Abstract
The antidiabetic effect and mode of action of Socrpio maurus palmatus body extract were evaluated in diabetic mice induced by alloxan. 24 male albino mice were divided into four groups. Group 1 was injected intraperitoneally with physiological saline. Group 2 was injected (i.p., daily for 5 weeks) with 300 mg/kg of the scorpion aqueous extract. Group 3 was received alloxan (150 mg/kg, i.p). Group 4 was diabetic and treated with scorpion extract (300 mg/kg, i.p., daily for 5 weeks). Several relevant biochemical parameters and histological examination of Langerhans islets were evaluated. The administration of scorpion extract significantly elevated the level of plasma insulin which was concomitant with a remarkable decrease in the level of blood glucose in diabetic mice. Furthermore, scorpion extract showed a notable ameliorative effect through enhancing the status of antioxidants and recovering the altered biochemical parameters in diabetic mice. Interestingly, scorpion extract significantly increased the number of β-cells and the size of pancreatic islets in diabetic mice. Accordingly, the obtained results demonstrate the antidiabetic effect of scorpion extract in alloxan-induced diabetic mice through its antioxidant and regeneration capacity.
1. Introduction
The lifelong progressive disease of diabetes mellitus (DM, one of the major causes of morbidity and death) is a chronic metabolic disturbance. DM is characterized by a high level of blood glucose due to the depletion of insulin secretion or insulin resistance (Type-1 DM and Type-2 DM, respectively) [Citation1]. There are about 230 million people worldwide have been affected by DM and the expected number of diabetic people will be 642 million by 2040 [Citation2–4]. Several risk factors are accompanied by DM including reactive oxygen (ROS) and nitrogen species (RNS) production, hyperglycemia, hypertension, dyslipidemia, decreased fibrinolytic activity, increased platelet aggregation, atherosclerosis, macro-and micro-vascular complications [Citation5–8].
Several approaches are being used for DM treatment through eating a healthy food and diet control, doing exercise, using standard hypoglycemic chemical drugs (e.g. sulfonylureas, biguanides and meglitinide analogues) or insulin injection, increasing pancreatic islet survival and β cell regeneration via INGAP (islet neogenesis associated protein) [Citation9,Citation10]. Moreover, traditional or alternative medicine is considered as an important source of future therapeutic agents to treat many diseases including DM [Citation11]. The extracts of several medicinal plants revealed potent antidiabetic activity using animal models [e.g. Citation11–14]. From animal sources, exendin-4 (39 amino acid, commercially known as Byetta®; $748–$795 for 1.2 ml pen cartridge) which derived from the venom of Gila monster (Heloderma suspectum) revealed potent antidiabetic activity and used in the treatment of type 2-DM [Citation15]. Xie and colleagues reported that scorpion extract (Buthus martensii kirsch) combined with gypsum revealed a novel antidiabetic activity in diabetic mice (induced by streptozotocin) through upregulating the expression of pancreatic PPARγ (peroxisome proliferator-activated receptor gamma) and PDX-1 (pancreatic and duodenal homeobox 1), improving islet regeneration and enhancing insulin secretion [Citation3]. In this regard, more investigations are urgently needed to develop therapeutic agents able to protect or regenerate pancreatic beta cells.
In Egypt, there are 24 scorpion species including the Scorpionidae scorpion of Scorpio maurus palmatus (S. m. palmatus). Several studies have been conducted to investigate the structural and biological characteristics of S. m. palmatus venom [e.g. Citation16–18]. However, no information is available about pharmacological properties of the whole body (except venom glands) of this scorpion. The main objective of this communication is to investigate potential antidiabetic effect of S. m. palmatus body extract in diabetic mice induced by alloxan.
2. Materials and methods
2.1. Experimental animals and scorpion collection
Male albino mice (18–25 g body weights) have been used in the present study. The animals were kept in polyethylene cages and fed on standard laboratory food with water ad libitum. They were fasted for 8 h before conducting the experiments. The guidelines of International Guiding Principles for Animal Research have been followed for care and maintenance of the experimental animals. About 100 adult specimens of the Egyptian scorpion S. m. palmatus were collected from the Western Mediterranean Costal Desert (Alexandria Governorate, Egypt). The scorpions were transferred to Zoology Department animal house (Faculty of Science, Suez Canal University, Ismailia, Egypt) and separately kept alive in plastic containers.
2.2. Preparation of scorpion extract
To lower the toxic effect, the telsons containing venom glands were discarded from each scorpion used to prepare the whole-body extract. Firstly, the scorpions were dried overnight at 60°C and then grinded to obtain scorpion powder. The pooled powder was soaked in warm water (2 h) and then filtered using 4 sheets of gauze. The solid material was extracted (n = 2) using the same conditions. The filtrates were pooled and centrifuged (3000 rpm, 15 min) to remove impurities and debris. The supernatant was lyophilized and kept in −20°C until use [Citation3].
2.3. Induction of diabetic mice using alloxan
Mice (18–25 g body weights) were subjected to fasting for 24 h with free access to water. Freshly solutions of alloxan (Sigma-Aldrich Chemical Co., USA) were prepared by dissolving alloxan monohydrate in physiological saline 0.9% NaCl and intraperitoneally (i.p.) injected (150 mg/kg) into the fasted mice. The blood glucose levels were measured 72 h post alloxan injection. The mice with steady elevated blood glucose level (>200 mg/dL) were considered as diabetic mice and used in the present study [Citation19,Citation20].
2.4. Experimental design
In order to examine the antidiabetic effect of scorpion extract, mice were divided into 4 groups (n = 6/group). The first group (normal negative control) was received intraperitoneally (i.p.) physiological saline NaCl 0.9% (200 µL i.p., daily for 5 weeks). The second animal group (scorpion extract) was received a daily dose of 300 mg/kg (i.p.) for five weeks. The third group (alloxan-diabetic mice) was received alloxan (150 mg/kg, i.p.). The fourth group (diabetic + scorpion extract) was diabetic and treated (i.p.) with scorpion extract at a daily dose 300 mg/kg for 5 weeks [Citation3]. The blood was collected from anesthetized mice (by diethyl ether) using retro-orbital method into K3-EDTA tubes (FL medical, ITALY) to avoid blood coagulation. The blood was centrifuged (5000 r.m.p for 10 min) for plasma collection which stored at –20°C until use. The plasma and pancreatic tissue samples were collected to measure various biochemical (plasma glucose and insulin, oxidative stress biomarkers, enzymatic and non-enzymatic antioxidants, total protein, AST, ALT, cholesterol and triglycerides) and histological (H&E stain of Langerhans islets) parameters in control and treated groups.
2.5. Estimation of biochemical parameters
The clinicobiochemical parameters of glucose, total protein, aspartate aminotransferase (AST), alanine aminotransferase (ALT), cholesterol and triglycerides [Citation21–23] were measured in plasma using colorimetric commercial kits following the steps described by the manufacture (Bio-Diagnostic Company, Dokki – Giza – Egypt). Plasma insulin level was estimated using mouse insulin ELISA kit purchased from Bio-Diagnostic Company [Citation24].
2.6. Assays of oxidative stress biomarkers
2.6.1. Lipid peroxidation (MDA assay)
The level of mice plasma lipid peroxidation was determined following the method described by Yagi [Citation25]. The absorbance of final product which produced from the reaction of thiobarbituric acid (TBA) and malonyldialdehyde (MDA, peroxidized lipid) was measured at 532 nm. The concentrations of MDA were expressed as µmol/mL.
2.6.2. Protein carbonyl content (PCC assay)
As a good marker of protein oxidation and damage, protein carbonyl content (PCC) in plasma was determined [Citation26]. Firstly, the protein was precipitated with 1% TCA (trichloroacetic acid) and the protein pellet was treated with 2,4-dinitrophenylhydrazine (DNPH, 1 mL of, 10 mM) and left for 1 h (room temperature and in the dark). The samples were reprecipitated with TCA (20%) and the pellets washed three times with a mixture of ethyl acetate: ethanol (1:1). The final washed pellet was dissolved in 1 mL of 6 M guanidine and the carbonyl content was measured at 370 nm.
2.6.3. Nitric oxide (NO assay)
The level of nitric oxide in plasma was determined according to the method of Griess reaction [Citation27]. One hundred microliter of Griess reagent (0.1% naphthylethylenediamine dihydrochloride + 1% sulfanilamide + 5% H3PO4) was mixed with 100 µL of filtered plasma (10 min, room temperature) and the absorbance was measured at 540 nm (sodium nitrite (NaNO2) was used as a standard).
2.7. Assays of non-enzymatic (GSH) and enzymatic antioxidants (GPx, SOD, CAT)
2.7.1. Blood reduced glutathione (GSH) and activity of glutathione peroxidase (GPx)
The blood content of GSH was determined according to the method described by Beutler et al. [Citation28]. Two hundred microliter of blood were added to 1.8 mL distilled water and 3 mL of precipitating solution (30 g NaCl, 0.2 g EDTA and 1.67 g glacial metaphosphoric acid dissolved in 100 mL distilled water). The mixture was then centrifuged (2200 g, 15 min, 4°C) and 100 µL of supernatant was added to 4 mL of Na2HPO4 and 0.5 mL DTNB reagent (dithiobis-2-nitrobenzoic acid, Sigma-Aldrich). The absorbance was measured at 412 nm (GSH was used as a standard). The activity of GPx in blood was determined according to the method of Paglia and Valentine [Citation29] using the commercial kit purchased from Bio-Diagnostic company (Dokki – Giza – Egypt) and following the procedures described by the manufacturer.
2.7.2. Cu/Zn-superoxide dismutase (Cu/Zn-SOD; EC 1.15.1.1) and catalase (CAT; EC 1.11.1.6)
The activity of blood SOD was determined by the method of Misra and Firdovich [Citation30]. This assay is based on the capability of SOD to inhibit the phenazine methosulphate-mediated reduction of nitroblue tetrazolium dye and the inhibition rate was measured at 560 nm. The amount of SOD needed to produce 50% inhibition is defined as one unit of enzyme activity which expressed as a unit/mL. Catalase activity was determined by following the decomposition of H2O2 at 240 nm [Citation31]. Blood samples were prepared in 50 mM phosphate buffer (pH 7) and Triton-X 100 (1%, v/v) was added to increase the observable CAT activity by releasing the enzyme from peroxisomes. Using a molar extinction coefficient of 43.6 mol, the enzyme activity was calculated and expressed as a unit/mL.
2.8. Histopathological examination
Four mice from each group were subjected to histopathological study. The pancreas was removed immediately from the animals after sacrificing and rinsed in ice-cold saline and fixed in 10% formaldehyde overnight for a maximum of 12 h. The dehydration and clearing of the tissues were processed routinely and embedded in paraffin wax. The pancreas was sectioned on the posterior, middle and the anterior parts. Paraffin sections were cut into 5-micron thickness and fixed on to glass slide. They were deparaffinized in xylene twice for 5 min and then rehydrated with graded alcohol and stained with hematoxylin and counterstained with eosin (H&E) dye. For histopathological changes, the stained sections were morphologically evaluated by comparing mice pancreas administered with the different treatments under light microscope (Olympus Bx53, Olympus Corporation, Tokyo, Japan) which provided with Camera Model U-LH HG and Imaging Software (Cell sense, Ver. 1.4.1) [Citation32]. For quantitative analysis, the following measurements have been evaluated: (1) volume density of islets to total tissue [Citation33]; (2) density of β-cells in islet tissue (number of β-cells to total islet cells) [Citation34] and (3) average area of islets diameter (4 islets in each section and totally 40 islets in each group) [Citation33,Citation35]. Islets volume was automatically calculated using the software, a total volume of 4 islets of each pancreatic region in the field (n = 12 islets per mouse) for 4 mice of each group was calculated. The microscopic slide fields have been sampled using a systematic random pattern (the measurement started from the slide corner and then moved in the X- and Y-directions at equal distances) [Citation36]. Moreover, microscopic images were taken using the Olympus camera microscope and displayed on the computer screen and number of β-cells per islets were manually calculated, summation of the total number of β-cells in the 40 islets were divided by their number for each mouse in the group.
2.9. Statistical analysis
Data were statistically analysed using Sigma Plot software (Version 11). For all measurements in control and treated groups, descriptive analyses (Mean ± SEM) were applied. The potential effect of scorpion extract on healthy and diabetic mice was initially evaluated using the Student’s unpaired t-test (control vs. each treated group). One-way ANOVA followed by Duncan's method (pairwise multiple comparison) was used to compare between control and treated groups. Differences between animal groups were considered statistically significant at P < 0.05.
3. Results
3.1. Effect of scorpion extract on the level of plasma glucose and insulin
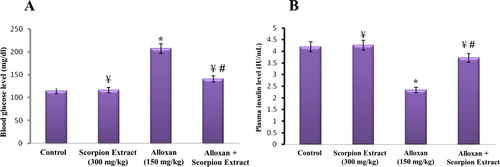
The effect of injection of Egyptian scorpion aqueous extract of Scorpio maurus palmatus (300 mg/kg, ip, daily for 5 weeks) on the level of mice blood glucose after five weeks of treatment was illustrated in Figure (A). It was noticed that the level of glucose was significantly increased (p < 0.05) in the alloxan-treated group (diabetic group; 206.4 ± 16 mg/dl) when compared with the healthy control group. Interestingly, there was a significant decrease in the level of blood glucose in the diabetic group treated with scorpion extract (alloxan + scorpion extract; 142.8 ± 11.6 mg/dl) (p <0.05). No significant changes were reported in glucose level between the normal control group and the scorpion extract-treated group. One-way ANOVA between the four animal groups showed a significant difference (p < 0.05) in the level of plasma glucose. More importantly to mention that these data was supported with the results of plasma insulin level (Figure (B)). There was a remarkable decrease (p < 0.05) in the level of plasma insulin in the alloxan-treated group (diabetic group; 2.33 ± 0.18 U/mL) when compared to the negative control group (4.26 ± 0.19 U/mL). The daily injection of scorpion extract for five weeks was able to significantly increase (p < 0.05) the plasma insulin level of diabetic mice (3.71 ± 0.22) when compared with the non-treated diabetic group. Highly significant difference in plasma insulin level was recorded between control and treated groups using one-way ANOVA (p < 0.05).
Figure 1. Effect of the Egyptian scorpion aqueous extract of Scorpio maurus palmatus on the level of mice blood glucose (A) and insulin (B). Scorpion extract was intraperitoneally injected at a daily dose of 300 mg/kg, and the level of plasma glucose and insulin was estimated after five weeks post-injection in healthy (control, scorpion extract) and diabetic (alloxan, alloxan + scorpion extract) mice groups. Data are presented as Mean ± SEM (6 mice/group). (*) Significant difference between control and each treated group using Student's unpaired t-test, (p < 0.05). (¥) A significant difference between alloxan group (150 mg/kg) and treated groups using Student’s unpaired t-test, (p < 0.05). (#) Significant difference between animal groups using one-way ANOVA, (p < 0.05) followed by a Duncan's method hoc test for pairwise multiple comparison.
3.2. Antioxidant effect of scorpion extract
The results in Figure and Table showed the notable antioxidant effect of scorpion extract (300 mg/kg, i.p.) in diabetic mice induced by alloxan. The data in Figure (A) presented the level of plasma lipid malondialdehyde (MDA, as a marker of lipid peroxidation) in control and treated mice groups. No significant difference in the level of MDA was detected between the negative control group (2.650 ± 0.267 (µmol/mL) and scorpion extract treated group (2.517 ± 0.142 µmol/mL). On the other hand, MDA level was significantly increased (p < 0.05) in the diabetic control group (alloxan) when compared with negative control group. The injection of scorpion extract induced significant decrease in the level of plasma level of MDA in alloxan + scorpion extract group (p < 0.05) when compared with diabetic control group. The same effect of scorpion extract was noticed in the plasma protein carbonyl content (PCC, as a marker of protein damage) (Figure (B)). The scorpion extract significantly reduced (p < 0.05) the concentration of PCC in alloxan + scorpion extract group (27.5 ± 1.7 µmol/mL) when compared with diabetic control group (35.1 ± 1.9 µmol/mL). The effect of scorpion extract on the level of plasma nitric oxide (NO) in control and diabetic groups has been also examined (Figure (C)). The plasma level of NO was significantly increased (p < 0.05) in mice group received scorpion extract (52.3 ± 2.4 µmol/L) when compared with negative control group (42.5 ± 2.1 µmol/L). In contrast, there was a significant decrease (p < 0.05) in the level of NO in diabetic mice group when compared with the negative control group. The level of NO returned to its normal range in the diabetic group treated with scorpion extract as shown in Figure (C). Effect of scorpion extract on the status of blood non-enzymatic (GSH) and enzymatic (GPx, SOD, CAT) antioxidants in control and treated groups is represented in Table . The level of GSH and activity of GPx, SOD and CAT were significantly decreased (p < 0.05) in the diabetic mice. However, all parameters significantly elevated (p < 0.05) in the diabetic group treated with scorpion extract when compared to the diabetic control group. The analysis of variance using one-way ANOVA revealed significant difference in the level/activity of MDA, PCC, NO, GSH and of GPx, SOD and CAT between experimental groups (p < 0.001).
Figure 2. Effect of the Egyptian scorpion aqueous extract of Scorpio maurus palmatus (300 mg/kg, ip, daily for five weeks) on the level of oxidative stress biomarkers [lipid peroxidation (A), protein carbonyl content (B), nitric oxide (C)] in control and treated mice. Data are presented as Mean ± SEM (6 mice/group). (*) Significant difference between control and each treated group using Student's unpaired t-test, (p < 0.05). (¥) Significant difference between alloxan group (150 mg/kg) and treated groups using Student's unpaired t-test, (p < 0.05). (#) Significant difference between animal groups using one-way ANOVA, (p < 0.05) followed by a Duncan's method hoc test for pairwise multiple comparison.
![Figure 2. Effect of the Egyptian scorpion aqueous extract of Scorpio maurus palmatus (300 mg/kg, ip, daily for five weeks) on the level of oxidative stress biomarkers [lipid peroxidation (A), protein carbonyl content (B), nitric oxide (C)] in control and treated mice. Data are presented as Mean ± SEM (6 mice/group). (*) Significant difference between control and each treated group using Student's unpaired t-test, (p < 0.05). (¥) Significant difference between alloxan group (150 mg/kg) and treated groups using Student's unpaired t-test, (p < 0.05). (#) Significant difference between animal groups using one-way ANOVA, (p < 0.05) followed by a Duncan's method hoc test for pairwise multiple comparison.](/cms/asset/69733625-dcf8-4467-8b3c-6f7316258b7e/tusc_a_1599184_f0002_oc.jpg)
Table 1. Effect of the Egyptian scorpion aqueous extract of Scorpio maurus palmatus (300 mg/kg, ip, daily for 5 weeks) on the status of antioxidants in experimental groups.
3.3. Biochemical effect of scorpion extract
Effect of the aqueous scorpion extract (300 mg/kg, ip, daily for 5 weeks) on the estimated biochemical parameters of total protein, AST, ALT, cholesterol and triglycerides is presented in Table . Diabetic mice displayed a significant decrease (p < 0.05) in the level of total protein and a significant increase (p < 0.05) in the activities of both AST and ALT as well as the level of cholesterol and triglycerides, as compared to negative control group. All biochemical measurements in the diabetic + scorpion extract group were returned close to normal control values. The difference in the levels of biochemical parameters was significantly (p < 0.05) confirmed between different animal groups using one-way ANOVA.
Table 2. Effect of the Egyptian scorpion aqueous extract of Scorpio maurus palmatus (300 mg/kg, ip, daily for 5 weeks) on some plasma biochemical parameters of experimental groups.
3.3. Histopathological effect of scorpion extract
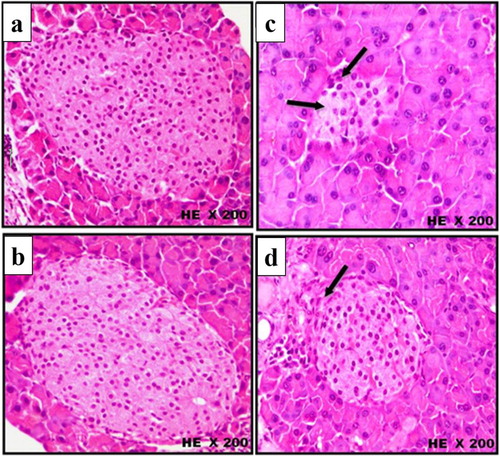
The microscopic histological examinations (H&E) of pancreatic tissue of control and treated mice groups are illustrated in Figures –. The histopathological sections of pancreatic tissue from control and scorpion extract groups showed normal size of pancreatic islets (mean = 2.280 and 2.480 µm2, respectively; Figure (A,B)), normal intensity of β-cells (mean = 58 and 61 cell/ islet, respectively; Figure (A,B)), normal rounded β-cells shape (Figure (a,b)) and normal rounded shape islets surrounded by a very fine layer of connective tissue (Figure (a,b)). On the other hand, injection of alloxan-induced significant pathological changes in pancreatic tissues of diabetic mice appeared as: (i) a notable reduction in the size of pancreatic islets (mean = 1.390 µm2; Figure (A,B)), (ii) significant decrease in the number of β-cells (mean = 26 cell/islet; Figure (A,B)) and (iii) irregular shaped islets crowded with small oval-shaped cells (Figures and ). Interestingly, the administration of scorpion extract significantly improved morphological and cellular features of pancreatic tissue in diabetic mice treated with the aqueous scorpion extract. The average size of pancreatic islets (1.980 µm3) and the number of β-cells (mean = 48 cell/ islet) significantly increased (p < 0.05) in the diabetic + scorpion extract group when compared with diabetic untreated group (Figures and ).
Figure 3. (A) Histological changes in islets of Langerhans of mice pancreas after treatment intraperitoneally with scorpion extract for five 5 weeks showing the difference of islets size. (a) Negative control group, (b) Scorpion extract (300 mg/kg), (c) Diabetic group by alloxan and (d) Diabetic received scorpion extract. Hematoxyline and Eosin (H&E) stain at magnification power ×100. (B) Quantitative analysis of the islets size in experimental animal groups. The average area of islets was determined by measuring diameter of 4 islets in each section and totally 40 islets in each group. Data are presented as Mean ± SEM (n = 40 islets/group). (*) Significant difference between control and each treated group using Student's unpaired t-test, (p < 0.05). (¥) Significant difference between alloxan group (150 mg/kg) and treated groups using Student's unpaired t-test, (p < 0.05). (#) Significant difference between animal groups using one-way ANOVA, (p < 0.05) followed by a Duncan's method hoc test for pairwise multiple comparison.
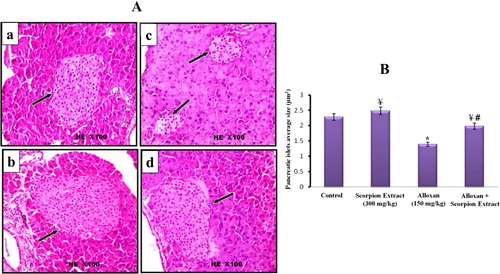
Figure 4. (A) Histological changes in islets of Langerhans of mice pancreas after treatment intraperitoneally with scorpion extract for five 5 weeks showing intensity of β-cells in experimental groups. (a) Negative control group, (b) scorpion extract (300 mg/kg), (c) diabetic group by alloxan and (d) diabetic received scorpion extract. Hematoxyline and Eosin (H&E) stain at magnification power ×200. (B) Comparison between the average number of β-cells in control and treated mice groups. Data are presented as Mean ± SEM (n = 40 islets/group). (*) Significant difference between control and each treated group using Student's unpaired t-test, (p < 0.05). (¥) Significant difference between alloxan group (150 mg/kg) and treated groups using Student's unpaired t-test, (p < 0.05). (#) Significant difference between animal groups using one-way ANOVA, (p < 0.05) followed by a Duncan's method hoc test for pairwise multiple comparison.
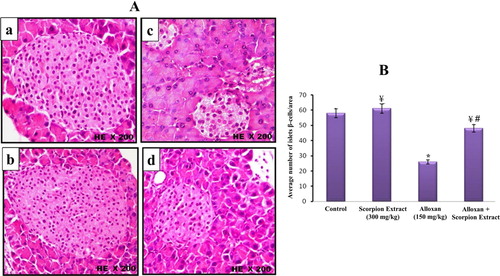
Figure 5. Histological changes in islets of Langerhans of mice pancreas after treatment intraperitoneally with scorpion extract for five five weeks showing β-cells shape in experimental groups. (a) Negative control group, (b) scorpion extract (300 mg/kg), (c) diabetic group by alloxan and (d) diabetic received scorpion extract. Hematoxyline and Eosin (H&E) stain at magnification power ×200.
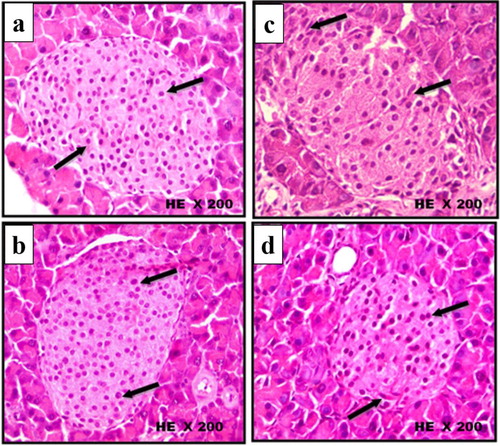
Figure 6. Histological changes in islets of Langerhans of mice pancreas after treatment intraperitoneally with scorpion extract for five weeks showing islets shape and covering connective tissue. (a) Normal control group, (b) scorpion extract (300 mg/kg), (c) diabetic group by alloxan and (d) diabetic received scorpion extract. Hematoxyline and Eosin (H&E) stain at magnification power ×200.
4. Discussion
With the prolonged usage, antidiabetic medicines have various deleterious side effects including abdominal discomfort, hypoglycaemia, nausea, headache, and gaining weight [Citation37]. Thus, it is urgent need to discover novel antidiabetic agents that having high therapeutic index with minimum side effects. The present work describes the potential antidiabetic efficacy of the Egyptian scorpion S. m. palmatus body extract using alloxan-induced diabetic mice model. Alloxan (commonly used as a hyperglycemic agent in experimental animals and induction of Type-1 DM) selectively enters β-cells through the glucose transporter of GLUT2. Alloxan has a strong necrotizing effect on β-cells via its specific suppression of glucokinase and generation of ROS Therefore, reduction of β-cells number resulted in insulin deficiency followed by various metabolic disorders in carbohydrates, proteins and fats [Citation38].
The data from Figure clearly revealed that the injection of scorpion aqueous extract significantly increased the level of plasma insulin which was concomitant with a remarkable decrease in blood glucose of alloxan + scorpion extract group when compared with the diabetic group. The hypoglycemic activities of scorpion extract could be attributed to (i) activation of pathways involved in β-cells regeneration [Citation39]; (ii) enhancing the activity of enzymes responsible for glucose utilization by insulin-dependent pathways and (iii) secretion of insulin from β-cells [Citation40]. Interestingly, our findings were confirmed by histopathological examinations. Scorpion extract improved morphological and cellular features of pancreatic tissue and increased both number and volume of pancreatic islets β-cells. Similar results were obtained by Xie and colleagues [Citation3] who reported hypoglycemic activity of scorpion extract (Chinese scorpion of B. martensii kirsch) combined with gypsum in streptozotocin-induced diabetic mice. This extract exerted its antidiabetic effect through enhancing the regeneration of beta-cells and increased the expression of both PPARγ and PDX-1. The activation of PPARγ restores the function of beta-cell in diabetic mice through (i) inhibiting stress on endoplasmic reticulum; (ii) protecting structure of euchromatin and (iii) regulation the transcription of PDX-1 [Citation41,Citation42]. PDX-1 regulates the development of pancreas and differentiation of beta cells [Citation43].
More importantly to mention that in addition to antidiabetic activity, scorpion extract of S. m. palmatus showed a strong antioxidant activity in diabetic animals (through decreasing lipid and protein oxidation and enhancing the activity of endogenous antioxidants). The extract neutralized free radicals and ROS induced by alloxan and protect macromolecules (lipids, proteins and DNA) from damage. Oxidative stress is considered as one of the main causes of pancreatic cell death, and numerous studies clearly revealed the relationships between oxidative stress and β-cell loss/apoptosis [Citation44]. Subsequently, the antioxidant therapy is currently used (as one of the important therapeutic strategies) in diabetes treatment and management [Citation13].
In addition to antidiabetic and antioxidant activities, the daily injection of scorpion extract significantly improved biochemical measurements (total protein, ALT and AST) and regulated dyslipidemia induced by alloxan. The scorpion extract significantly decreased the levels of cholesterol and triglycerides in diabetic mice and might have a good effect in decreasing the incidence of cardiovascular diseases in diabetic patients [Citation45]. Various drugs are currently being used to control the level of lipids in diabetic individuals such as statins (3-hydroxy-3-methylglutaryl coenzyme A inhibitors) which inhibit the main process of LDL cholesterol in liver and lowering its level in the blood [Citation46].
Several previous studies have been discussed significant biological functions of scorpion venom [Citation47] and the other body organs including heamolymph. Abdou and colleagues [Citation48] reported that heamolymph of the Egyptian scorpion Androctonus australis significantly neutralized toxicological effects induced by the snake venom of Cerastes cerastes through lowering the elevated levels of oxidative stress biomarkers (MDA, PPC and NO) and liver enzymes (ALT and AST) of envenomed rats. Moreover, Schenk and co-workers [Citation49] characterized three unusual lipoproteins (called apoPiLp I, II and III which belong to the HDL-class with molecular masses of 230, 130 and 120 kDa, respectively) from the scorpion haemolymph of Pandinus imperator. Using different in vitro approaches, several biological functions have been reported for lipoproteins such as (i) blood clotting and fibrinolytic processes modulation [Citation50]; (ii) motoneurons L-type Ca2+-channels activator leading to acetylcholine release from synaptic vesicles via exocytosis [Citation49] and (iii) the most striking biological feature of lipoproteins is the repairing of damaged tissue, wound healing and development of blood vessels through their specific binding with receptors and macromolecules located at the surface of cells (such as fibroblasts, endothelial cells, macrophages and platelets) [Citation51]. From the structural point of view, lipoprotein domains might possess growth factor-like characteristics like different growth factors (such as hepatocyte growth factor) [Citation52]. Interestingly, these findings strongly support our present results about the possible regeneration efficacy of scorpion extract on the β-cell of pancreatic islets. Taken together, the results of in vivo experiments provide the first scientific evidence supporting an antihyperglycemic effect of the scorpion aqueous extract of S. m. palmatus in alloxan-induced diabetic mice. Antidiabetic efficacy of scorpion extract could be related to its antioxidant capacity and the ability to enhance regeneration of pancreatic islets and increase insulin secretion. Further investigations are urgently needed to characterize potential molecules responsible for these activities and develop them into effective antidiabetic agents.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Ahmad K. Mohammed http://orcid.org/0000-0002-1423-2756
Sherifa H. Ahmed http://orcid.org/0000-0001-6004-0395
Yaser S. Binnaser http://orcid.org/0000-0003-4730-4653
Ismail M. Abdel-Nabi http://orcid.org/0000-0002-5558-2197
References
- Aylward GW. Progressive changes in diabetics and their management. Eye. 2005;19(10):1115–1118. doi: 10.1038/sj.eye.6701969
- Wild S, Roglic G, Green A, et al. Global prevalence of diabetes. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047
- Xie W, Zhao Y, Gu D, et al. Scorpion in combination with gypsum: novel antidiabetic activities in streptozotocin-induced diabetic mice by Up-regulating pancreatic PPARγ and PDX-1 expressions. Evid based Complem Altern Med. 2011;2011, Article ID 683561, 9 pages, 2011.
- Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024
- Williams G, Pickup JC. Handbook of diabetes. 3rd ed. Malden (MA): Blackwell Publishing Co; 2004.
- Aronson D. Hyperglycemia and pathobiology of diabetic complications. Adv Cardiol. 2008;45:1–16. doi: 10.1159/000115118
- Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008;26:77–82. doi: 10.2337/diaclin.26.2.77
- Rajalakshmi M, Eliza J, Priya CE, et al. Anti-diabetic properties of Tinospora cordifolia stem extracts on streptozotocin-induced diabetic rats. Afr J Pharm Pharmacol. 2009;3:171–180.
- Krentz AJ. Comparative safety of newer oral antidiabetic drugs. Expert Opin Drug Saf. 2006;5(6):827–834. doi: 10.1517/14740338.5.6.827
- Rafaeloff R, Pittenger GL, Barlow SW, et al. Cloning and sequencing of the pancreatic islet neogenesis associated protein (INGAP) gene and its expression in islet neogenesis in hamsters. J Clin Invest. 1997;99(9):2100–2109. doi: 10.1172/JCI119383
- Kumar AY, Nandakumar K, Handral M, et al. Hypoglycaemic and anti-diabetic activity of stem bark extracts Erythrina indica in normal and alloxan-induced diabetic rats. Saudi Pharm J. 2011;19:35–42. doi: 10.1016/j.jsps.2010.10.001
- Hegde K, Arathi A, Mathew A. Evaluation of antidiabetic activity of hydro alcoholic extract of Chrysophyllum cainito fruits. Int J Pharm Sci Res. 2016;7:4422–4428.
- Doan HV, Riyajan S, Iyara R, et al. Antidiabetic activity, glucose uptake stimulation and α-glucosidase inhibitory effect of Chrysophyllum cainito L. stem bark extract. BMC Complement Altern Med. 2018;18:267. doi: 10.1186/s12906-018-2328-0
- Ayeleso TB, Ramachela K, Mukwevh E. Aqueous-Methanol extracts of orange-fleshed sweet potato (Ipomoea batatas) ameliorate oxidative stress and modulate type 2 diabetes associated genes in insulin resistant C2C12 cells. Molecules. 2018;23:2058. doi: 10.3390/molecules23082058
- Yap MKK, Misuan N. Exendin-4 from Heloderma suspectum venom: from discovery to its latest application as Type II diabetes combatant. Basic Clin Pharmacol Toxicol. 2018. 2018 Nov 12. doi:10.1111/bcpt.13169.
- Abdel-Rahman MA, Omran MAA, Abdel-Nabi IM, et al. Intraspecific variation in the Egyptian scorpion Scorpio maurus palmatus venom collected from different biotopes. Toxicon. 2009;53(3):349–359. doi: 10.1016/j.toxicon.2008.12.007
- Abdel-Rahman MA, Quintero-Hernandez V, Possani LD. Venom proteomic and venomous glands transcriptomic analysis of the Egyptian scorpion Scorpio maurus palmatus (Arachnida: Scorpionidae). Toxicon. 2013;74:193–207. doi: 10.1016/j.toxicon.2013.08.064
- Harrison PL, Abdel-Rahman MA, Strong PN, et al. Characterisation of three alpha-helical antimicrobial peptides from the venom of Scorpio maurus palmatus. Toxicon. 2016;117:30–36. doi: 10.1016/j.toxicon.2016.03.014
- Liu JP, Zhang M, Wang WY, et al. (2004). Chinese herbal medicines for type 2 diabetes mellitus. Cochrane Database of Systematic Reviews, no. 3, Article ID CD003642.
- Sophia D, Manoharan S. Hypolipidemic activities of Ficus racemosa linn bark in alloxan induced diabetic rats. Afr J Tradit Complement Altern Med. 2007;4(3):279–288. doi: 10.4314/ajtcam.v4i3.31220
- Richmond W. Estimation of serum cholesterol and lipid profile. Clin Chem. 1973;19:1350–1359.
- Reitman S, Frankel S. A colorimetric method for the determination of serum glutatmic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56
- Fassati P, Prencipe L. Enzymatic colorimetric method for the determination of triglycerides. Clin Chem. 1982;28:2077.
- Friesen NT, Buchau AS, Schott-Ohly P, et al. Generation of hydrogen peroxide and failure of antioxidative responses in pancreatic islets of male C57BL/6 mice are associated with diabetes induced by multiple low doses of streptozotocin. Diabetologia. 2004;47:676–685. doi: 10.1007/s00125-004-1367-x
- Yagi K. Lipid peroxides and human diseases. Chem Phys Lipids. 1987;45(2–4):337–351. doi: 10.1016/0009-3084(87)90071-5
- Reznick AZ, Packer L. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Meth Enzymol. 1994;233:357–363. doi: 10.1016/S0076-6879(94)33041-7
- Green LC, Wagner DA, Glogowski J, et al. Analysis of nitrate, nitrite and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-X
- Beutler E, Doron O, Kelly B. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61(5):882–888.
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169.
- Misra HP, Firdovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175.
- Aebi H. Catalase. In: Bergmeyer HU, editor. Methods of enzymatic analyses. New York: Academic Press; 1974. p. 673–683.
- Zuofa Z, Guoying L, Huijuan P, et al. Anti-diabetic activities of ethanol extract of dry matters of culture broth of Coriolus versiolor in Submerged culture. Braz Arch Biol Technol. 2011;54(4):701–708. doi: 10.1590/S1516-89132011000400008
- Findlay JA, Thomas NW. Histology and cytology of the islets of Langerhans in the Mongolian gerbil (Meriones unguiculatus). Acta Anat (Basel). 1980;108(4):446–462. doi: 10.1159/000145344
- Chakravarthy BK, Gupta S, Gambhir SS, et al. Pancreatic beta cell regeneration. A novel antidiabetic mechanism of Petercarpus marsupium. Indian J Pharma. 1980;12:123–128.
- Gholamali J, Maleki M, Sirus S. Effect of walnut leaf, coriander and pomegranate on blood glucose and histopathology of pancreas of alloxan induced rats. Afr J Trad Comp Alt Med. 2007;4(3):299–305.
- Gundersen HJ, Bagger P, Bendtsen TF, et al. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988;96(10):857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x
- Cobble ME, Peters AL. Clinical practice in type 2 diabetes: after metformin and lifestyle, then what? J Fam Pract. 2009;58(11):S7–S7.
- Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51:216–226. doi: 10.1007/s00125-007-0886-7
- Grijesh K, Mall PK, Veeru P. Antidiabetic and hypolipidemic activity of Gymnema sylvestre in alloxan induced diabetic rats. Global J Biotech Biochem. 2009;4(1):37–42.
- Thomson M, Al-Amin ZM, Al-Qattan KK, et al. Anti-diabetic and hypolipidaemic properties of garlic (Allium sativum) in streptozotocin-induced diabetic rats. Int J Diabetes Metab. 2007;15:108–115.
- Evans-Molina C, Robbins RD, Kono T, et al. Peroxisome proliferator-activated receptor gamma activation restores islet function in diabetic mice through reduction of endoplasmic reticulum stress and maintenance of euchromatin structure. Mol Cell Biol. 2009;29:2053–2067. doi: 10.1128/MCB.01179-08
- Gupta D, Jetton TL, Mortensen RM, et al. In vivo and in vitro studies of a functional peroxisome proliferator-activated receptor gamma response element in the mouse pdx-1 promoter. J Biol Chem. 2008;283: 32462–32470. doi: 10.1074/jbc.M801813200
- Kaneto H, Matsuoka TA, Miyatsuka T, et al. PDX-1 functions as a master factor in the pancreas. Front Biosci. 2008;13:6406–6420. doi: 10.2741/3162
- Sakurai K, Katoh M, Someno K, et al. Apoptosis and mitochondrial damage in INS-1 cells treated with alloxan. Biol Pharm Bull. 2001;24:876–882. doi: 10.1248/bpb.24.876
- Gimeno-Orna JA, Faure-Nogueras E, Sancho- Serrano MA. Usefulness of total cholesterol/HDL-cholesterol ratio in the management of diabetic dyslipidaemia. Diabetic Med. 2005;22(1):26–31. doi: 10.1111/j.1464-5491.2004.01341.x
- Shah RV, Goldfine AB. Statins and risk of new-onset diabetes mellitus. Circulation. 2012;126(18):e282–e284. doi: 10.1161/CIRCULATIONAHA.112.122135
- Abdel-Rahman MA, Harrison PL, Strong PN. Snapshots of scorpion venomics. J Arid Environ. 2015;112(PB): 170–176. doi: 10.1016/j.jaridenv.2014.01.007
- Abdou RH, Abd El-Ghany FS, Abdel-Nabi M, et al. Toxicological effects of the horned viper venom Cerastes cerastes and their neutralization with the Egyptian scorpion Androctonus Australis heamolymph. Indian J Appl Res. 2015;5(3):38–41.
- Schenk S, Gras H, Marksteiner D, et al. The Pandinus imperator haemolymph lipoprotein, an unusual phosphatidylserine carrying lipoprotein. Insect Biochem Mol Biol. 2009;39:735–744. doi: 10.1016/j.ibmb.2009.08.009
- Koschinsky ML. Novel insights into Lp(a) physiology and pathogenicity: more questions than answers? Cardiovasc Hematol Disord Drug Targets. 2006;6:267–278. doi: 10.2174/187152906779010764
- Schmidt K, Noureen A, Kronenberg F, et al. Structure, function, and genetics of lipoprotein (a). J Lipid Res. 2016;57:1339–1359. doi: 10.1194/jlr.R067314
- Donate LE, Gherardi E, Srinivasan N, et al. Molecular evolution and domain structure of plasminogen-related growth factors (HGF/SF and HGF1/MSP). Protein Sci. 1994;3:2378–2394. doi: 10.1002/pro.5560031222
