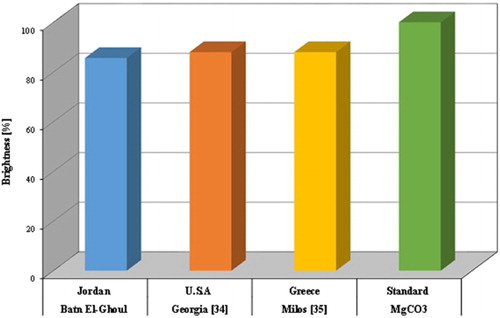
Measurements of brightness against MgCO3 of the analysed kaolin samples before and after leaching process are represented in . These results verify the positive effect of the proposed processes for the qualitative upgrading of Batn El-Ghoul kaolin. The kaolin achieved a remarkable brightness of an average of 85.6 for the leached (treated) samples by sodium hydrosulphite (Na2S2O4). Comparison of the average results of brightness of leaching kaolin from Batn El-Ghoul with a well-known leaching kaolin was represented in .
Figure 7. Brightness measurements of the analysed samples before and after chemical leaching compared with standard of brightness (MgCO3).
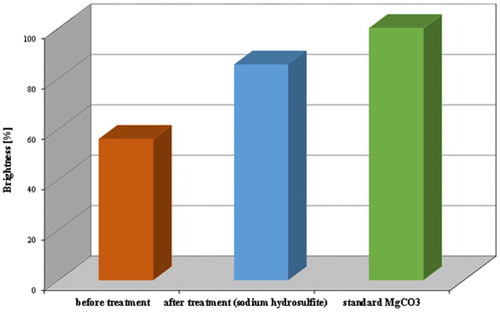
Figure 8. Typical leached brightness percentage of refined Batn El-Ghoul kaolin compared with a standard of brightness (MgCO3) and well-known leached kaolins.
Chemical leaching effects are visually indicated by a definite change in colour of kaolins – from yellow, yellowish brown and reddish brown to off white–white. This phenomenon confirms that the treatment by chemical leaching with sodium hydrosulphite (Na2S2O4) gives a satisfactory reduction in the Fe2O3 content of up to 95% of the initial level ( and ). This leads to a significant increase in the brightness of the kaolin product ( and ).
The iron-oxide content of kaolin is the main cause of the colouration that reduces the commercial value of the mineral product. For some parts of Batn El-Ghoul kaolin deposit, a considerable proportion of the kaolin concentrates contain more than 8 wt. % Fe2O3; the impurity represents an economic problem and requires upgrading of these parts containing more iron from Batn El-Ghoul deposit to meet commercial specifications.
In general, the concentration of the fraction (<44 µm) of the studied kaolins as well as the elimination of the iron and titanium oxides (the colouring materials) using sedimentation and chemical leaching techniques will improve properties so that they are accepted for the production of fine ceramics and for their use in paper industry.
The product of the leaching, iron dithionite or complex ferrous iron can either be recovered from solution or be precipitated as iron hydroxide, and the leaching agents can be recycled to the process.
4. Conclusions
The leaching process appears to be a potentially efficient technology for removing iron impurities from kaolin. After the tests of chemical leaching, the results were considered satisfactory because the kaolin had their brightness values increased between 23% and 32%. These results confirm that the iron occurs in the form of oxides or free minerals and does not substitute in the kaolin structure. In addition, this suggests that the chemical leaching used above is an optimal beneficiation process for removal of the colouring material from Batn El-Ghoul kaolin.
The chemical leaching process of the low-quality kaolin (high colouring impurities) gave the following results:
The brightness of kaolin improved from 56% to 86%.
Removal of 94% of total iron gives a product containing 0.48% Fe2O3, which is suitable for industrial applications.
The kaolin content increased by 14–17% units (with an average from 84% to 93%) after acid ageing.
Brightness properties are improved for use in fine ceramics and as a filler materials in paper, plastic and rubber industries.
Acknowledgements
The author expresses his gratitude to the Tafila Technical University (TTU), Jordan for the technical support of this research work. Special thanks to the Institute of Mineralogy, Leibniz Hannover University, Germany as well as the Department of Physics at Taibah University, KSA for allowing us the use of their research facilities.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Bundy WM. The diverse industrial applications of kaolin. In: HH Murray, W Bundy, C Harvey, editors. Kaolin, genesis and utilization. Boulder (CO): The Clay Mineral Society, Special Publication 1; 1993. p. 43–73.
- Murray HH, Keller W. Kaolins, kaolins, and kaolins. In: H Murray, W Bundy, C Harvey, editors. Kaolin genesis and utilization. Boulder (CO): The Clay Mineral Society; 1993. p. 1–24.
- Grimshaw RW. The chemistry and physics of clays and other ceramic materials. 4th ed. London: Ernest Benn; 1971.
- Grim RE. Clay mineralogy. 2nd edition New York: McGraw-Hill Book Company, International Series in the Earth and Planetary Sciences; 1968, p. 596.
- Muller JP, Calas G. Genetic significance of paramagnetic centers in kaolinites. In: H Murray, W Bundy, C Harvey, editors. Kaolin genesis and utilization. Boulder (CO): The Clay Minerals Society; 1993. p. 261–290.
- Clozel B, Allard T, Muller JP. Nature and stability of radiation-induced defects in natural kaolinites: new results and a reappraisal of published works. Clays Clay Miner. 1994;42:657–666. doi: 10.1346/CCMN.1994.0420601
- Conceição S, Santos NC, Old J, et al. Properties of paper coated with kaolin: the influence of the rheological modifier. Appl Clay Sci. 2005;30:165–173. doi: 10.1016/j.clay.2005.03.010
- Shoumkov S, Dimitrov Z, Brakalov L. High-gradient magnetic treatment of kaolin. Interceram. 1987;6:26–28.
- Prasad MS, Reid KJ, Murray HH. Kaolin: processing, properties and applications. Appl Clay Sci. 1991;6:87–119. doi:10.1016/0169-1317(91)90001-P.
- Borggaard OK. Selective extraction of amorphous iron oxides by EDTA from a Danish sandy loam. J Soil Sci. 1979;30:727–734. doi: 10.1111/j.1365-2389.1979.tb01022.x
- N. Farhount, Beneficiation of kaolins of islands of Milos [PhD thesis]. Athens: National Technical University; 1989.
- Veglio F, Toro L. Process development of kaolin bleaching using carbohydrates in acid media. Int J Miner Process. 1994;41:239–255. doi: 10.1016/0301-7516(94)90031-0
- Veglio F, Passariellio B, Toro L, et al. Development of a bleaching process for a kaolin of industrial interest by oxalic, ascorbic and sulphuric acids: preliminary study using statistical methods of experimental design. Ind Eng Chem Res. 1996;35:1680–1687. doi: 10.1021/ie950427s
- Ambikadevi VR, Lalithambika M. Effect of organic acids on ferric iron removal from iron-stained kaolinite. Appl Clay Sci. 2000;16:133–145. doi: 10.1016/S0169-1317(99)00038-1
- Saikia NJ, Bharali DJ, Sengupta P, et al. Characterization, beneficiation and utilization of a kaolinite clay from Assam, India. Appl Clay Sci. 2003;24:93–103. doi: 10.1016/S0169-1317(03)00151-0
- Guo MR, Lin YM, Xu XP, et al. Bioleaching of iron from kaolin using Fe (III)-reducing bacteria with various carbon nitrogen sources. Appl. Clay Sci. 2010;48:379–383. doi: 10.1016/j.clay.2010.01.010
- He QX, Huang XC, Chen ZL. Influence of organic acids, complexing agents and heavy metals on the bioleaching of iron from kaolin using Fe (III)-reducing bacteria. Appl Clay Sci. 2011;51:478–483. doi: 10.1016/j.clay.2011.01.012
- Musiał I, Cibis E, Rymowicz W. Designing a process of kaolin bleaching in an oxalic acid enriched medium by Aspergillus niger cultivated on biodiesel-derived waste composed of glycerol and fatty acids. Appl Clay Sci. 2011;52:277–284. doi: 10.1016/j.clay.2011.03.004
- Štyriaková I, Štyriak I, Kraus I, et al. Biodestruction and deferritization of quartz sands by Bacillus species. Miner Eng. 2003;16:709–713. doi: 10.1016/S0892-6875(03)00165-1
- Stucki JW. Chapter 11 – Properties and behaviour of iron in clay minerals. In: Bergaya F, Lagaly G, editors. Handbook of clay science. Amsterdam: Elsevier Science; 2013. p. 559–611.
- Zegeye A, Yahaya S, Fialips CI, et al. Refinement of industrial kaolin by microbial removal of iron-bearing impurities. Appl Clay Sci. 2013;86:47–53. doi: 10.1016/j.clay.2013.08.041
- Lee EY, Cho KS, Wook Ryu H. Microbial refinement of kaolin by iron-reducing bacteria. Appl Clay Sci. 2002;22:47–53. doi: 10.1016/S0169-1317(02)00111-4
- Kostka JE, Haefele E, Viehweger R, et al. Respiration and dissolution of iron (III)-containing clay minerals by bacteria. Environ Sci Technol. 1999;33:3127–3133. doi: 10.1021/es990021x
- de Mesquita LMS, Rodrigues T, Gomes SS. Bleaching of Brazilian kaolins using organic acids and fermented medium. Miner Eng. 1996;9:965–971. doi: 10.1016/0892-6875(96)00087-8
- Groudev SN. Biobeneficiation of mineral raw materials. Miner Metall Process. 1999;16:19–28.
- Bertolino LC, Rossi AM, Scorzelli RB, et al. Influence of iron on kaolin whiteness: an electron paramagnetic resonance study. Appl Clay Sci. 2010;49:170–175. doi: 10.1016/j.clay.2010.04.022
- Thurlow C. China clay from Cornwall and Devon: an illustrated account of the modern China clay industry. Cornwall: Cornish Hillside publications; 2001.
- Makarov SV, Silaghi-Dumitrescu R. Sodium dithionite and its relatives: past and present. J Sulfur Chem. 2013;34:444–449. doi:10.1080/17415993.2012.749878.
- Makarov SV, Horvath A, Silaghi-Dumitrescu R, et al. Sodium dithionite, rongalite and thiourea oxides: chemistry and application. London: World Scientific; 2016. p. 244.
- Gougazeh M, Buhl JC. Geochemical and mineralogical characterization of the Jabal Al-Harad kaolin deposit, southern Jordan, for its possible utilization. Clay Miner. 2010;45:281–294. doi: 10.1180/claymin.2010.045.3.301
- M. Gougazeh, Kaolin and feldspar deposits in Jordan: characterization, mining, beneficiation and upgrading process technology, production and economic evaluation for industrial utilization [PhD dissertation]. Aachen: Verlag Mainz, Wissenschaftsverlag; 2001. p. 420.
- C.I. Basilio, Recent advances in the processing of kaolin, Thiele Kaolin Company, special presentation at Virginia Tech, 1997.
- R-H. Yoon, J. Shi, ‘ Processing of kaolin clay’. In: P. Somasundran, editor. Advances in mineral processing. Littleton (CO): Society of Mining Engineers, Inc. 3–5; 1986. p. 366–379.
- Asdell B.K, Wet Processing for brighter kaolin products. Mining Engineering-Annual Meeting; 1967 Nov; Los Angeles. p. 59–65.