?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
An elegant and eco-friendly photocatalytic degradation approach for the most commonly used herbicide, Atrazine (ATZ); allowed a selective and partial degradation into atrazine-2-hydroxy (Hydroxyatrazine, HAT), which was the sole obtained metabolite. The effect of tungstosilicates and tungstophosphates as photocatalysts under 60 min UV irradiation at two different wavelengths; 254 and 366 nm; was investigated by measuring the absorbance at 10-min intervals, using 720-UV spectrophotometry. ATZ conversion rate was the highest when irradiating with the higher-energy 254 nm UV light. This resulted into 54% degradation in the presence of the silicon-based α-Keggin dodecatungstosilicate [α-SiW12O40]4−, whereas 17% was decomposed in its absence. The nature of the heteroatom as well as the structural type of the studied polyoxometalates had a significant effect on the degradation percentage. Accordingly, 31% of ATZ was only decomposed in presence of the phosphorus-analogue [α-PW12O40]4− whereas the cyclic super-lacunary octatetracontatungstooctaphosphate [P8W48O184]40− increased the degradation to 41%.
1. Introduction
The contamination of our water supplies as well as our food chain by various persistent organic pollutants has emphasized the attention of many researchers since several decades [Citation1–8]. Herbicides are phototoxic chemicals that have been identified as organic pollutants due to their ubiquitous influence on the environment and their ability to remain nondegradable for long periods of time [Citation9–12]. Atrazine, 6-chloro-N-ethyl-N’-(1-methylethyl)-triazine-2,4-diamine (ATZ), was ranked as the second mostly used agricultural herbicide [Citation13–16]. Its usage has heavily intensified in many countries especially in the Middle East region [Citation17]. Moreover, this pesticide was found to be an endocrine disruptor that can affect reproduction and development [Citation18]. It is highly persistent in the environment, lasting from days up to several years, due to the stability of the s-triazine ring that doesn't undergo any natural degradation. Therefore, the use of this herbicide is restricted in many countries nowadays [Citation19]. The US Environmental Protection Agency announced that the legal limit for ATZ in drinking water is just 3 ppb [Citation20]. In spite of these regulations, ATZ have been reported to exceed the recommended value in many countries [Citation21–23].
UV irradiation has been an alternative technology for water purification and treatment of various hazardous contaminants in the presence of different catalysts. Many studies have reported the use of ozone gas, hydrogen peroxide, zinc oxide and titanium oxide as photocatalysts for the degradation of Atrazine by both oxidative and reductive pathways [Citation24–27]. Accordingly, the degradation of ATZ can produce 11 different metabolites depending on the used degradation process [Citation28]. Table summarizes the obtained metabolites from different degradation processes. Our study adds yet another approach, however unique, for the degradation of ATZ using for the very first time a highly-selective and discrete metal–oxo cluster facilitating the degradation into only one major metabolite, atrazine-2-hydroxy (HAT), which can be easily monitored.
Table 1. Atrazine degradation products of some reported degradation methods in the literature.
Hence, metal oxide clusters, known as polyoxometalates (POMs), have drawn the attention of many researches as photocatalysts in various reactions of both organic and inorganic pollutants [Citation35–37]. It has been found that these POMs offer many advantages as homogeneous photocatalysts, mainly due to being highly redox active [Citation38–41]. Additionally, POMs experience strong light absorption in the UV region as well as in the visible region for those containing molybdenum and vanadium [Citation42]. The irradiation of POM with UV light below 400 nm activates their oxidizing ability by the formation of OH radicals that are able to decompose a certain organic substrate, denoted here by “S”, according to the following scheme. Furthermore, the structural framework of the POMs is totally maintained during the photoredox chemical reaction thus reversible electron transfer occurs easily [Citation43,Citation44].
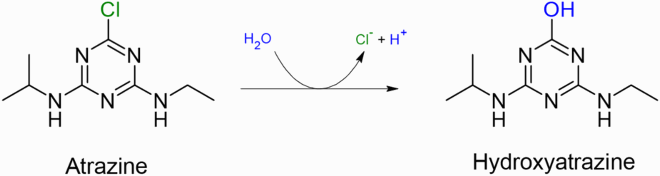
The reported study here illustrates the photocatalytic effect of several tungstosilicates and tungstophosphates on the degradation of Atrazine in the presence of UV irradiations at either 254 nm or 366 nm. The nature of the heteroatom present in the examined clusters as well as the effect of tungsten atoms have been investigated in our work. The degradation product of ATZ is also identified, as evident according to the following reaction:
2. Materials and methods
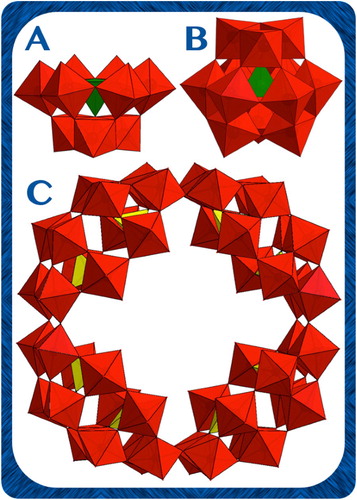
All chemicals were purchased from Sigma Aldrich and were used without any further purification. The examined POMs (Figure ); [SiW9O34]10− {SiW9}, [PW9O34]9− {PW9}, [SiW12O40]4− {SiW12}, [PW12O40]3− {PW12}, and [P8W48O184]40− {P8W48}; were synthesized according to published procedures in the literature [Citation45,Citation46] and fully characterized by FTIR spectroscopy to confirm identity at the fingerprint region as well as purity [Citation47–50]. Standard solution of Atrazine was prepared in acetonitrile solvent (HPLC-grade) at 20 ppm and stored in dark at 0°C, no more than 15 days. Aqueous solutions of the mixture (Atrazine + POM) were freshly prepared by mixing the same mass (2.5 mg) of the tested POM salts in 25 mL of 2 × 10−5 M ATZ solution.
Figure 1. Polyhedral representation of the used POM photocatalysts. (A) XW9 (X = Si, P), (B) XW12 (X = Si, P), and (C) P8W48. Colour code, polyhedra: WO6 (red), XO4 (green or yellow) (X = Si or P).
A custom-built photoreactor was used to study the photocatalytic degradation of the herbicide. Two 8 W UV-Xenon lamps were used for irradiation with different emissions (254 and 366 nm) equipped with a circulating water flow thermostat. All experiments were conducted in closed vials and repeated thrice. The variation in the reaction mixture was monitored by measuring the absorbance via full spectrum every 10 min for an hour using 720-UV spectrophotometry.
3. Results and discussion
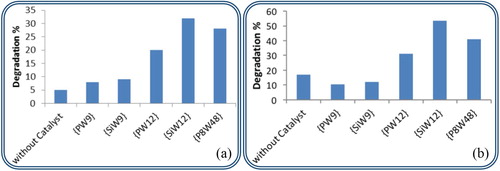
Atrazine solution was irradiated at both wavelengths separately for 60-min period in the absence of any photocatalyst and its full UV spectrum was measured. The obtained results suggest that the wavelength has a significant effect on the degradation percentage. 17% of ATZ was degraded upon irradiation at 254 nm whereas only 5% were being decomposed at 366 nm UV light.
Figure (a) represents the degradation percentage of ATZ in the absence and presence of the examined photocatalysts upon irradiation at 366 nm UV light. Better results were obtained after irradiation using 254 nm (Figure (b)) since the energy of a UVC light is much higher than that of UVA causing faster degradation and more efficient processes [Citation5].
Figure 2. (a,b) The degradation percentage of ATZ in the absence and presence of photocatalyst under UV irradiation at 366 and 254 nm, respectively.
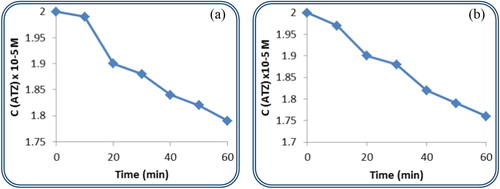
The effect of nonatungstophosphate {PW9} and nonatungstosilicate {SiW9} on the decomposition of the ATZ herbicide is illustrated in Figure (a,b), respectively, under 254 nm UV irradiation. These two metal oxide clusters belong to the same Keggin structural family, both of which are trilacunary. The behaviour of both catalysts is the same till 30 min of irradiation. As time proceeds the tungstosilicate succeeded to degrade 14% of ATZ whereas the presence of tungstophosphate resulted in 11% degradation.
Figure 3. (a,b) The variation in concentration of ATZ in the presence of {PW9} and {SiW9}, respectively, with irradiation time.
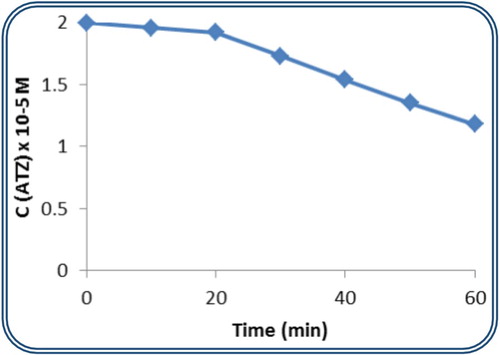
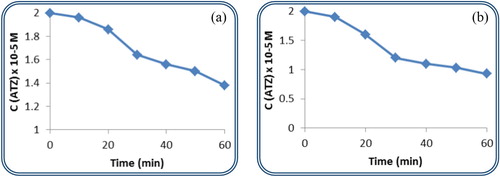
The plenary and complete Keggin-structured polyoxometalates {PW12} and {SiW12} have been investigated as photocatalysts for the treatment of Atrazine. Their effect is shown in Figure (a,b), respectively. The concentration of ATZ drops directly after 10 min of irradiation in the presence of dodecatungstosilicate to reach 0.93 × 10−5 M at 60 min. The degradation percentage reaches 54% in the presence of this photocatalyst. However, the dodecatungstophosphate catalyst reaches a modest 31% of ATZ degradation.
Figure 4. (a,b) The variation in concentration of ATZ in the presence of {PW12} and {SiW12}, respectively, with irradiation time.
On the other hand, the effect of the cyclic superlacunary octatetracontatungstooctaphosphate salt {P8W48} was also examined in this study. This polyanion was able to degrade 41% of ATZ under the studied experimental conditions (Figure ). The behaviour of this catalyst in the reaction mixture is somehow different than the other photocatalysts in our work. The concentration of ATZ gradually dropped in a linear manner after 20 min of UV irradiation.
As seen from the above results, the Si-based POMs gave better results than P-based ones. This can be attributed to the important influence of the heteroatom nature on the photocatalytic activity of the polyoxometalates [Citation51]. The bond length of X-O affects the electron density on the W-Oaxial that in turn highly affects the redox properties of these metal oxide clusters. The long Si-O bond lengths compared to that of P-O increases the electron density at the tungsten atoms in the Keggin-structured {SiW12} [Citation52].
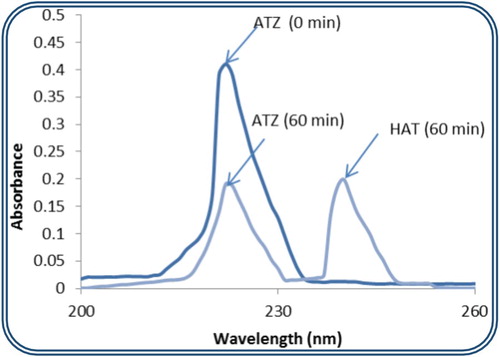
The degradation product resulting from the photodegradation of ATZ in the presence of {SiW12} as the best photocatalyst in our study was determined. As shown in Figure , the only peak appearing at t0 is related to ATZ whose maximum wavelength was found to be 222 nm. However, at the end of our experiment the absorbance at 222 nm drops indicating a loss in ATZ concentration and a new peak forms at 244 nm. This new peak is due to the formation of HAT [Citation27] as the only metabolite for the ATZ decomposition under our experimental conditions.
4. Conclusion
Polyoxometalates, being discrete, environmentally benign, and thermally stable, are promising photocatalysts for the selective degradation of Atrazine to Atrazine-2-hydroxy, as the only produced metabolite. The use of Keggin-type dodecatungstosilicate anion resulted in 54% decomposition under simple experimental conditions, which could be easily mimicked under natural circumstances. The catalytic effect of octatetracontatungstooctaphosphate polyanion {P8W48} on the degradation of atrazine was firstly reported here. This complex succeeded to degrade 41% of the studied pollutant. UVC irradiation showed better effect than UVA on the catalytic activity of the studied polyoxometalates. Finally, the kinetic behaviour of these polyanions as well as other affecting parameters on the photodegradation of Atrazine is going to be reported in our next paper.
Acknowledgment
The authors would like to thank Beirut Arab University for research support and facilities, as well as Nour El Ghouch for her kind and much appreciated help.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Loubna Youssef http://orcid.org/0000-0002-5503-5708
Ghassan Younes http://orcid.org/0000-0001-6927-3523
Rami Al-Oweini http://orcid.org/0000-0003-1891-3716
References
- Readman JW, Albanis TA, Barcelo D, et al. Herbicide contamination of Mediterranean estuarine waters: results from a MED POL pilot survey. Mar Pollut Bull. 1993;26:613–619. doi:10.1016/0025-326X(93)90500-J
- Gray N. Drinking water quality. problems and solutions. Chichester: John Wiley & Sons; 1994. p. 132–148.
- Encyclopedia of surface and colloid science. New York: Marcel Dekker; 2002.
- Ali I, Aboul-Enein HY. Instrumental methods in metal ions speciation: chromatography, capillary electrophoresis and electrochemistry. New York: Taylor & Francis; 2006.
- Chen C, Yang SG, Guo YP, et al. Photolytic destruction of endocrine disruptor atrazine in aqueous solution under UV irradiation: products and pathways. J Hazard Mater. 2009;172:675–684.
- Ali I, Aboul-Enein HY, Gupta VK. Nanochromatography and nanocapillary electrophoresis: pharmaceutical and environmental analyses. Hoboken (NJ): Wiley & Sons; 2009.
- Gupta VK, Ali I. Environmental water: advances in treatment, remediation and recycling. Amsterdam: Elsevier; 2012.
- Basheer AA. Chemical chiral pollution: impact on the society and science and need of the regulations in the 21st century. Chirality. 2018;30(4):402–406. doi:10.1002/chir.22808
- Ali I, Jain CK. Groundwater contamination and health hazards by some of the most commonly used pesticides. Curr Sci. 1998;75(10):1011–1014.
- Ali I, Aboul-Enein HY. Determination of chiral ratio of o,p-DDT and o,p-DDD pesticides on polysaccharides chiral stationary phases by HPLC under reversed-phase mode. Environ Toxicol. 2002;17(4):329–333. doi:10.1002/tox.10069
- Gupta PK. Reproductive and developmental toxicology. Academic Press: Elsevier; 2011. p. 503–521.
- Ali I, ALOthman ZA, Al-Warthan A. Sorption, kinetics and thermodynamics studies of atrazine herbicide removal from water using iron nano-composite material. Int J Environ Sci Technol. 2016;13(2):733–742. doi:10.1007/s13762-015-0919-6
- Neskovic NK, Elezovic I, Karan V, et al. Acute and subacute toxicity of atrazine to carp (Cyprinus carpio L.). Ecotoxicol Environ Safety. 1993;25:173–182.
- Jain CK, Ali I. Determination of pesticides in water, sediments and soils by gas chromatography. Int J Environ Anal Chem. 1997;68(1):83–101. doi:10.1080/03067319708030482
- Sanagi MM, Muhammad SS, Hussain I, et al. Novel solid-phase membrane tip extraction and gas chromatography with mass spectrometry methods for the rapid analysis of triazine herbicides in real waters. J Sep Sci. 2015;38(3):433–438. doi:10.1002/jssc.201400912
- Ali I, Al-Othman ZA, Al-Warthan A. Removal of secbumeton herbicide from water on composite nanoadsorbent. Desalin Water Treat. 2016;57(22):10409–10421. doi:10.1080/19443994.2015.1041164
- Pathak RK, Dikshit AK. Atrazine and its use. Int J Res Chem Environ. 2012;2:1–6.
- Hofman RS, Capel PD., Larson SJ. Comparison of pesticides in eight U.S. urban streams. Environ Toxicol Chem. 2000;19:2249–2258.
- The list of priority substances in the field of Water Policy 2455/2001/EC (2001).
- ChemicalSummary, Atrazine, Toxicityand ExposureAssessment for Children’s Health. In: USEPA, editor. U.S.2007.
- Toxicological Profile for Atrazine. U.S. Department of health and human services; 2003.
- Indicator Fact Sheet (WHS1a) Pesticides in Groundwater, (2004).
- Atrazine and Its Metabolites in Drinking Water, 2011.
- Arantegui J, Prado J, Chamarro E, et al. Kinetics of the UV degradation of atrazine in aqueous solution in the presence of hydrogen peroxide. J Photochem Photobiol A Chem. 1995;88:65–74.
- Beltrán FJ, González M, Rivas FJ, et al. Aqueous UV radiation and UV/H2 O2 oxidation of atrazine first degradation products: deethylatrazine and deisopropylatrazine. Environ Toxicol Chem 1996;15:868–872.
- Balmer ME, Sulzberger B. Atrazine degradation in irradiated iron/oxalate systems: effects of pH and oxalate. Environ Sci Technol. 1999;33: 2418–2424.
- Moreira AJ, Borges AC, Gouvea LFC, et al. The process of atrazine degradation, its mechanism, and the formation of metabolites using UV and UV/MW photolysis. J Photochem Photobiol A Chem. 2017;347:160–167.
- Burrows HD, Canle M, Santaballa JA, et al. Reaction pathways and mechanisms of photodegradation of pesticides. J Photochem Photobiol B Biol. 2002;67:71–108.
- Xu LJ, Chu W, Graham N. Atrazine degradation using chemical-free process of USUV: analysis of the micro-heterogeneous environments and the degradation mechanisms. J Hazard Mater. 2014;275:166–174.
- Zhanqi G, Shaogui Y, Na T, et al. Microwave assisted rapid and complete degradation of atrazine using TiO2 nanotube photocatalyst suspensions. J Hazard Mater. 2007;145:424–430.
- Ta N, Hong J, Liu T, et al. Degradation of atrazine by microwave-assisted electrodeless discharge mercury lamp in aqueous solution. J Hazard Mater. 2006;138:187–194.
- Hu E, Cheng H, Hu Y. Microwave-Induced degradation of atrazine sorbed in mineral micropores. Environ Sci Technol. 2012;46:5067–5076.
- Hu E, Cheng H, Hu Y. Catalytic effect of transition metals on microwave-induced degradation of atrazine in mineral micropores. Water Res. 2014;57:8–19.
- Li K, Chen T, Yan L, et al. Design of graphene and silica co-doped titania composites with ordered mesostructure and their simulated sunlight photocatalytic performance towards atrazine degradation. Colloids Surf A Physico Chem Eng Asp. 2013;422:90–99.
- Troupis A. Photocatalytic reduction—recovery of silver using polyoxometalates. Appl Catal B Environ. 2003;42:305–315.
- Zhang J, Goh J-K, Tan W. Mechanistic analysis of the electrocatalytic properties of dissolved α and β isomers of [SiW12O40]4- and Solid [Ru(bipy)3]2[α-SiW12O40] on the reduction of nitrite in acidic aqueous media. Inorg Chem. 2006;45:3732–3740.
- Song L, Zhang S, Wu X, et al. One-step synthesis of composite semiconductor AgBr/Ag5P3O10 heterojunctions and their photocatalytic activity, kinetic analysis, photocatalytic mechanism under visible light radiation. Chem Eng J. 2013;214:336–342.
- Kortz U, Müller A. Introduction: a special issue dedicated to Michael T. Pope. J Cluster Sci. 2006;17(2):139–141. doi:10.1007/s10876-006-0065-x
- Kortz U. Polyoxometalates. Eur J Inorg Chem. 2009;2009(34):5056), doi:10.1002/ejic.200990096
- Kortz U, Müller A, van Slageren J, et al. Polyoxometalates: fascinating structures, unique magnetic properties. Coord Chem Rev. 2009;253(19-20):2315–2327. doi:10.1016/j.ccr.2009.01.014
- Kortz U, Liu T. The best of polyoxometalates. Eur J Inorg Chem. 2013;2013(10-11):1559–1560. doi:10.1002/ejic.201300230
- Al-Oweini R, Aghyarian S, El-Rassy H. Immobilized polyoxometalates onto mesoporous organically-modified silica aerogels as selective heterogeneous catalysts of anthracene oxidation. J Sol-Gel Sci Technol. 2012;61(3):541–550. doi:10.1007/s10971-011-2657-7
- Al-Oweini R, Sartorel A, Bassil BS, et al. Photocatalytic water Oxidation by a mixed-valent MnIII3MnIVO3 manganese oxo core that mimics the Natural Oxygen-Evolving Center. Angewandte Che - International Edition. 2014;53(42):11182–11185. doi:10.1002/anie.201404664
- Natali M, Bazzan I, Goberna-Ferrón S, et al. Photo-assisted water oxidation by high-nuclearity cobalt-oxo cores: tracing the catalyst fate during oxygen evolution turnover. Green Chem. 2017;19(10):2416–2426. doi:10.1039/c7gc00052a
- Canny J, Teze A, Thouvenot R, et al. Disubstituted tungstosilicates. 1. Synthesis, stability, and structure of the lacunary precursor polyanion of a tungstosilicate.gamma.-SiW10O368-. Inorg Chem. 1986;25:2114–2119.
- Ginsberg AP. Inorganic syntheses. Berkeley Heights (NJ): Wiley-Interscience; 1990; Vol. 27.
- Al-Oweini R, El-Rassy H. Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si(OR)4 and R′′Si(OR′)3 precursors. J Mol Struct. 2009;919(1-3):140–145. doi:10.1016/j.molstruc.2008.08.025
- Al-Oweini R, Bassil BS, Palden T, et al. The manganese(III)-containing tungstophosphate [MnIII 3(H2O)5(A-α-PW9O 34)2]9. Polyhedron. 2013;52:461–466. doi:10.1016/j.poly.2012.08.050
- Al-Oweini R, Bassil BS, Friedl J, et al. Synthesis and characterization of multinuclear manganese-containing tungstosilicates. Inorg Chem. 2014;53(11):5663–5673. doi:10.1021/ic500425c
- Al-Oweini R, Bassil BS, Itani M, et al. The mixed-valent 10-manganese(III/IV)-containing 36-tungsto-4-arsenate(V), [MnIII6MnIV4O4(OH)12(H2O)12(A-[beta]-AsW9O34)4]22. Acta Crystallogr Sect C. 2018;74(11):1390–1394. doi:10.1107/S2053229618014183
- Poblet JM, López X, Bo C. Ab initio and DFT modelling of complex materials: towards the understanding of electronic and magnetic properties of polyoxometalates. Chem Soc Rev. 2003;32:297–308.
- Allmen KV, Moré R, Mìller R, et al. Nickel-containing Keggin-type polyoxometalates as hydrogen evolution catalysts: Photochemical structure-activity relationships. ChemPlusChem. 2015;80:1389–1398.