?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In term of the Lambert W function, simple and reliable analytical expression for the estimation of the specific heat capacity of the CuAlS2 semiconductor is proposed by using the Lambert Boltzmann distribution. The obtained results have been compared with the corresponding experimental results in the 100-300 K temperature range. The estimated results are in good agreement with the experimental results over the entire temperature range. However, accurate values of the heat capacity cannot be calculated using the standard Boltzmann formula.
1. Introduction
Among with I-III–VI2 semiconductors compounds, disulphide of copper and aluminum (CuAlS2) is of special technological interest. It is known that these semiconductor compounds are suitable for many technical applications, such as light emitting devices [Citation1], optical filters [Citation2] and solar cells [Citation3], they are also being studied as materials for Spintronics [Citation4]. The CuAlS2 semiconductor is also suitable for the use as window material for solar cells and light-emitting diodes since it has a wide direct gap of 3.5 eV.
For optimizing the synthesis of the CuAlS2 semiconductor compound and estimating its thermodynamic characteristics, the evaluating data on the heat capacities of this semiconductor compound is of great importance. So far, the heat capacity of CuAlS2 has been measured by the adiabatic calorimeter and were more refining using the least squares method [Citation5]. However, no theoretical methods were used to calculate the heat capacity of CuAlS2.
The aim of the present study is to present a simple and reliable analytical expression for the estimation of the specific heat capacities of semiconductor compounds such as CuAlS2 in terms of the Lambert W function using the Lambert Boltzmann distribution in the temperature range 100-300 K.
2. Heat capacities Cv and Cp expression and its application to the semiconductor compound CuAlS2
The expression for internal energy U and heat capacity Cv of indistinguishable particles are, respectively,
The constant volume heat capacity is expressed as
(1)
(1)
Here k is the Boltzmann constant, T the temperature, N the Avogadro number and Z is the partition function. This latter was determined for the Lambert-Boltzmann distribution which is the revised Boltzmann statistics for a finite number of particles established by Kakorin [Citation6].
In the literature to derive the exponential Boltzmann distribution, the Stirling approximation, ln(N!)=N ln(N)-N is used. By applying a more exact version of Stirling’s approximation and using the exact form log N!, respectively, Kakorin derives two more accurate versions of the Botlzmann distribution for a finite number of particles. The first of these involves the Lambert W function [Citation7]. In particular, the Lambert partition function is [Citation6],
(2)
(2)
Here NW is the number of particles,
is the Lambert maximum energy, β =1/kT,
where
, k are, respectively, the Einstein temperature and the Boltzmann constant and W denotes the Lambert W function. This function solves the equation [Citation7].
The Lambert W function allows the explicit solution of entire classes of differential equations, which actually only could be solved numerically and is experiencing today a renaissance in various fields of science and engineering [Citation8–18].
(3)
(3)
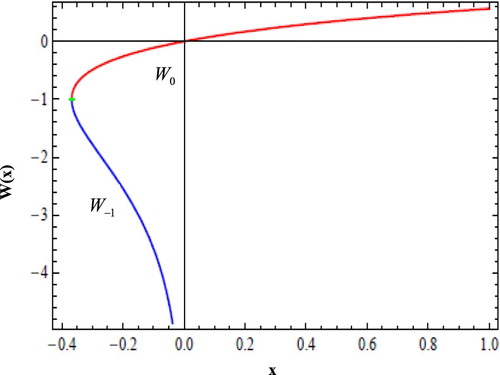
The Lambert W function is a complex function with an infinite number of branches, only two of them having real values. If x is real, then for , there are two possible real values of
, as displayed in Figure . The branch satisfying
is denoted
and the branch satisfying
is denoted
. Also
.
Also, the Cv versus Cp relationship can be obtained from the Nernst–Lindemann equation [Citation19,Citation20]
(4)
(4) Implying that
(5)
(5) where A0 = 5.1 × 10−3 J−1. K. mol and Tm is the melting temperature. The square root in Equation (5) can be expanded conveniently into a Taylor series expansion [Citation21],
(6)
(6)
Inserting Equation (2) into Equation (1) yields
(7)
(7) and by replacing Cv in Equation (6), we obtain the expression of the heat capacity Cp as:
(8)
(8)
Numerical values of the principal Lambert W function can be computed using packages such as Mathematica and Maple.
3. Results and discussion
Firstly, the method was applied to calculate the values of the specific heat capacity at constant volume Cv which we compared with experimental data [Citation5].
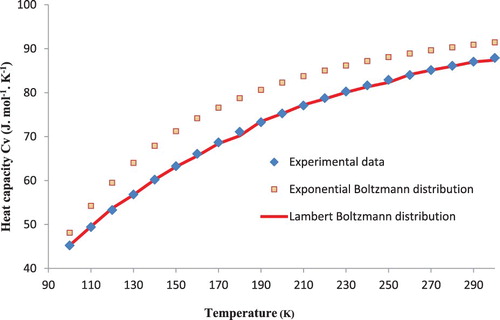
The experimental and our calculated values of heat capacity Cv are presented in Table . It is evident from this table that the result obtained is in excellent agreement with the experiment data [Citation5] in the temperature range from 100 to 300 K with a large discrepancy of 0.7% at 120 K, 160 K and 250 K.
Table 1. Heat capacities Cv of CuAlS2 with Einstein and melting temperatures are θE = 306 K and Tm = 1495 K.
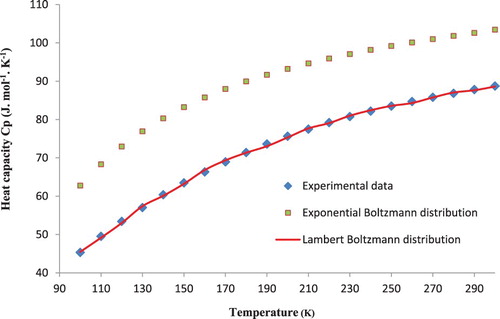
In an attempt to confirm the method of calculation proposed in this paper, the specific heat capacity at constant pressure Cp for the semiconductor compound CuAlS2 is calculated in the temperature range 100–300 K. In comparison with the experimental data available in the literature, the calculated result is shown in Table . Our results are in excellent agreement with experimental data obtained by Korzun et al. [Citation5] over the temperature range from 100 to 300 K with the largest discrepancy of 0.9% at 170 K.
Table 2. Heat capacities Cp of CuAlS2 with Einstein and melting temperatures are θE =306 K and Tm=1495 K.
For further improvement for this method of calculation, Figures and show respectively the comparison of the value of heat capacity at constant pressure Cp and the heat capacity at constant volume Cv calculated by the standard Boltzmann formula and the Lambert Boltzmann distribution with the experimental data [Citation5]. The Boltzmann formula gives significantly larger discrepancies with the data and a large disagreement appears between both results. This confirms that the standard Boltzmann distribution is unable to accurately represent the specific heat.
4. Conclusion
A new method has been presented to accurately calculate the specific heat capacities of the semiconductor compound CuAlS2 using the Lambert-Boltzmann distribution which is a generalization of the exponential Boltzmann distribution when the number of particles is finite. The calculated results of specific heat capacities relatively to the semiconductor compound CuAlS2 have been compared with those reported in the literature and are found to be in good agreement. By comparison, the standard Boltzmann formula is systematically over-biased.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Ishibashi N, Nishi T, Hayashi N, et al. Electrically induced optical absorption in Al-CuAlS 2-Au diode. Jpn J Appl Phys Pt 2, Lett. 1999;38:L626–L628. doi: 10.1143/JJAP.38.L626
- Horinaka H, Yamamoto N. Japanese research review for pioneering. Ternary and multinary compounds in the 21st Century. Tokyo: IPAP; 2001. p. 348.
- Guillemoles J-F, Kronik L, Cahen D, et al. Stability issues of Cu(In,Ga)Se2-based solar cells. J Phys Chem B. 2000;104:4849–4862. doi: 10.1021/jp993143k
- Zhao Y-J, Zunger A. Electronic structure and ferromagnetism of Mn-substituted CuAlS2, CuGaS2, CuInS2, CuGaSe2, and CuGaTe2. Phys Rev B. 2004;69:104422. doi: 10.1103/PhysRevB.69.104422
- Korzum BV, Mianzelen RR, Sheleg AU, et al. Heat capacity and thermodynamic functions of the CuAlS2 semiconductor in the temperature range from 80 to 300 K. Phys Stat Sol (b). 2005;242:1990–1995. doi: 10.1002/pssb.200540064
- Kakorin S. Revision of Boltzmann statistics for a finite number of particles. Am J Phys. 2009;77:48–53. doi: 10.1119/1.2967703
- Corless RM, Gonnet GH, Hare DEG, et al. On the Lambert W function. Adv Comp Math. 1996;5:329–359. doi: 10.1007/BF02124750
- Morales DA. Exact expressions for the range and the optimal angle of a projectile with linear drag. Can J Phys. 2005;83:67–83. doi: 10.1139/p04-072
- Vial A. Horizontal distance travelled by a mobile experiencing a quadratic drag force: normalized distance and parametrization. Eur J Phys. 2007;28:657–663. doi: 10.1088/0143-0807/28/4/005
- Warburton RDH, Wang J. Analysis of asymptotic projectile motion with air resistance using the Lambert W function. Am J Phys. 2004;72:1404–1407. doi: 10.1119/1.1767104
- Parker GW. Projectile motion with air resistance quadratic in the speed. Am J Phys. 1977;45:606–610. doi: 10.1119/1.10812
- Hadj Belgacem C, Fnaiech M. Exact analytical solution for the critical layer thickness of a lattice-mismatched heteroepitaxial layer. J Elec Mater. 2010;39:2248–2250. doi: 10.1007/s11664-010-1290-5
- Hadj Belgacem C, Fnaiech M. Solution for the critical thickness models of dislocation generation in epitaxial thin films using the Lambert W function. J Mat Sci. 2011;46:1913–1915. doi: 10.1007/s10853-010-5026-y
- Hadj Belgacem C. Range and flight time of quadratic resisted projectile motion using the Lambert W function. Eur J Phys. 2014;35:055025. doi: 10.1088/0143-0807/35/5/055025
- Hadj Belgacem C. Explicit solution for critical thickness of semicircular misfit dislocation loops in strained semiconductors heterostructures. Silicon. 2016;8:397–399. doi: 10.1007/s12633-015-9281-z
- Hadj Belgacem C. Analysis of projectile motion with quadratic air resistance from a nonzero height using the Lambert W function. J Taibah Univ Sci. 2017;11:328–331. doi: 10.1016/j.jtusci.2016.02.009
- Hadj Belgacem C. Theoretical Models for Anomalously High Ideality Factor in Au/SnO2-Si(n)/Al Solar cell. Silicon. 2018;10:1063–1066. doi: 10.1007/s12633-017-9572-7
- Hadj Belgacem C. Dertmination of commercial Silicon Diode (4007IN) Parameters from the I-V and P-V Characteristics. Silicon. 2018;10:1469–1474. doi: 10.1007/s12633-017-9628-8
- Rabe KM, Joannopoulos JD. Ab initio relativistic pseudopotential study of the zero-temperature structural properties of SnTe and PbTe. Phys Rev B. 1985;32:2302–2314. doi: 10.1103/PhysRevB.32.2302
- Nernst W, Lindemann AF, Elektrochem Z. Spezifische Warme und Quantentheorie. Angew Phys Chem. 1911;17:817–827.
- Passler P. Dispersion-related theory for heat capacities of semiconductors. Phys Status Solidi B. 2007;244:4605–4623. doi: 10.1002/pssb.200743174