ABSTRACT
In this study, a mice model was devised to elucidate and compare the adverse effects caused by fungi isolated from outdoor locations in Lagos, Nigeria. The effects caused by Aspergillus flavus, Aspergillus penicilloides, Penicillium chrysogenum and Penicillium citrinum were investigated. Both the doseresponse and time-course of the inflammatory and toxic responses were investigated and histopathological analysis was also performed on the lungs. Biochemical parameters and histopathology revealed that all the microbes studied provoked inflammation after a single dose, but the magnitude and its characteristic features were different. The spores of P. chrysogenum provoked the most intense acute inflammation indicated by a strong and rapid monocytes cells production in the lungs. In summary, the results show that the different fungus have different potential to cause inflammatory and toxic responses after airway exposure in mice and suggest that fungi present in the environments can induce inflammation in lungs and cause systemic toxicity.
1. Introduction
In spite of several studies that have associated respiratory symptoms with air quality in different work environments, only limited information exists on biological exposure of allergenic fungi and associated health effects from different locations in Sub-Saharan Africa especially Nigeria. Epidemiological studies have shown that a few hundred million people around the world are exposed to biological agents such as fungi. Unfortunately, there are no quantitative health-based guideline values or thresholds for acceptable levels of microbial contamination. Among the most common fungal species present in the environment were those from genera Aspergillus and Penicillium, which are broadly present in nature including air, soil, cereal grains, hay and other plant materials or foodstuff. Fungi are hard to avoid as millions are found in pillows, leaf surfaces, compost, air conditioning, heating systems, damp houses and in all locations. They thrive in warm and damp environments, both outdoors and indoors. Humans are exposed to fungi throughout life via inhalation, digestion and other sources of entry. Fungi cause lung disease by different mechanisms such as allergic asthma [Citation1,Citation2], infections (e.g. aspergillosis) [Citation3,Citation4], and inflammation (e.g. hypersensitivity pneumonitis, and farmer’s lung disease) [Citation5,Citation6]. Mycoses is a diseases condition in which fungi pass the resistance barriers of human and animals and establish infections with varied clinical manifestations. Garcia and Blanco [Citation7] reported that they are widely distributed in the environment and therefore very difficult to eradicate. Aerosol dispersal of pathogens such as bacteria and fungi pose important health problems and it is associated with a variety of adverse health effects, especially inflammatory responses in the lower airways. Exposure to microbial spores has been suspected to be one reason for inflammation, but the inflammatory and toxic potential of some fungi have not been well characterized. This research therefore aims at evaluating the inflammatory and Immunoglobulin E response in lungs of albino mice induced by four different fungi species isolated from different locations in Southwest Nigeria.
2. Isolation and identification of fungi
Fungal spores were sampled from the air in two states in Southwestern Nigeria by an open plate method using Dichloran glycerol-18 and Potato dextrose agar (PDA). The commonly isolated species which are: (1) A. flavus, (2) A. penicilloides, (3) P. chrysogenum and (4) P. citrinum were screened further and used for the experiment. These species were selected since they were the most frequently isolated organisms found in all the sampled locations in Oyo and Lagos, Nigeria. After the sampling, the DG-18 and PDA plates were incubated at 25°C up to 7 days. With the use of lactophenol blue solution, a wet mount preparation of the fungi was prepared and this was observed microscopically. Identification of the same was based on the appearance of the colony, microscopic examination of the spore and the hyphal characteristics. Molecular characterization using ITS 1 and 4 primers were also used to confirm the fungi identity.
All the fungal strains were cultured and identified in the Mycology Laboratory of the Department of Botany, University of Lagos, Nigeria.
3. Animals
Sixty-three male BALB/c mice (10–13 g) were used in the study. The animals were obtained from the breeding colony of Department of Veterinary pathology and Medicine, University of Ibadan. They were housed in metal cages on wooden chips one week before the experiment started. Animals received water and food (maintenance diet for rats and mouse, Ladokun feed, Ibadan, Nigeria) ad libitum. The mice were on a 12-h light and dark rhythm at the average room temperature. They were anesthetized with ketamine (10 mg/kg) intraperitoneally (i.p.) before fungal spores were instilled intranasally.
4. Fungi cell counting
Fungi cells were counted by haemocytometer. Spore concentration of different spore suspensions was evaluated by using a Neubauer’s chamber (haemocytometer), according to the classical procedure [Citation8].
4.1. Fungi description
4.2. Fungi characteristics
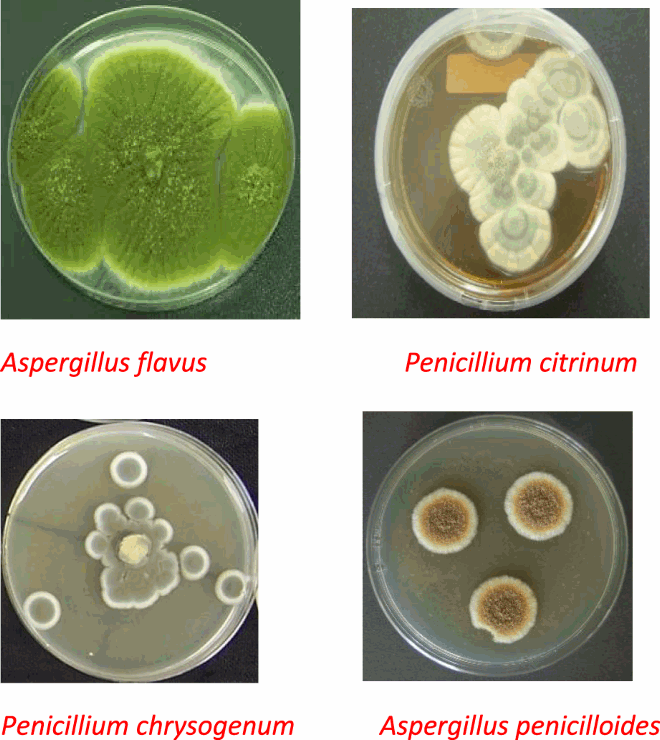
4.2.1. Aspergillus flavus
The colony is granular, flat, often with radial grooves. Yellow at first but quickly becoming bright to green with time. Conidial head is typically radiate, later splitting to form loose columns, having heads with phalides.
4.2.2. Penicillium citrinum
The colony is fast growing in shades of green and white. Cultures consisting of chains of single-celled conidia are produced in basipetal succession from a specialized conidiogenous cell called phialide. The phialides are produced singly in groups or from branched metulae giving a brush-like appearance.
4.2.3. Aspergillus penicilloides
Spore heads are radiate rather than columnar. Conidia at first elliptical becoming globose. Phialides and conidia are often swollen.
4.2.4. Penicillium chrysogenum
Coniodophores are at the end of each branch accompanied by green spherical constricted units called conidiospores which are the main dispersal route of the fungi.
4.3. Instillation
Mice were divided into nine different cages with seven mice in each cage. Animals were exposed to 50 µl/animal of each fungal spores by intranasal instillation with a pipette. The control was without exposure.
4.4. Surgical preparation and blood collection
After the exposure period, the animals were anesthesized with an intra-peritoneal injection of pentobarbital and exsanguinated by cardiac puncture. Blood was collected and serum separated.
4.5. Dissection for histopathological analysis
For histopathology, a portion of the lungs was collected and infused with 4% neutral-buffered formaldehyde and later stained with haematoxylin and eosin.
4.6. Immunoglobulin E determination
The ChemiLuminescence Enzyme Immunoassay (CLIA) method was used for IgE determination in mice.
4.7. Statistical analysis
Data obtained were analysed using multiple analysis of variance (ANOVA) and means were separated using Duncan Multiple Rnge Test (DMTR) with the level of significance at p < 0.05 (95% confidence interval).
4.8. Study approval
All experiments were conducted in accordance with guidelines set forth by the Guide for the Care and Use of Laboratory Animals under a protocol approved by the Animal Care and Use Committee of the University of Ibadan.
5. Results
The fitted model for the effect of four fungi treatments at different inoculum load on the lungs of balb/c albino mice produced a significant (p < 0.01) effect as shown in Table . Similarly, the time of innoculation showed a significant effect on the lungs of Aspergillus flavus inoculated mice at 3 × 10−7 concentration and also Aspergillus penicilloides at both concentrations. Spores of Penicillium citrinum and P. chrysogenum showed no significant effect on the lungs for the period of study while replicate recorded no significant difference for all inoculated spores at both concentrations (Table ).
Table 1. Effect of fungi treatments at different inoculum load on the lungs of balb/c albino mice.
Positive and highly significant (p < 0.01) correlation exists between time and Aspergillus flavus inoculated mice at 3.2 × 105 concentration (r = 0.80), A. penicilloides inoculated mice at 2.3 × 107 concentration had (r = 0.88) and 3.2 × 105 concentration had value of (r = 0.77) with time, while the association with A. flavus at 2.3 × 107concentration (r = −0.54) was negative but significant at p < 0.05 level. Also, strong and significant (p < 0.01) relationship was recorded between A. flavus and A. penicilloides (r = 0.77), while P. citrinum at 3.2 × 105 concentration as well showed significant (p < 0.05) but negative increase with P. chrysogenum. However, A. penicilloides at 3.2 × 105 concentration was only found significant (p < 0.05) and negatively correlated with A. flavus at 2.3 × 107 concentration (r = ��0.64) (Table ).
Table 2. Association between fungi, inoculum load and time, and their effect on the lungs of balb/c albino mice.
Evaluations of the Principal Component Axis (PCA) to the variation in the extent of infection recorded on the lungs of balb/c albino mice inoculated with four different fungi treatments showed that; Prin 1 with an eigen value of 3.43 contributed 38.13% of the total variation and it increases with increasing concentration of A. flavus. Prin 2 contributed 26.87% of the variation, although showed a negative association with A. flavus, A. penicilloides and P. citrinum but significantly increases with increase in P. chrysogenum inoculated mice at 2 × 10−5 concentration. While Prin 3 also showed a positive relationship with A. flavus, it however showed a negative association with P. chrysogenum at 3 × 10−7 concentration. Prin 4 contributed 9.73% of the total variation. It also increased with an increase in P. citrinum at concentration 1 but decrease with increase in P. chrysogenum at 3 × 10−7 concentration. Prin 5 contributed 4.67% of the variation, although showed a negative association with P. chrysogenum and P. citrinum. While Prin 6 with eigen value 0.22 also showed a positive relationship with A. penicilloides, it however showed negative association with P. chrysogenum, P. citrinum and A. penicilloides at 3 × 10−7 concentration. Prin 7 contributed 1.29% of the total variation. Prin 8 with an eigen value of 0.07 contributed 0.83% of the total variation and it increases with increasing concentration 1 of Penicillium citrinum. Prin 9 contributed 0.00% of the variation, although showed a positive association with A. penicilloides at concentration 1 and Penicillium citrinum at 2 × 10−5 concentration (Table ).
Table 3. Contribution of Principal Component Analysis (PCA) to the variation in the extent of infection recorded on the lungs of balb/b albino mice inoculated with different fungi treatments.
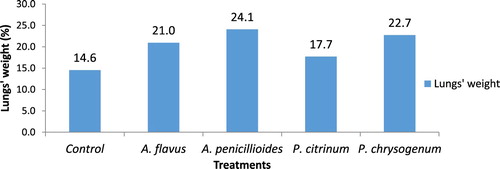
The weight of lungs showed that A. penicilloides had the heaviest lungs compared with the rest which was followed by P. chrysogenum (22.7) while the lowest weight was found in Penicillium citrinum inoculated lungs (17.7) and control lungs was 14.6 (Figure ).
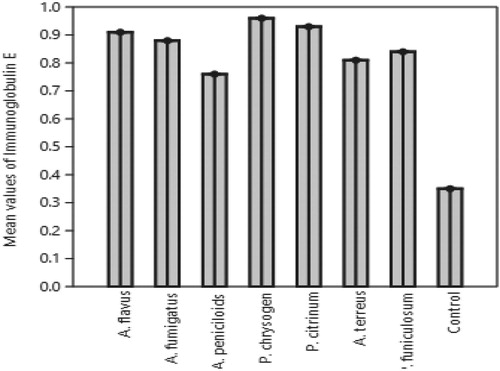
The mean IgE value induced by Penicillium chrysogenum fungal protein in mice was highest and followed by that of Penicillium citrinum, A. flavus, A. fumigatus, P. funiculosum, A. terreus while A. penicilloides protein was the least induced and were all significantly different from control samples (Figure ).
All the fungi inoculated resulted in significant (p < 0.05) increase in the monocytes and basophils contents compared to the control. P. citrinum significantly increased the eosinophils of mice as P. chrysogenum inoculated mice also had an increase in lymphocytes cells and white blood cell was also significantly increased followed by P. citrinum inoculated mice. The neutrophils cells were higher in the A. penicillioides, P. chrysogenum and P. citrinum inoculated mice. Both haemoglobin and red blood cells were also increased in mice inoculated with P. chrysogenum and A. flavus compared to the control mice (Table , Figures ).
Figure 3. Plate 1(a and c): Aspergillus penicilloides (2.3 × 107): Lung. The airways (alveoli and bronchioles) are clear. There is however moderate haemorrhages and multiple foci of alveolar macrophages laden with dark pigment materials. Plate 2(b and d): Aspergillus penicilloides (3.2 × 105): Lung. There is extensive severe thickening of alveolar interstitium. There is an accumulation of inflammatory cells around blood vessels suggestive of vasculitis. Numerous unstained clear fungal hyphae are trapped in the necrotic debris (Mg: 100×).
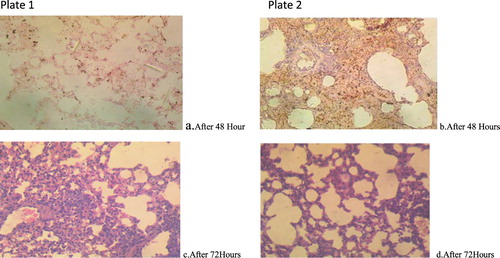
Figure 4. Plate 3(a and b): Penicillium chrysogenum (2.3 × 107): There are severe widespread haemorrhages into the alveoli. There are a few foci of formation of giant cells. Plate 4(c and d): Penicillium chrysogenum (3.2 × 105): There is extensive severe thickening of alveolar interstitium. There are numerous foci of macrophages laden with dark pigments. Numerous unstained clear fungal hyphae are trapped in the necrotic debris. There is moderate congestion of blood vessels.
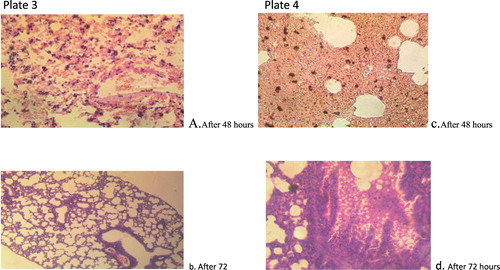
Figure 5. Plate 5(a and b): P. citrinum (2.3 × 107): There are multiple foci of necrosis and accumulation of necrotic debris. Embedded within the debris are clear unstained fungal hyphae. There are multiple foci of alveolar macrophages laden with dense aggregates of black pigments. Plate 6(c and d): P. citrinum (3.2 × 105):There are multiple foci of necrosis and accumulation of necrotic debris. There are extensive foci of alveolar macrophages laden with mild aggregates of black pigment (Mg: 100×).
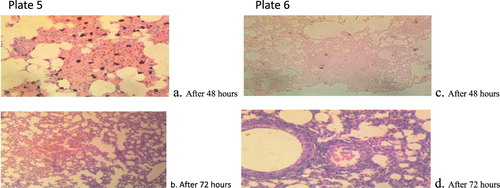
Figure 6. Plate 7(a and b): Aspergillus flavus (2.3 × 107): There are foci of thickened alveolar septae and alternate foci of over-distended alveoli [emphysema] (Mg: 100×). Plate 8(c and d): A. flavus (3.2 × 105): Lung. There is widespread haemorrhage and over-distension of the alveoli indicative of pulmonary emphysema (Mg: 100×).
![Figure 6. Plate 7(a and b): Aspergillus flavus (2.3 × 107): There are foci of thickened alveolar septae and alternate foci of over-distended alveoli [emphysema] (Mg: 100×). Plate 8(c and d): A. flavus (3.2 × 105): Lung. There is widespread haemorrhage and over-distension of the alveoli indicative of pulmonary emphysema (Mg: 100×).](/cms/asset/948f4f81-9c83-40d0-854c-cf963af9b124/tusc_a_1573458_f0006_oc.jpg)
Figure 7. Plate 9: Control: Alveoli are clear and devoid of exudates. There is no congestion (Mg: 100×). (Concentration 1 = 2.3 × 107 Concentration 2 = 3.2 × 105).
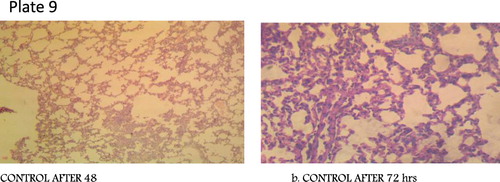
Table 4. Effects of different fungi treatments on Immune cells in balb/c albino mice.
Essentially, the pathology shown by the tissues appears to be similar although with varying degrees of severity. All of them had intra-lesional unstained fungal hyphae. Pathologies include thickening of alveolar sepatae, which causes impairment in vascular exchange and/or respiratory movements. This in turn results in forced expansion of adjacent alveoli which is manifested morphologically as the over-distension of alveoli.
6. Discussion
Fungal spores identified and used in this work are among the invasive airborne fungal spores that have been implicated in nosocomial (hospital) infection of patients with solid organ transplants [Citation9,Citation10]. Exposure to these fungi has been associated with a variety of adverse health outcomes including respiratory, heamatological, immunological, and neurological system disorders and/or diseases [Citation11,Citation12]. In this study, four different fungi species were inoculated into mice for immune response.
All the mice inoculated with fungi spores produced higher neutrophilic cells. Hoselton et al. [Citation13] and Amin [Citation14] reported that continuous exposure to fungal spores such as A. fumigatus results in the accumulation of neutrophils, basophils, and mast cells which are characteristic symptoms associated with the early phase inflammatory reaction while the late phase reaction is observed by the accumulation of Th2 lymphocytes which is also in agreement with this work. Traynor and Huffnagle [Citation15] also reported that tissue phagocytes have an essential role in host defence against fungi infection which could be the reason why more neutrophils and lymphocytes were produced as host defence in this study. Although, despite the enormous capacity of the macrophages to kill fungi conidia, their effectiveness is not 100% [Citation16]. This defensive mechanism greatly reduces pathogenicity of the inoculated fungi by impeding germination and development of the fungus [Citation17].
The result from the blood analysis of inoculated mice revealed that monocytes were produced in large numbers in all the fungal spores inoculated mice lungs compared to control. Similar result was also obtained for basophils which were produced in higher quantities. For eosinophils, Penicillium citrinum inoculated mice recruited most eosinophils to the centre of infection. Eosinophils have been considered end-stage cells in immunopathology of fungal allergic asthma. Histopathology revealed that all the microbes studied provoked inflammation after fungal instillation once, but the magnitude and its characteristic features were different. Also, vascular changes observed in the histology of the lungs ranged from congestion to pulmonary haemorrhages to vasculitis (inflammation of the pulmonary blood vessels). Gupta et al. [Citation18] attributed the vasculities found in a similar work to be mediated by mononuclear inflammatory cells which manifested as thickening of the walls of blood vessels. Hyperplasia of the bronchiolar epithelium was observed in one of the sections. This is likely to be due to localization of the fungal hyphae in the lumen of the bronchioles. Fokkens et al. [Citation19] also identified hyperplasia as the major symptoms of fungal allergy in their experiment. The hyperplasia is a response to the irritation caused by the fungal spores as well as the need to replace necrotic epithelial cells of the bronchioles. There are very few reports of A. flavus, A. penicilloides, P. chrysogenum and P. citrinum synthesis of IgE in balb/c albino mice. Immunoglobulin E is a class of immunoglobulin essential for the allergic response. Normal levels of IgE are highly variable in the population. Factors regulating IgE levels include age, gene-by-environment interactions, genetic factors (such as certain polymorphisms), racial factors (higher levels are seen in African Americans and persons of Filipino descent), sex (males tending to have higher levels) and season (IgE levels may increase during pollen season in allergic individuals). IgE can also accompany other immunologic deficiency diseases, including common variable immunodeficiency. The clinical spectrum of hypersensitivity reactions elicited by fungi is very broad and includes IgE-mediated type I allergy. Irregular levels of Immunoglobulin E (IgE) shows a dysregulation of IgE synthesis and could be seen in a variety of immunological disorders. Elevated levels of IgE may be seen in atopic disease. Very high levels of IgE may be found in patients with food allergy, allergic fungal disease and atopic eczema. There are also certain reports that IgE antibody can provide immunity against B. burgdorferi infection in children that lasts throughout adulthood [Citation20], and also contribute to the expulsion of intestinal parasites such as N. americanus [Citation21]. In this investigation, P. chrysogenum was observed to produce higher immunoglobulin E compared to A. flavus, A. penicilloides and P. citrinum.
However, as discussed here, differences in the immune system of mice could be essential in the fight against specific fungal diseases.
7. Conclusion
The impact of fungal allergy on human health have been clearly investigated in this work. There is no doubt that fungi are involved in many allergic disorders which is clearly shown in this work. It has now been evident that inhalation of fungal spores may lead to or exacerbate immunologic/ allergic reactions, cause toxic effects, or cause infections.
Acknowledgement
The authors are grateful to Prof E. Farombi and Dr Ife Awogbindin of Biochemistry Department, University of Ibadan for their cooperationand support.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Jaakkola M, Nordman H, Pilpari R, et al. Indoor dampness and molds and development of adult onset asthma: a population-based incident case-control study. Environ Health Persp. 2002;110:543–547. doi: 10.1289/ehp.02110543
- Zureik M, Neukirch C, Leynaert B, et al. Sensitization to airborne moulds and severity of asthma: cross sectional study from European Community respiratory health survey. Br Med J. 2002;325(7361):411–414. doi: 10.1136/bmj.325.7361.411
- Fraser RS. Pulmonary aspergillosis: pathologic and pathogenetic features. Pathol Annu. 1993;28:231–277.
- Sumi Y, Natura H, Takeuchi M, et al. Granulomatous lesions in the lung induced by inhalation of mold spores. Virchows Arch. 1994;424:661–668. doi: 10.1007/BF00195782
- Fan LL. Hypersensitivity pneumonitis in children. Curr Opin Pediatr. 2002;14:323–326. doi: 10.1097/00008480-200206000-00008
- Patel AM, Ryu J H, Reed CE. Hypersensitivity pneumonitis: current concepts and future questions. J Allergy Clin Immunol. 2001;108:661–670. doi: 10.1067/mai.2001.119570
- Garcia ME, Blanco JL. Principales enfermedades fu´ngicas que afectan a los animales dome´sticos. Rev Iberoam Micol. 2000;17:S2–S7.
- Araujo R, Acacio G, Rodrigues A. A fast, practical and reproducible procedure for the standardization of the cell density of an Aspergillus suspension. J Med Microbiol. 2004;53:783–786. doi: 10.1099/jmm.0.05425-0
- Sanchez H, Bush R. A review of alternaria sensitivity. Revista iberoamericana de micologia. 2000;2:56–59.
- Cashel P, Newhouse BS, Levetin E. Correlation of environmental factors with asthma and rhinitis symptoms in Tulsa, Okhlahoma. Ann Allerg Asthma Im. 2004;92:356–366. doi: 10.1016/S1081-1206(10)61575-X
- Dutkiewicz J, Górny RL. Biologiczne czynniki szkodliwe dla zdrowia—klasyfikacjai kryteria oceny narażenia [Biological factors hazardous to human health—classification and criteria of exposure assessment]. Med Pr. 2002;53:29–39.
- Samson RA, Hoekstra ES, Frisvad JC. Introduction to food- and airborne fungi. 7th ed. Utrecht: Centraalbureau voor Schimmelcultures; 2004. p. 126.
- Hoselton SA, Samarasinghe AE, Seydel JM. An inhalation model of air- way allergic response to inhalation of environmental Aspergillus fumigatus conidia in sensitized BALB/c mice. Med Mycol. 2010;48:1056–1065. doi: 10.3109/13693786.2010.485582
- Amin K. The role of mast cells in allergic inflammation. Respir Med. 2012;106:9–14. doi: 10.1016/j.rmed.2011.09.007
- Traynor T, Huffnagle GB. Role of chemokines in fungal infections. Med Mycol. 2001;39(1):41–50. doi: 10.1080/mmy.39.1.41.50
- Latge JP. The pathobiology of Aspergillus fumigatus. Trends Microbiol. 2001;9:382–389. doi: 10.1016/S0966-842X(01)02104-7
- Romani L. Immunity to fungal infections. Nat Rev Immunol. 2004;4(1):1–23. doi: 10.1038/nri1255
- Gupta R, Sharma V, Sridhara S. Identification of serine protease as a major allergen of Curvularia lunata. Allergy. 2008;59:421–427. doi: 10.1046/j.1398-9995.2003.00378.x
- Fokkens WJ, Lund VJ, Mullo J. European position paper on rhinosinusitis and nasal polyps. Rhinology. 2012;50:225–226.
- Bluth MH, Robin J, Ruditsky M, et al. Ige anti-Borrelia burgdorferi components (p18, p31, p34, p41, p45, p60) and increased blood CD8 + CD60+ T cells in children with Lyme disease. Scand J Immunol. 2007;65:376–382. doi: 10.1111/j.1365-3083.2007.01904.x
- Pritchard DI, Walsh EA. The specificity of the human IgE response to Necator americanus. Parasite Immunol. 1995;17:605–607. doi: 10.1111/j.1365-3024.1995.tb01005.x