?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The mechanism formation of adenine is determinate in the gas phase, by using density functional theory (DFT) method at the B3LYP hybrid together with 6-311g(d,p) basis set level. Thus, we have investigated the structures and mechanisms pathway as well as the barrier energies involved in this study. The results indicate that adenine formation in the space of primitive Earth is very difficult of energetic point of view of both cases tautomers. In addition, the high activation energies induce a very late reaction, so this conclusion increases suggest that the components necessary for the origin of life may have been formed in stars or by the help of the heat of Earth throughout its geologic formation or in solution phase. Subsequently, the prebiotic syntheses of adenine product by oligomerization process under interstellar conditions is an inefficiently in the gas phase.
1. Introduction
Where adenine molecule synthesized, this latter molecule represents a constituent of DNA [Citation1], RNA, and many coenzymes [Citation2]. However, there are literally two choices: is produced within the clouds themselves or is produced elsewhere and transported to the clouds. Moreover, adenine and other products are probably responsible for life begin on Earth. Whereas, numerous experiments have demonstrated that amino acids, nucleotides, carbohydrates, and other essential compounds form under simulated primitive earth conditions from simple starting materials, hydrocarbons, HCN, cyano compounds, aldehydes, and ketones [Citation3,Citation4]. HCN, a high-energy prebiotic precursor, is produced in appreciable amounts. The HCN pentamer adenine is one of the most abundant biochemical molecules. The abiotic synthesis of adenine from a solution of HCN and ammonia was first reported by Oró and colleagues in 1960 [Citation5–8]. Equally, the pentamerization of HCN will provide adenine in long processes formation [Citation9–15]. Adenine synthesis from HCN may occur in the gas phase because HCN has been detected in interstellar clouds [Citation16].
In previous works, we attempt to evaluating the mechanisms and processes of two, three and four HCN reaction [Citation17,Citation18] in order to highlight the possibility of oligomers formation in dense interstellar conditions in the gas phase, then we have shown that each successive step, HCN dimerization and the sequential HCN additions to give dimer, trimer and tetramer is quite exothermic. Moreover, we have been concluded that the production of such precursors in the gas phase necessity high-energy demand in Earth conditions, and that can do by the action of stars, electric discharges on simulated primitive atmospheres [Citation17–19]. Although, the reaction mechanisms involving several intermediates and transition states which are difficult to detect and identify experimentally, however can be studied effectively computationally. These allow selection among various possibilities. Our investigations have identified a plausible detailed step-by-step mechanism for the formation of adenine based on density functional theory computations. Therefore, in this paper, we will study the adenine formation in the gas phase in dense interstellar clouds from the most stable tetramer HCN isomer [Citation18], similarly the mechanism pathways of adenine formation will be evaluated and an accomplished kinetic study will be realized in order to investigate the possibility occurrence of adenine under interstellar conditions.
2. Computational methods
The geometry optimizations of such critical points were performed using the density functional formalism with the B3LYP exchange-correlation energy functional [Citation20,Citation21]. All the calculations were realised with GAUSSIAN G09 program package [Citation22] and visualization of the output files is performed using the Gauss-View 5.0.8 software. All computed harmonic frequencies of fully optimized minima were real, whereas transition structures (states) had a single imaginary frequency. The surface mapping was determined using a 6-311G(d,p) basis set level and the critical points (minima and transition states) were optimized and checked by calculating the intrinsic reaction coordinates (IRCs) with this basis set. All energies have been corrected for zero-point energy (ZPE) contributions calculated at the same level.
3. Results and discussion
3.1. Mechanistic studies of adenine formation from tetramer HCN (AICN)
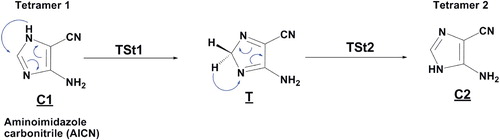
The synthesis of adenine from the pentamerization of HCN has been studied entirely in this paragraph. Moreover, the mechanism reaction characterizing of adenine formation is determinate, and then all structures were fully optimized, and vibrational analyses were performed; thus, the relative energies of the minima and TSs are shown in Figures and . Adenine was shown to be formed from five HCN molecules and the most stable dimer, trimer, and tetramer isomers. Such as the bimolecular complexes of HCN may be isomerized to covalently bound HCN dimer(s) and then to trimer, tetramer and pentamer (adenine) sequentially after an addition of two, three, four and five HCN molecules, respectively [Citation17,Citation18]. In addition, we have shown in this study that the tetramer aminoimidazole carbonitrile 1 (AICN) can tautomerizes easily to tetramer aminoimidazole carbonitrile 2 (AICN) (figure ) with barrier energy not exceeded 5 kcal/mol, although C2 is less stable than C1 about –2 kcal/mol, consequently these tautomers are approximately equal in space, so we will study the two possibilities of adenine formation from tetramer 1 and 2 separately, also we will elucidate the most favourable pathway kinetically and thermodynamically. Subsequently, the detailed step-by-step mechanism for the formation of HCN pentamer from tetramer (1,2) (AICN) is depicted in Figures and .
Figure 2. Proposed steps for the formation of adenine D1 from tetramer (1) with HCN molecule, in the gas phase.
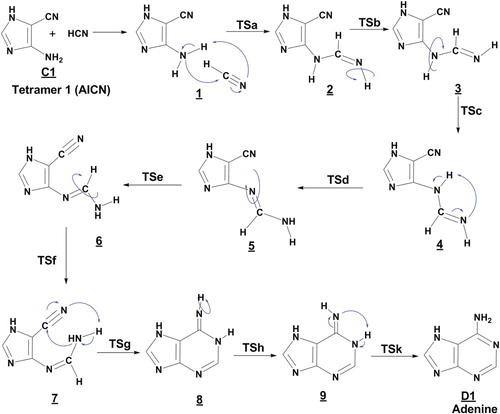
Figure 3. Proposed steps for the formation of adenine D2 from tetramer (2) with HCN molecule, in the gas phase.
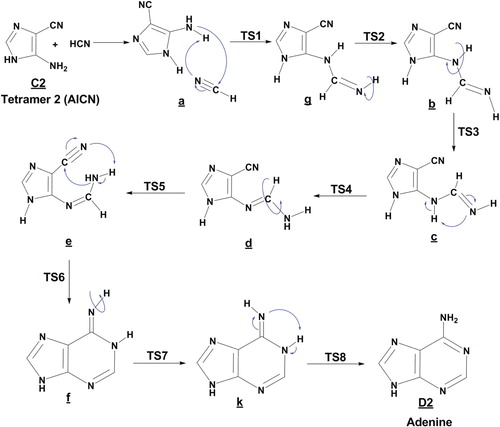
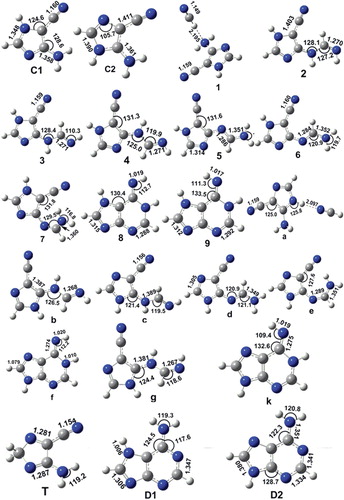
Figure 4. Geometric structures of selected transition states optimised from DFT methods. All the transition states are planar. The distances are in Å and the angles are in degrees, the magnitude of the imaginary frequency is in cm–1.
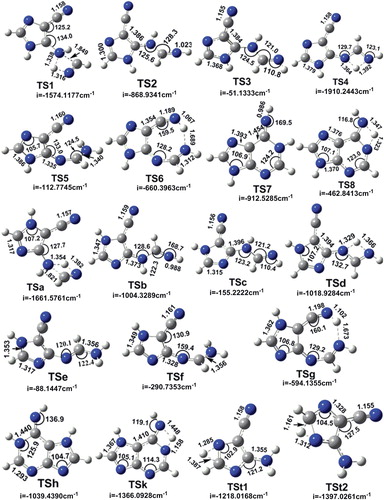
Furthermore, and for more detail, all the mechanisms and structures involved in the adenine formation will be shown. Thus, the minima and transition state of each complex of Figures 1, 2 and 3 are calculated using DFT method and 6-311G(d,p) basis set level, and each TS of HCN isomerization is evaluated by imaginary frequency in the Hessian matrix. The distance and angle parameters of different TSs and minima are drawn and presented in Figures and .
3.2. Barrier energy
In this section, we will study briefly the energetic demand of adenine formation with respect to the proposed mechanisms pathway for both cases of tetramers HCN molecules as noted previously in this work. Therefore, each step of pentamerization process is identified energetically as well as the relative energies of different complexes (minima and transition state) shown in and are determined and represented in Figures and .
Figure 6. Potential energy diagram for the formation of adenine (D1) from tetramer C1(4HCN) with HCN molecule, derived from DFT calculations. The energies are in kcal/mol.
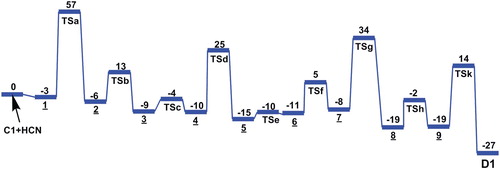
Figure 7. Potential energy diagram for the formation of adenine (D2) from tetramer C2(4HCN) with HCN molecule, derived from DFT calculations. The energies are in kcal/mol.
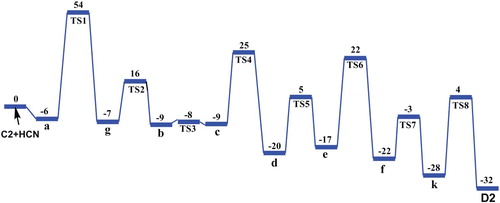
Taking into account the results noted in Figures and , we have revealed that the formation of adenine D(1,2) can be formed from both tetramer AICN (C1 and C2), by the addition of a one HCN molecule. Then, the formation of adenine from the tautomer C1, has a higher overall reaction barrier in which HCN addition to tetramer give the complex 1 then the addition step 1/2 (TSa) passing through a four-centered TS and is represents the rate-limiting step of 57 kcal/mol to give the rotamers 2 and 3, hydrogen shifts from NH and rearrangement of backbone N-C-N in the isomer 4 lead to formation of rotamers 5 and 6, the ring closure procedure occur on the nitrile CN and C-NH2 sequence of compound 7 to form pseudo-adenine 8 with (Z)-imino that requires passing by activation energy to be 34 kcal/mol, this process to be considered more energetic, a subsequent isomerization to its (E)-imino followed by a hydrogen shift from N to NH in the 9 compound lead lastly to form adenine D1 (Figure ).
The formation of adenine from the tautomer C2 has a lower overall reaction barrier compared to that of tautomer C1. The HCN addition step a/g (TS1) is passing through four-centered TS and is the rate-limiting step (Figure ). After isomerizations to rotamer (b); (c) is now formed, hydrogen shift from backbone C–NH–C to N, followed by rearrangement give the rotamer (d). The second ring closure occurs through a hydrogen shift from NH2 to N of the nitrile group of (e), then the rearrangement of pseudo-adenine with (Z)-imino, form (f). After isomerization to its E-isomer (k) followed by hydrogen shift to imine NH from backbone C–N–C, adenine in form D2 is finally formed. Interestingly, when the reaction starts from C1, the overall activation energy is 57 kcal mol–1 which higher than that (54 kcal mol–1) for the reaction starting from C2. This meant that its rate constant would be somewhat smaller than the reaction starting from C2.
Consequently, according to this study the overall activation energies of the pathways obtained from C2 is smaller than C1 tetramer, in despite, the formation of adenine in the interstellar clouds in gas phase is very higher energetically in the normal conditions. In this context, and in order to identify the possibility of adenine occurrence in space spontaneously, to this end we will calculate the rate coefficient of adenine formation.
3.3. Rate coefficients for adenine formation
In this paragraph, we will study the possibility of adenine product to occur efficiently in the interstellar clouds, however and independently of what particular chemically bound formed of such covalent or a simple Van Der Waals of the HCN pentamer is produced, and at this end, the activation energies found for adenine products are 54 and 57 kcal/mol (at 298 K) for D2 and D1 adenine, respectively. In this context, a kinetic analysis was performed. First, the rate constant (k) for the formation of the product cited above from HCN + 4HCN was calculated by the Arrhenius equation as is described below:
where A is the pre-exponential factor, E0 is the critical energy, R is the gas constant, and T is the temperature. A was calculated from the Langevin model [Citation23]:
where e is the electronic charge, α is the polarizability of the neutral reactant, and μ is the reduced mass of the reactants. Thus calculated A value for the reactions of HCN dimer is 1 × 10−9 cm3 molecule–1 s–1 [Citation23]. The overall critical energies are 54 and 57 kcal/mol at 298 K and were used for E0. Therefore, the calculated rate constants are 9.13 × 10−10 cm3 molecule–1 s–1 and 9.09 × 10−10 cm3 molecule–1 s–1 at 298 K of D2 and D1 respectively. So the calculated rate constant of the D2 is (9.13 × 10−10 s–1), on the one hand indicating that the isomerization process of D2 is faster than D1 adenine or iminoacetonitrile (dimer) products and are very slow compared to HCN isomer, trimer and tetramer production, consequently the estimated rate constants is too small, close to zero, for the reaction to occur at 298 K, on the other hand, the activation energies to produce adenine are very expansive to occur efficiently in the Earth conditions in the low temperature.
4. Conclusion
The adenine occurrence in space and how life began on Earth are the purpose of this work, to this end, we have established the mechanisms formation of adenine from both tautomers tetramers C1 and C2 addition to HCN. Thus, the proposed processes pathways and kinetic evaluation lead to conclude that adenine in form D2 is more accepted pathways thermodynamically and kinetically under the conditions prevalent in cloud cores in the oligomerization process. Despite, this compound may not occur efficiently in the atmosphere because of the highest activation energies, besides, adenine formation is a very slow reaction. Consequently, adenine product is likely never going to be overcome spontaneously in interstellar clouds but could happen on planetary bodies close to stars, through geologic heating on the early Earth, or through plasma discharges (lightning) or in another different phase.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Harańczyk M, Miller JH, Gutowski M. Differences in electrostatic potential around DNA fragments containing adenine and 8-oxo-adenine: An analysis based on regular cylindrical projection. J Mol Graph Mod. 2007;26:282–289. doi: 10.1016/j.jmgm.2006.12.005
- Leung W, Hamazaki T, Ostrov DA, et al. Identification of adenine nucleotide translocase 4 inhibitors by molecular docking. J Mol Graph Mod. 2013;45:173–179. doi: 10.1016/j.jmgm.2013.08.016
- Eschenmoser A, Loewenthal E. Chemistry of potentially prebiological natural products. Chem Soc Rev. 1992;21:1–16. doi: 10.1039/cs9922100001
- Orgel LE. Prebiotic chemistry and the origin of the RNA world. Crit Rev Biochem Mol Biol. 2004;39:99–123. doi: 10.1080/10409230490460765
- Oró J. Synthesis of adenine from ammonium cyanide. Biochem Biophys Res Commun. 1960;2:407–412. doi: 10.1016/0006-291X(60)90138-8
- Oro J. Mechanism of synthesis of adenine from hydrogen cyanide under possible primitive earth conditions. Nature. 1961;191:1193–1194. doi: 10.1038/1911193a0
- Oro J, Kimball AP. Synthesis of purines under possible primitive earth conditions. I. adenine from hydrogen cyanide. Arch Biochem Biophys. 1961;94:217–227. doi: 10.1016/0003-9861(61)90033-9
- Oró J, Kimball AP. Synthesis of purines under possible primitive earth conditions. II. Purine intermediates from hydrogen cyanide. Arch Biochem Biophys. 1962;96:293–313. doi: 10.1016/0003-9861(62)90412-5
- Glaser R, Hodgen B, Farrelly D, et al. Adenine synthesis in interstellar space: mechanisms of prebiotic pyrimidine-ring formation of monocyclic HCN-pentamers. Astrobiology. 2007;7:455–470. doi: 10.1089/ast.2006.0112
- Roy D, Najafian K, von Ragué Schleyer P. Chemical evolution: the mechanism of the formation of adenine under prebiotic conditions. Proc Natl Acad Sci. 2007;104:17272–17277. doi: 10.1073/pnas.0708434104
- Ferris JP, Hagan WJ. HCN and chemical evolution: the possible role of cyano compounds in prebiotic synthesis. Tetrahedron. 1984;40:1093–1120. doi: 10.1016/S0040-4020(01)99315-9
- Rehder D. Chemistry in space: from intersteller matter to the origin of life. 1st ed. Weinheim: Wiley-VCH; 2010.
- Ghosh KK, Ghosh SN. A mechanism for the formation of glycine, adenine and guanine in interstellar space. Life Sci Space Res. 1980;18:37–42. doi: 10.1016/B978-0-08-024436-5.50008-1
- Smith IWM, Talbi D, Herbst E. The production of HCN dimer and more complex oligomers in dense interstellar clouds. Astron Astrophys. 2001;369:611–615. doi: 10.1051/0004-6361:20010126
- Kikuchi O, Watanabe T, Satoh Y, et al. Ab initio GB study of prebiotic synthesis of purine precursors from aqueous hydrogen cyanide: dimerization reaction of HCN in aqueous solution. J Mol Struct. Theochem. 2000;507:53–62. doi: 10.1016/S0166-1280(99)00356-5
- Irvine WM. The composition of interstellar molecular clouds. Space Sci Rev. 1999;90:203–218. doi: 10.1023/A:1005258300558
- Benallou A. Understanding the most favourable dimer of HCN for the oligomerization process in the gas phase of interstellar clouds. Comp Theor Chem. 2016;1097:79–82. doi: 10.1016/j.comptc.2016.10.016
- Benallou A. The mechanism determination of trimer and tetramer HCN for adenine formation in the gas phase of interstellar space. Comp Theor Chem. 2017;1101:68–73. doi: 10.1016/j.comptc.2016.12.029
- Abelson PH. Chemical events on the primitive earth. Proc Natl Acad Sci USA. 1966;55:1365–1372. doi: 10.1073/pnas.55.6.1365
- Becke AD. A new mixing of Hartree–Fock and local density-functional theories. J Chem Phys. 1993;98:1372–1377. doi: 10.1063/1.464304
- Lee C, Yang W, Parr RG. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B. 1988;37:785–789. doi: 10.1103/PhysRevB.37.785
- Frisch MJ, et al. GAUSSIAN 09, revision A. 02. Wallingford (CT): GAUSSIAN Inc.; 2009.
- Herbst E. The chemistry of interstellar space. Chem Soc Rev. 2001;30:168–176. doi: 10.1039/a909040a