Abstract
Several studies have reported the occurrence of metal-resistant bacteria and their genes in different wastewater, but there is a dearth of information on wastewater generated from printing operations as a probable source. This study aimed at fingerprinting metal-resistance encoding genes in bacteria recovered from wastewaters of selected printeries in Ibadan, Nigeria. Wastewaters from 10 selected printeries in Ibadan were collected monthly for 12 months. The metal composition of wastewater was determined using Atomic Absorption Spectrophotometry. Metal-resistant bacteria were isolated on metal-supplemented nutrient medium, and characterized using 16S rRNA gene sequencing. Metal-resistance genes were detected using specific primers and the presence of plasmids was determined using alkaline-lysis method. Forty metal-resistant bacteria belonging to six genera; Bacillus, Klebsiella, Pseudomonas, Citrobacter, Providencia and Proteus were identified. cusCBA, encoding resistance to copper and silver was detected in nine bacteria, while pbrA (encoding lead resistance) was detected in seven Pseudomonas aeruginosa isolates. chrA, encoding resistance to chromate ions, was detected in Proteus mirabilis PW3a and two isolates of Pseudomonas aeruginosa, while chrB was detected in Providencia vermicola PWAP3 and Proteus mirabilis PW4c. Bacillus stratosphericus PW1b possessed the copper-resistance genes, pcoA and pcoR. Thirty-six bacteria (90%) of the total bacteria possessed plasmids larger than 10 Kb in size. In conclusion, wastewater generated from printing operations could be a potential source of metal-resistant bacteria and their genes.
1. Introduction
Printing and the processes associated with it occupy a pivotal position in everyday life as no day is complete without human beings having a direct or indirect contact with various forms of printed items. These items include packaged consumer products, books, newspapers, journal articles, computer prints, photocopies and a host of other products. Inks, the organic or inorganic pigments employed in printing process contain coloured and colourless pigment particles dispersed in suitable solvents [Citation1]. Modern inks are very complex compounds in terms of their composition, as they contain along with pigments or dyes, other ingredients generally referred to as vehicles. These materials include: humectants (to control drying), pH modifiers, polymeric resins (for proper binding), anti-foaming agents to regulate foam efficiency, wetting agents such as surfactants (which control surface properties), biocides (to inhibit microbial growth) and thickeners (to control the application and flow of the ink) [Citation2].
Wastewater discharged from printing processes and other related operations has been reported to contain potentially hazardous components such as residual chemicals, dyestuff, solvent residues, pigmented wiping materials, and some toxic heavy metals such as silver (Ag), copper (Cu), zinc (Zn), chromium (Cr), cadmium (Cd), lead (Pb), etc. Metals, apart from the naturally occurring ones, are products of anthropogenic activities such as chemical manufacturing, pigment and dye production, battery manufacturing, automobiles and petrochemicals. All these represent some of the major sources of input of metals into the environment [Citation3]. Pigments used in printing operations, especially the inorganic pigments are usually metallic salts precipitated from solutions; in addition, the organic pigments also contain some metallic compounds in their chemical structures [Citation4]. This makes it possible for bacteria in wastewater generated by printeries to develop metal resistance as a means of coping with the toxicity of metals in the wastewater. Thus wastewaters from printing operations are potential sources of metal-resistant bacteria. This feature notwithstanding has its application in the use of bacteria for metal clean up and bioremediation of metal-contaminated environments.
Although metal resistance has been widely studied in relation to industrial wastewater in Nigeria, none of the studies has investigated printing industry wastewater as a source of metal-resistant bacteria. In addition, no study has investigated the genes responsible for metal resistance in bacteria isolated from wastewater generated by printing industries. The objective of this study was to isolate metal-resistant bacteria from wastewater collected from small and medium scale printing industries in Ibadan, Southwestern Nigeria and assess the incidence of genes encoding resistance to metals in these bacteria.
2. Materials and methods
2.1. Study site
Wastewater samples were collected from 10 printeries located in Mokola, an area which is a hub for small and medium scale printing operations and the University of Ibadan printery, both located in Ibadan, Oyo State, Nigeria. The high concentration of printeries at Mokola was responsible for the selection of the area for sample collection. The University of Ibadan printery is located within the University of Ibadan premises. The printeries collect their wastewater in holding tanks which are emptied into a central drainage channel which connects the entire community to the Ogunpa River in Ibadan, Oyo state, Nigeria.
2.2. Wastewater sample collection
Wastewater samples were collected into pre-sterilized sample containers from the final effluent holding tanks of the printeries and transported in ice chests to the Environmental Microbiology and Biotechnology Laboratory, Department of Microbiology, University of Ibadan. Samples were analysed within six hours of collection.
2.3. Metal composition of printing press industry wastewater
The metal composition of selected printing wastewater was determined using the Atomic Absorption Spectrophotometer (AAS) (UNICAM 929, London Atomic Absorption Spectrophotometer powered by SOLAAR software). The wastewater samples were digested using the nitric acid method [Citation5]. The digested filtrates were then analysed using the cathode lamp of each metal.
2.4. Isolation of bacteria from printing wastewater
Aliquots of serially diluted printing wastewater were plated on nutrient agar (Pronadisa Laboratorios Conda, SA) using the standard pour plate technique. Morphologically distinct colonies of bacteria growing on the plates were repeatedly streaked on fresh plates to obtain pure cultures which were stored in 15% glycerol stock at −80°C for further studies.
2.5. Minimum Inhibitory concentration (MIC) of the metals on bacteria
The isolated bacteria were subjected to increasing concentrations of selected metals on Mueller Hinton agar supplemented with filter-sterilized soluble salts of CuSO4, PbNO3, CdCl2, K2Cr2O7, AgNO3 and ZnSO4. The starting concentration for each metal was 50 μg/mL. The culture growing on the last concentration was transferred to the next higher concentration until the isolates failed to show visible growth. The Minimum Inhibitory Concentration (MIC) was taken as the lowest concentration of the metals that prevented the growth of the bacteria [Citation6,Citation7].
2.6. Identification of the metal-resistant bacteria
The isolates were identified using PCR amplification/sequencing of the 16S rRNA [Citation8]. The PCR products were sequenced (Inqaba Biotech, South Africa) and the sequences were blasted against reference sequences in the GenBank for identification (http://www.ncbi.nlm.nih.gov/BLAST/). Extraction of the DNA was carried out using the ZR 96 Fungal/Bacterial DNA Kit (Zymo Research Corporation, USA). The sequences were submitted to the GenBank and accession numbers were assigned.
2.7. PCR amplification of metal-resistance genes/detection of plasmids
Metal-resistance encoding genes were amplified by PCR with primers targeting the chromium-zinc-cadmium resistance genes czcA, czcB, and czcD; silver resistance genes silCBA, agrCBA and cusCBA; copper resistance genes pcoA and pcoR; chromate resistance genes chrA and chrB, and lead resistance gene pbrA. The reaction mixture in each case contained 12.5 μL of Master Mix, 7.5 μL of PCR quality (Nuclease-free) water, 1.0 μL each of both forward and reverse primers and 3 μL of the DNA template. The annealing temperature for the PCR assays are as follows: 57°C (czcA, czcB, czcD, pcoA, pcoR, chrA and chrB), 55°C (silCBA, agrCBA and cusCBA) and 58°C (pbrA). All reactions included a negative (sterile water) control and a positive control where available. The oligonucleotide primers used in this study are shown in Table . Plasmid DNA was extracted from the bacteria using the alkaline-lysis method [Citation9]. This was necessary because most of the genes targeted (unless stated otherwise) were plasmid-based. Plasmid sizes were determined by comparison with a DNA marker (Thermo Scientific).
Table 1. Oligonucleotide primers used in this study.
3. Results
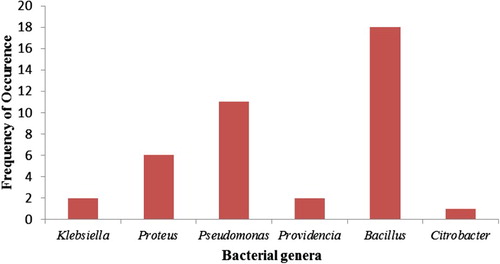
A total of 40 bacteria showing different levels of resistance to five selected metals were obtained. The 16S rRNA gene sequencing showed that the isolates belonged to six genera namely: Bacillus (18), Pseudomonas (11), Proteus (6), Klebsiella (2), Providencia (2) and Citrobacter (1) as shown in Figure .
Table shows the mean metal concentration of wastewater obtained from 3 selected printeries of the 10 sampled. The copper concentration of the wastewater was highest for PPW1 and PPW2 (3.07 and 4.52 mg/L respectively), while zinc was highest in PPW3 (2.22 mg/L) in comparism to the other metals. The least concentration of metal was silver for all the wastewater sampled. In most of the cases, the metal concentrations were more than the National Environmental Regulations (NER) limit [Citation15], except in few instances.
Table 2: Mean metal concentration of wastewater of three selected printeries (mg/L).
3.1. Minimum inhibitory concentration (MIC) of the metals on the bacteria
The bacteria showed varying degree of tolerance to the tested metals. Bacillus stratosphericus PW1b showed the highest level of tolerance to copper (Cu) with an MIC of (650 µg/mL), while the MIC for all the other bacteria ranged from 100 to 500 µg/mL. In the case of lead (Pb), 5 of the isolates were resistant at concentrations <500 µg/mL with the remaining having MIC values ranging from 500 to 550 µg/mL. At a concentration of 500 µg/mL, 37.5% (15) of the total bacteria obtained in this study were able to grow on zinc. However, the MIC range for zinc was between 100 and 400 µg/mL for the remaining isolates. Fourteen of the 40 metal-resistant bacteria, representing 35% were resistant to cadmium with MIC value of 500 µg/mL. With the exception of Proteus mirabilis PW4c (MIC: 0 µg/mL) and Pseudomonas aeruginosa PW5c (MIC: 450 µg/mL) all the other bacteria showed a MIC ranging between 100 and 400 µg/mL for cadmium. Twenty-six of the total bacteria (65%) grew in the presence of 400 µg/mL of silver, with the rest growing at MIC <400 µg/mL (Table ).
Table 3: Minimum Inhibitory Concentration (MIC) of the metals on the bacteria (μg/mL), metal-resistance genes detected and presence of plasmids.
3.2. Detection of metal resistance genes and plasmid profile
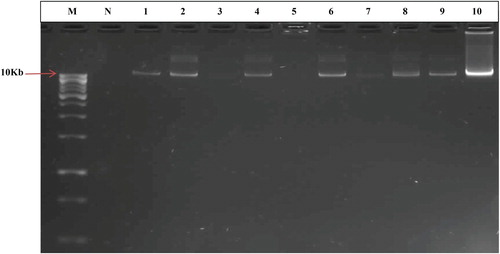
The silver resistance genes, silCBA and agrCBA, and the chromium-zinc–copper resistance genes czcA, czcB and czcD were not detected in any of the isolates. However, copper resistance genes, pcoA and pcoR were both detected in Bacillus stratosphericus PW1b. chrA and chrB encoding chromium resistance were detected in three bacteria (Proteus mirabilis PW3a, Pseudomonas aeruginosa PW5c and Pseudomonas aeruginosa PW3d) and two bacteria (Providencia vermicola PWAP3 and Proteus mirabilis PW4c) respectively. cusCBA was detected in nine isolates namely: Proteus mirabilis PW4c, Bacillus aerius PW1a, B. stratosphericus PW2bb, Bacillus aerophilus PW2a, Klebsiella oxytoca PW4a, Proteus mirabilis PW5e, Providencia vermicola PW2b, Bacillus aerophilus PW3c and Bacillus stratosphericus PW1b. pbrA was detected in seven Pseudomonas aeruginosa strains spread across the sampling sources. The plasmid profile showed that 36 of the 40 metal-resistant bacteria possessed plasmids with sizes larger than 10Kb (Figure ).
Figure 2. Plasmid profile of selected metal-resistant bacteria on 0.8% agarose gel. Lanes represent the following: M: DNA marker (Thermo Scientific), N: Negative control, 1: B. cereus PW5b, 2: P. mirabilis PWN3A, 3: B. aerophilus PWN1A, 4: B. thuringiensis PWN2B, 5: B. stratosphericus PW1e, 6: B. cereus PWA3, 7: B. stratosphericus PW2bb, 8: B. subtilis PWN1C, 9: B. thuringiensis PWN1B, 10: P. aeruginosa PWAP1.
4. Discussion
Various genera of metal-resistant bacteria have been isolated from different sources notably wastewater. Several authors have reported the isolation of metal-resistant bacteria from various sources [Citation7,Citation16–18]. Bacteria adapt to metal stress in their environment and respond to it by developing several resistances or coping mechanisms to its toxicity [Citation19]. This has made the study of bacteria in metal-contaminated environment an interesting one. Furthermore, the presence of metal contaminants in the immediate environment could also act as a precursor in the stimulation of resistance to metal species by bacteria. The printing press wastewater employed in this study had a considerable level of metal contaminants present, and this could have propelled the bacteria therein to develop adaptive features against those metals.
The cop resistance determinants which share a functional similarity with pco resistance determinants have been confirmed to be responsible for copper resistance in Pseudomonas syringae [Citation20], whereas pco are responsible for copper resistance in Escherichia coli. In contrast however, other researchers reported that there is a slight difference in the mechanisms of action of cop- and pco- encoded copper resistance in bacteria. The cop genes are believed to encode the sequestration of copper and higher accumulation [Citation21], whereas that encoded by pco is an energy-dependent export and lower accumulation of copper in the bacterial cell [Citation22,Citation23]. The proposed mechanism of copper resistance in Escherichia coli requires the cooperation of both the plasmid and chromosomal functions to initiate resistance in an integrated fashion [Citation24]. Plasmid-mediated resistance to copper has been reported in several species of bacteria especially Pseudomonas syringae pv tomato and E. coli and documented by several authors [Citation14,Citation24–28]. On the contrary however, chromosomal resistance to copper has also been described in Enterococcus hirae [Citation29,Citation30]. This might be responsible for the phenotypic resistance to copper by some bacteria in this study, even without the possession of the copper resistance determinants.
The range of bacterial hosts in which the pco determinant could function, might be limited to those genera closely related to E. coli, such as Citrobacter, Salmonella, and Shigella, all Gram negative organisms. This is in sharp contrast with the findings from this study, in which Bacillus stratosphericus PW1b, a totally unrelated Gram positive organism, was found to possess the pco gene determinants. The presence of these genes in gram positive group of bacteria could be attributed to the plasmid-borne nature of the gene which might have broadened its host spectrum [Citation24]. Though Chihomvu and his co-workers in 2015 in their study on Klip River in South Africa, reported the detection of the copper resistance gene, pcoA in Lysinibacillus sp. KR25, this study is the first report of the detection of pco genes in any Gram positive bacterium isolated from printing wastewater.
The chrBAC operon is a set of genes harboured by the pMOL28 plasmid of the multi-metal resistant Cupriavidus metallidurans CH3. chrA chromate resistance protein has been detected in strains of Pseudomonas aeruginosa [Citation31]. Two Pseudomonas strains in this study were observed to possess the chrA gene; however Proteus mirabilis PW3a in this study was also detected to possess the same gene. Based on the literatures at our disposal, this is likely to be the first report of the detection of chrA gene in Proteus mirabilis especially from printing wastewater. In addition, it has been reported that bacterial resistance to chromium may be due to either chromosomal mutations [Citation32] or plasmid-mediated [Citation33,Citation34]. The presence of plasmids encoding chromate resistance has also been reported in certain species of Pseudomonas, Alcaligenes, Salmonella, Bacillus and Escherichia coli by several authors [Citation34–39]. In this present study, chrB which regulates the chrA transporter [Citation40] was detected in Providencia vermicola PWAP3 and Proteus mirabilis PW4c and this corroborated the report on the possession of the chr operon on the plasmids of species of Gram negative bacteria [Citation41]. The same authors also reported the detection of the genes in some Bacillus strains isolated from tannery effluent.
The CBA-transport systems which are involved in the export of metal ions, xenobiotics and drugs are exclusively found in Gram negative bacteria. The need for Gram negative cells to safeguard the cytoplasm and translocate metals and other toxicants across their outer membrane has necessitated this system. Contrary to this report however, the cusCBA was detected in some strains of bacteria that are not Gram negative in this study. Five of the seven bacteria possessing the cusCBA in this study were gram-positive, while the remaining belongs to the gram-negative genera. The 6413 bp gene was detected in Proteus mirabilis PW4c, Bacillus aerius PW1a, Bacillus stratosphericus PW2bb, Bacillus aerophilus PW2a, Klebsiella oxytoca PW4a, Proteus mirabilis PW5e, Providencia vermicola PW2b, Bacillus aerophilus PW3c, and Bacillus stratosphericus PW1b. The gene which has also been detected in Escherichia coli is also carried by the pMOL30 plasmid of the well-studied, multi-metal resistant Cupriavidus metallidurans CH34 [Citation42,Citation43].
All the Pseudomonas strains possessing the cusCBA in this present study showed varying resistance to copper and silver as outlined by their MIC to copper and silver ions; and this corroborates the report of some authors who opined that the cus determinant is induced by copper and silver, though the inducement by silver is to a lesser extent compared to copper [Citation44–46]; Their findings were partly corroborated by other reports on the clear contribution of the cus to copper resistance under anaerobic condition [Citation46], they went further to report that the detoxification of copper by the cus system occurred in an oxygen-rich atmosphere. Contrary to this however, it has been reported that the protein of the cus system only mediates resistance to silver and that the conferment of resistance to copper could not be ascertained, even when the copA which encodes the copper-detoxifying P-type ATPase was disrupted in a mutant background. They however showed in their finding that copper was a better inducer of the expression of cus than silver, suggesting a probable involvement of the genes in the resistance of bacteria to copper; as reported by other researchers [Citation47,Citation48].
The pbr proteins are a group of proteins encoded in the widely studied metal-resistant Cupriavidus metallidurans CH34, and they include; PbrT, PbrA, PbrB, PbrC, PbrD and PbrR. The pbrA, is a PIB-type ATPase in Cupriavidus metallidurans, and is the main lead efflux transporter [Citation10]. The gene was detected in 7 of the 40 (17.5%) metal-resistant bacteria obtained in this study. The bacteria found to possess the pbrA gene were all strains of Pseudomonas aeruginosa. Strains of Pseudomonas marginalis and Bacillus megaterium have been observed to show extracellular lead exclusion and intracellular cytoplasmic lead accumulation respectively. Pb-resistant strains of other bacteria e.g. Staphylococcus aureus, Citrobacter freundii and Vibrio harveyi have also been reported [Citation49–52].
Though it was initially thought that the Pbr efflux system was Pb(II) specific. The participation of the Pbr efflux in the protection of the cell wall against Cd (II) and Zn (II) has been reported [Citation53]. The specific mechanisms of Pb(II) resistance require the mutual cooperation of pbrA and pbrB genes hence the two are majorly involved in mediating lead resistance in bacteria e.g. in the metal-resistant Cupriavidus metallidurans [Citation10]. It should be stressed however that the functional roles of the other genes in the Pbr efflux system e.g. PbrT, PbrC and PbrD are still an issue of debate among researchers, because their absence does not in any way impair the ability of the system to neutralize toxic ions in Cupriavidus metallidurans [Citation10,Citation53] However from this present study, all the bacteria were able to tolerate different concentration of lead, even without the possession of the pbrA. This suggests that there might probably be other mechanisms of resistance to the metal possessed by the strains and the possibility of the resistance being chromosome-mediated or being mediated by other gene variants.
Conclusion
This study has highlighted printing industries in Ibadan, Nigeria as a potential contributor of metal-resistant bacteria and their genes into the environment, highlighting an urgent need for the enforcement of regulations regarding wastewater discharge, especially in developing countries of the world where wastewater from the manufacturing sector is discharged into the environment without prior treatment.
Acknowledgements
The authors are also grateful to the University of Fort Hare for providing the platform for the molecular component of this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Abimbola O. Adekanmbi http://orcid.org/0000-0002-2871-1002
Olawale O. Adelowo http://orcid.org/0000-0002-5235-8122
Additional information
Funding
References
- Ahmed S. Technology of printing inks, coating and adhesives. Polymer Sci. 2007.
- J.T. Kunjappu, Essays in ink chemistry. New York: Nova Science Publishers; 2001.
- Jern WNG. Industrial wastewater treatment. Singapore: Imperial College Press; 2006.
- Herbst W, Hunger K. Industrial organic pigments. Third Edition. Copyright © 2004. Weinheim: WILEY-VCH Verlag GmbH and Co. KGaA; 2004. ISBN: 3-527-30576-9.
- Hseu Z. Evaluating heavy metal contents in nine composts using four digestion methods. Biores Technol. 2004;95:53–59. doi: 10.1016/j.biortech.2004.02.008
- Aleem A, Isar J, Malik A. Impact of long-term application of industrial wastewater on the emergence of resistance traits in Azotobacter chroococcum isolated from rhizosphere soil. Bioresour Technol. 2003;86:7–13. doi: 10.1016/S0960-8524(02)00134-7
- Singh V, Chauhan PK, Kanta R, et al. Isolation and characterization of Pseudomonas resistant to heavy metals contaminants. Int J of Pharm Sci Rev and Res. 2010;3:164–167.
- Lane DJ. 16S/23S rRNA sequencing. In: Stackebrandt E and Goodfellow M, editors. Nucleic acid techniques in bacterial systematics. Chichester: 1991. p. 115–175.
- Kado CI, Liu ST. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981;145(3):1365–1373.
- Borremans B, Hobman JL, Provoost A, et al. Cloning and functional analysis of the pbr lead resistance determinant of Ralstonia metallidurans CH34. J Bacteriol. 2001;183:5651–5658. doi: 10.1128/JB.183.19.5651-5658.2001
- Nies A, Nies DH, Silver S. Nucleotide sequence and expression of a plasmid encoded chromate resistance determinants from Alcaligenes eutrophus. J Biol Chem. 1989;265(10):5648–5653.
- Mijnendonckx K. Adaptive silver resistance in Cupriavidus metallidurans. Unpublished Ph.D thesis. Laboratory of Food and Environmental Microbiology (UCL), Microbiology Unit (SCK•CEN), Université Catholique de Louvain – Belgian Nuclear Research Centre. November 7, 2013; 2013.
- Brown NL, Rouch DA, Lee BTO. Copper resistance determinants in bacteria. Plasmid. 1992;27:41–51. doi: 10.1016/0147-619X(92)90005-U
- Brown NL, Barrett SR, Camakaris J, et al. Molecular genetics and transport analysis of the copper resistance determinant (pco) from Escherichia coli plasmid pRJ1004. Mol Microbiol. 1995;17:1153–1166. doi: 10.1111/j.1365-2958.1995.mmi_17061153.x
- National Environmental Regulations (NER). Effluent limitation standards for textile, wearing Apparel Sector. National Environmental Standards and Regulatory Agency (NESREA). Abuja, Nigeria; 2009.
- Dinu LD, Anghel L, Jurcoane S. Isolation of heavy metal resistant bacterial strains from the battery manufactured polluted environment. Rom. Biotechnol Letters. 2011.
- Adekanmbi AO, Falodun OI. Physicochemical, Microbiological and heavy metal studies on water samples and bacteria obtained from Dandaru River in Ibadan. South-Western Nigeria. Afr J Microbiology Res. 2015;9:1357–1365. doi: 10.5897/AJMR2015.7388
- Oriomah C, Adelowo OO, Adekanmbi AO. Bacteria from spent engine-oil-contaminated soils possess dual tolerance to hydrocarbon and heavy metals, and degrade spent oil in the presence of copper, lead, zinc and combinations thereof. Ann Microbiol. 2015;65:207–215. doi: 10.1007/s13213-014-0851-x
- Raja EC, Anbazhagan K, Selvam GS. Isolation and characterization of a metal-resistant Pseudomonas aeruginosa strain. World J of Microbiol Biotechnol. 2006;22:577–585. doi: 10.1007/s11274-005-9074-4
- Chihomvu P, Stegmann P, Pillay M. Identification and Characterization of heavy metal resistant bacteria from the Klip River. Proc Int Conf Ecol, Environ Biol Sci. 2015: 25–26.
- Cooksey DA. Copper uptake and resistance in bacteria. Mol Microbiol. 1993;7:1–5. doi: 10.1111/j.1365-2958.1993.tb01091.x
- Rouch DA. Plasmid mediated copper resistance in Escherichia coli. Ph.D. Thesis. The University of Melbourne, Parkville, Australia; 1986.
- Rouch DA, Lee BTO, Camakaris J. Genetic and molecular basis of copper resistance in Escherichia coli. Mol Biol Chem. 1989: 439–446.
- Williams IR, Morgan AG, Rouch DA, et al. Copper-resistant enteric bacteria from United Kingdom and Australian piggeries. Appl Environ Microbiol. 1993;59:2531–2537.
- Cooksey DA, Azad HA, Cha J, et al. Copper resistance gene homologs in pathogenic and saprophytic bacterial species from tomato. Appl Environ Microbiol. 1990;56:431–435.
- Brown NL, Rouch DA, Lee BTO. Copper resistance determinants in bacteria. Plasmid. 1992;27:41–51. doi: 10.1016/0147-619X(92)90005-U
- Silver S, Lee BTO, Brown NL, et al. Bacterial plasmid resistances to copper, cadmium and zinc. In Chemistry of copper and zinc Triads. The Royal Soc Chem. 1993: 33–53.
- Cooksey DA. Molecular mechanisms of copper resistance and accumulation in bacteria. FEMS Microbiol Rev. 1994;14(4):381–386. doi: 10.1111/j.1574-6976.1994.tb00112.x
- Odermatt A, Suter H, Krapf R, et al. An ATPase Operon involved in copper resistance by Enterococcus hirae. Ann N Y Acad Sci. 1992;471:484–486. doi: 10.1111/j.1749-6632.1992.tb43836.x
- Odermatt A, Suter H, Krapf R, et al. Primary structure of two P-type ATPases involved in copper homeostasis in Enterococcus hirae. J of Biol Chem. 1993;268:12775–12779.
- Alvarez AH, Moreno-Sanchez R, Cervantes C. Chromate efflux by means of the ChrA chromate resistance protein from Pseudomonas aeruginosa. J Bacteriol. 1999;81:7398–7400.
- Ohta N, Galsworthy PR, Pardee AB. Genetics of sulfate transport in Salmonella typhimurium. J of Bacteriol. 1971;105:1053–1106.
- Cervantes C, Silver S. Plasmid chromate resistance and chromate reduction. Plasmid. 1992;2:65–71. doi: 10.1016/0147-619X(92)90007-W
- Mondaca MA, Gonzalez CL, Zaror CA. Isolation, characterization and expression of a plasmid encoding chromate resistance in Pseudomonas putida T2441. Lett Appl Microbiol. 1998;26:367–371. doi: 10.1046/j.1472-765X.1998.00349.x
- Cervantes C, Ohtake H. Plasmid determined resistance to chromate in Pseudomonas aeruginosa FEMS. Microbiol Lett. 1988;56:173–176. doi: 10.1111/j.1574-6968.1988.tb03172.x
- Collard JM, Corbiser P, Diel SL, et al. Plasmids for heavy metal resistance in Alcaligenes eutrophus CH34: mechanisms and applications. FEMS Microbiol Rev. 1994;14:404–414. doi: 10.1111/j.1574-6976.1994.tb00115.x
- Ghosh A, Singh A, Ramteke PW, et al. Characterization of large plasmids encoding resistance to toxic heavy metals in Salmonella abortus equi. Biochem Biophy Res Comm. 2000;272:6–1. doi: 10.1006/bbrc.2000.2727
- Verma T, Garg SK, Ramteke PW. Effect of ecological factors on conjugal transfer of chromium resistant plasmid in Escherichia coli isolated from tannery effluent. Appl Biochem Biotechnol. 2002;102:103), :5–20.
- Kamala-Kannan S, Lee KJ. Metal tolerance and antibiotic resistance of Bacillus species isolated from Sunchon Bay sediments. South Korea Biotechnol. 2008;7:149–152.
- Juhnke S, Peitzsch N, Hübener N, et al. New genes involved in chromate resistance in Ralstonia metallidurans strain CH34. Arch Microbiol. 2002;179:15–25. doi: 10.1007/s00203-002-0492-5
- Verma T, Garg SK, Ramteke PW. Genetic correlation between chromium resistance and reduction in Bacillus brevis isolated from tannery effluent. J Appl Microbiol. 2009;107:1425–1432. doi: 10.1111/j.1365-2672.2009.04326.x
- Janssen PJ, Van Houdt R, Moors H, et al. The complete genome sequence of Cupriavidus metallidurans strain CH34, a master survivalist in harsh and anthropogenic environments. PLoS One. 2010;5:e10433. doi: 10.1371/journal.pone.0010433
- Van Houdt R, Mergeay M. Plasmids as Secondary Chromosomes. Mol Life Sci. 2012: 1–4.
- Munson GP, Lam DL, Outten FW, et al. Identification of a copper-responsive two-component system on the chromosome of Escherichia coli K-12. J Bacteriol. 2000;182:5864–5871. doi: 10.1128/JB.182.20.5864-5871.2000
- Grass G, Rensing C. Genes involved in copper homeostasis in Escherichia coli. J Bacteriol. 2001;183:2145–2147. doi: 10.1128/JB.183.6.2145-2147.2001
- Outten FW, Huffman DL, Hale JA, et al. The independent cue and cus systems confer copper tolerance during aerobic and anaerobic growth in Escherichia coli. J Biol Chem. 2001;276:30670–30677. doi: 10.1074/jbc.M104122200
- Franke S, Grass G, Nies DH. The product of the ybdE gene of the Escherichia coli chromosome is involved in detoxification of silver ions. Microbiol. 2001;147:965–972. doi: 10.1099/00221287-147-4-965
- Silver S. Bacterial silver resistance: molecular biology and uses and misuses of silver compounds. FEMS Microbiol Rev. 2003;27:341–353. doi: 10.1016/S0168-6445(03)00047-0
- Levinson HS, Mahler I, Blackwelder P, et al. Lead resistance and sensitivity in Staphylococcus aureus. FEMS Microbiol Lett. 1996;145:421–425. doi: 10.1111/j.1574-6968.1996.tb08610.x
- Levinson HS, Mahler I. Phosphatase activity and lead resistance in Citrobacter freundii and Staphylococcus aureus. FEMS Microbiol Lett. 1998;161:135–138. doi: 10.1111/j.1574-6968.1998.tb12939.x
- Roane TM. Lead resistance in two bacterial isolates from heavy metal contaminated soils. Microbial Ecol. 1999;37:218–224. doi: 10.1007/s002489900145
- Mire CE, Tourjee JA, O’Brien WF, et al. Lead precipitation by Vibrio harveyi: evidence for novel quorum-sensing interactions. Appl Environ Microbiol. 2004;70:855–864. doi: 10.1128/AEM.70.2.855-864.2004
- Hynninen A, Touze T, Pitkanen L, et al. An efflux transporter PbrA and a phosphatase PbrB cooperate in a lead-resistance mechanism in bacteria. Mol Microbiol. 2009;74:384–394. doi: 10.1111/j.1365-2958.2009.06868.x