?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In bearing structures of automobile dump truck semi-trailers engaged in the transport of agricultural goods, mineral fertilizers, a large part of metal structures is under the direct influence of aggressive environments in combination with dynamic loads that arise when driving on roads. The peculiarities of corrosion processes when contacting steel surfaces of dump semi-trailers with mineral fertilizers, as well as their aqueous solutions, are insufficiently studied. Also studied mechanisms of electrochemical corrosion of materials of metal structures of agricultural vehicles to ensure their performance in conditions of prolonged aggressive environments. The authors determined that the mineral fertilizer medium is the most aggressive material used in cargo transportation in the agrarian sector mineral fertilizer and ammonium sulphate cause intense corrosion damage of typical materials of metal structures of automobile dump-carriers, which adversely affects reliability. The obtained results allow a better understanding of the causes of agricultural machines corrosion and can be used in assessing their residual resource.
1. Introduction
In bearing structures of automobile dump semi-trailers used in the transport of agricultural goods, mineral fertilizers, a large part of metal structures is under the direct influence of aggressive environments in combination with dynamic loads that arise when driving on roads [Citation1,Citation2]. This leads to significant corrosion and corrosion-fatigue damage. It is known that because of the influence of atmospheric corrosion and mechanical stresses, 70–80% of machine parts break down, 20–25% [Citation2] of which are breakdowns caused by overloads due to the loss of strength due to atmospheric corrosion. Thus, according to the estimation of corrosion damage of aggregates, parts and joints of metal structures of machine building metal constructions, in particular considered semi-trailers, the most prone to corrosion of sheet metal constructions, cars sheathing, pulleys, bushing roller chains, welded joints, details of pipe transport, other [Citation1,Citation3,Citation4].
Mineral fertilizers and pesticides are the most dangerous from the point of view of corrosive influence on the materials of dump semi-trailers involved in the transportation of agrarian goods [Citation5–9]. Therefore, the estimation of corrosion and electrochemical behaviour of typical metallic materials of vehicles in such corrosive-active environments is of great practical value. Inspection of machines from the standpoint of corrosion damage indicate that the most severely destroyed parts of metal structures are located in the lower part due to prolonged contact with aggressive media. The corrosion pitting, cracks, enlarged gaps in joints (Figure ) are often observed on surfaces. The most corrosive-active among mineral fertilizers are nitrophosphate and ammonium sulphate, less aggressive are ammonia water, and humidity is a catalyst for corrosion processes [Citation5]. The massive losses of steel metal in the transport sector are negligible, but the technical state of the corroded parts negatively affects overall reliability, with the combination of corrosion factors and cyclic loads having the greatest danger [Citation3,Citation4]. In this case, the service life may decrease by 40–60% [Citation10].
Figure 1. General view (a,b) and corrosion damage (c,d) of a dump semi-trailer used in transportation of fertilizers after one season of operation.

The peculiarities of corrosion processes when contacting steel surfaces of dump semi-trailers with mineral fertilizers, as well as their aqueous solutions, are insufficiently studied. Lack of reliable data makes it difficult to develop effective methods of anti-corrosion protection and does not allow to develop a long-term forecast of the life of the transport vehicles of this class. Despite existing research on the effects of working aggressive environments on reducing the strength and reliability of semi-trailers, the processes of corrosion metal structures under the influence of fertilizers are not sufficiently studied. In [Citation5,Citation6], only two steels were investigated, although in the manufacture of metal structures of the considered semi-trailers, the nomenclature of standard quality and high-quality steels is used in full. Therefore, there is a need for experimental research of a more comprehensive range of materials of metal structures of vehicles.
The aim of work is to study speeds and mechanisms of electrochemical corrosion of materials of metal structures of agricultural vehicles: standard quality steels – St3, St5 (DSTU 2651: 2005; DIN 17100) and high-quality steels – Steel 10, Steel 15, Steel 20, Steel 25 (GOST 1050- 88; DIN 17200) (Table ) in corrosive environments of ammonium sulphate and nitrophosphate. The obtained results allow better understanding of the causes of corrosion of elements of agricultural machines and can be used in assessing their residual resource.
Table 1. Chemical composition of tested steels.
2. Materials and methods
Testing was carried out on samples made of mentioned above materials under supply conditions. The samples were made in the form of discs with a diameter 20 mm and a thickness 0.16 mm. The surfaces were polished to the roughness Ra = 0.63 µm. The diameter of holes for fixation on a holding tool was 2 mm. The samples were cleaned and degreased using acetone, and then dried in a desiccator for 2 h. After that, they were weighed to within ±0.0004 g and stored in a desiccator up to 24 h.
As operating environments were used distilled water as standard test rainwater and saturated solutions of the most corrosion aggressive mineral fertilizers [Citation5] of ammonium sulphate (NH4)2SO4, containing 20.5% of nitrogen and 24% of sulphur with minor impurities of H2SO4, Ca2+, Mg2+ and SiO2; nitrophosphate is a mixture of NH4H2PO4, (NH4)2HPO4 and KNO3, which contains 35–52% of nitrogen, P2O5 and K2O.
Before and after the experiments the pH of solutions was measured using a pH meter. The rate of corrosion Km (kg/(m2·s)) was determined by a gasometrical method after exposure for 1, 7, 12 and 24 days in solutions of ammonium sulphate, nitrophosphate, and, for comparison, in distilled water and after the removal of corrosion products [Citation11,Citation12].
The calculation was performed using the formula
(1)
(1) where Δm is sample weight change after exposure to corrosive environment and afer the removal of corrosion products, kg; S is area of sample (m2); τ is exposure time (s).
The obtained value was converted using the depth index Π (mm/year)
(2)
(2) where k is the conversion factor of s per year.
To study the influence of mineral fertilizers under supply condition on the corrosion resistance of investigated standard and high-quality steels, the samples were vertically placing into dry fertilizers to control the occurrence of corrosion damages in 1, 7, 12, 24 days. The rate of corrosion was defined by (1) and (2).
Polarization studies were conducted using the potentiostat IP-Pro with appropriate software [Citation1]. The samples of steel pressed in PTFE were used as the working electrodes with the working surface area 0.0628 cm2. Before each measurement, the sample was grinded by the abrasive paper №0, cleaned with acetone and dried. The comparison electrode is the saturated silver chloride electrode, the auxiliary is the platinum one. To determine the corrosion current and Tafel constants of cathode and anodic reactions, the straight sections of polarization curves were used.
The current-controlled indexes of corrosion rate were converted to the mass ones using the formula
(3)
(3) where i is corrosion current (A/m2); k is coefficient (k = 1 if the testing time is expressed in seconds, and the area in cm2); A is atomic mass of metal (iron and steel A = 56); n is valence of metal (iron 2 or 3); F is Faraday constant.
The nature of corrosion damages of the samples surface was evaluated using the microscope EVO-40XUP (Zeiss) with X-ray microanalysis system INCA Energy 350 for the local chemical analysis of surfaces [Citation13,Citation14].
3. Theory/calculation
Many commercial chemicals are used in farming, including fertilizers, grain and silage preservatives, chemicals for pest, disease and weed control, and proprietary acid solutions for cleaning dairy equipment. Farm wastes and slurries can also be significantly corrosive [Citation13,Citation15]. Fertilizers are chemicals given to plants with the intention of promoting growth. They are usually applied either via the soil or by foliar spraying. Fertilizers typically provide in varying proportions the three major plant nutrients (nitrogen, phosphorus and potassium) and the secondary plant nutrients (calcium, sulphur, magnesium, iron, etc.) [Citation13,Citation15]. Some fertilizers are more corrosive than others, especially if they decompose or react to produce aggressive substances such as ammonia or hydrogen sulphide; if chloride ions are present (including potassium or ammonium chloride), or if acidic conditions prevail. For example, dihydrogen ammonium phosphate or ammonium nitrate can lead to increased corrosion via hydrolysis to acids (i.e. a fall in pH) [Citation13,Citation15]. The relative ratios of the essential plant nutrients can influence the corrosiveness of compound liquid fertilizers, there being some evidence that the greatest effects occur with fertilizer solutions containing about 15% nitrogen, especially when half the free nitrogen is derived from urea and half from ammonium nitrate [Citation13,Citation15]. Some typical reactions for liquid fertilizers are given in Table .
Table 2. Corrosive reactions of liquid fertilizers [Citation13, Citation15].
If fertilizers are kept dry, then no corrosion occurs, but being hygroscopic they can pick up moisture and hence may become corrosive. The hygroscopic point – the relative humidity (RH) at and above which moisture is absorbed – varies from one compound to another and the lower its value the more corrosive the fertilizer is likely to be. Ammonium nitrate starts to absorb moisture at 60% RH, while certain phosphates absorb moisture only above 90% RH. Moisture initially causes caking of the fertilizer, which can increase its abrasive properties [Citation13,Citation15]. Galvanizing of steel is generally beneficial in resisting corrosion, as can be noted from the results of laboratory tests, where metal was immersed in shallow pools of solution for 500 h at ambient temperatures (). Carbon steel is often used to contain fertilizers because it is cheap, but adequate surface cleaning, preparation and coating are necessary. Large fertilizer storage tanks should be constructed of materials, which are resistant to corrosion, puncture, or cracking.
Table 3. Steel corrosion rates in fertilizers determined laboratory tests at ambient temperature [Citation13, Citation15].
4. Results
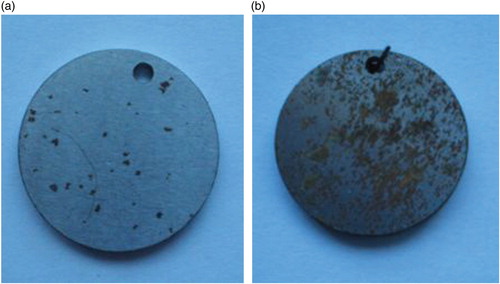
We performed two stages of corrosion tests: in dry mineral fertilizers and its saturated solutions. The samples made of high-quality steels were placed in crystalline salts of ammonium sulphate. The first damages as single pittings could be found in 24 h on all the samples. They transformed into pits during the next 4 days. In nitrophosphate single pittings occurred in the crystal form on the 7th day (Figure ). After exposure for 24 days the rate of corrosion of high-quality steels in dry ammonium sulphate stabilized at 0.006 mm/year, in dry nitrophosphate it stabilized at 0.0012 mm/year.
Figure 2. Overall view of 20 steel samples after 24 h of exposition in the nitrophosphate (dry) (a) and ammonium sulphate (dry) (b).
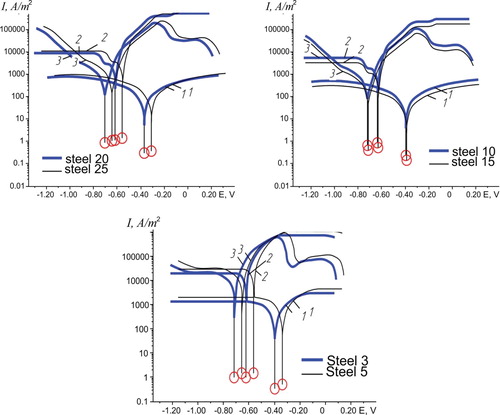
Saturated solutions of ammonium sulphate and nitrophosphate are acidic environments, consequently, pH = 4.35 and pH = 4.6. Therefore, the mechanism of initiation of corrosion in these solutions can differ from the comparable mechanism in distilled water. The corrosion rates of trailers metal structures materials under study are observed to be an order of magnitude higher in saturated solutions of ammonium sulphate and nitrophosphate than in standard test rainwater (Table ). The time dependence of corrosion rate is typical for neutral environments: with the exposure time increase from 1 to 24 days the corrosion rate gradually decreases.
Table 4. The corrosion rate (Km) of metal structures materials of tractor semi-trailers in rainwater model and saturated solutions of ammonium sulphate and nitrophosphate.
Two groups of steel were used as experimental materials. During the first day, the highest corrosion rate was observed in saturated solution of nitrophosphate. In the period from 1 to 24 days the rates of corrosion in saturated solution of ammonium sulphate are twice higher than in distilled water. Besides, in the period from 12 to 24 days the corrosion rates are found to be higher than in nitrophosphate solution. The given rates are 29–36 times higher than the corresponding rates in dry mineral fertilizers of ammonium sulphate and nitrophosphate due to the catalytic impact of water that acts as a polar solvent and promotes the dissociation of ammonium sulphate and nitrophosphate (Figure ).
Figure 3. Dependence of 20 steel corrosion deep index on exposure time τ in solutions: 1 – distilled water; 2 – saturated ammonium sulphate solution; 2′ – ammonium sulphate (dry); 3 – saturated nitrophosphate solution; 3′ – nitrophosphate (dry).
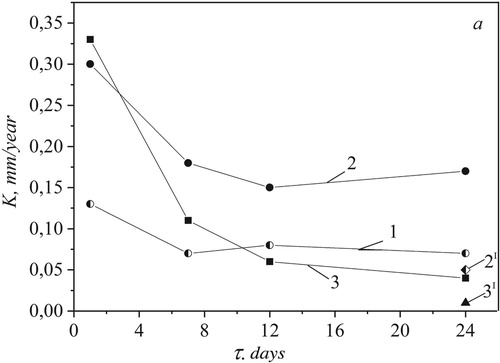
Dissociation products together with water are more corrosion active than the corresponding crystalline salts. After exposure for more than 7 days the values of corrosion rates in nitrophosphate solution are lower than in ammonium sulphate solution and water, due to the passivation effect of NO3-anions and the formation of phosphate layer on the steel surface by ions
The electronic picture of samples surfaces made of high-quality steel after exposure for 24 days in rainwater model, nitrophosphate and ammonium sulphate detects differences in the form of corrosion damages: ammonium sulphate causes deeper and wider pittings and pits compared to distilled water. Partial analysis proved the presence of sulphur, traces of calcium and oxygen on the pit bottom, in contrast to pittings formed in distilled water, in which calcium and oxygen dominate. The surface of samples after exposure to nitrophosphate solution is covered by single pittings, at the bottom of which K, Ca, Na, O, Cl, N are revealed.
The area of pittings on the surface of Steel 20 after exposure to nitrophosphate is smaller in comparison with ammonium sulphate. This fact contradicts the statements [Citation6,Citation16,Citation17] on the highest level of corrodibility of nitrophosphate. Perhaps, different duration of tests causes this difference.
5. Discussion
Corrosion in saturated solution of ammonium sulphate and nitrophosphate occurs by electrochemical mechanism. Steady-state potentials were determined during 1200–1500 s, the most negative values were observed in ammonium sulphate solution (Figure (a,b); Table ). In comparison to distilled water these values are negative by more than 0.300 V. Therefore, it can be concluded that thermodynamic stability of high-quality steels and standard steels is low in the given fertilizer solution.
Table 5. Electrochemical properties of metal structures materials of agricultural vehicles in solutions of ammonium sulphate and nitrophosphate.
Polarization curves of standard steels compared to high-quality steels have more negative potentials (Figure (a,b); Table ). Cathode curves of steels groups under study in distilled water and saturated solution of ammonium sulphate and nitrophosphate are different: in the ammonium sulphate on the cathodic curves, for example, of Steel 15 within the potentials −(0.800–0.980) V, the area of maximum diffusion current is expressed less clearly than in Steel 5 and both steels in distilled water and nitrophosphate. The maximum diffusion current in nitrophosphate solution in Steel 20 is 1·104 A/m2, in Steel 5 – 1.5·104 A/m2, in ammonium sulphate solution – correspondently, 5 · 103 A/m2 and 1.5·104 A/m2.
Table 6. Comparing the rate of corrosion of metallic metals in environments of mineral fertilizers obtained by a gravimetric method (Km (kg/m2 ·s)), electrochemical method (icor (A/m2)) and converted from current indexes (Ki (kg/m2 ·s)).
Anode polarization curves in solutions of nitrophosphate and ammonium sulphate significantly differ from the anode curves in rainwater model. In ammonium sulphate solution the potential of early passivation of Steel 3 is 0.390 V, of 20 Steel – 0.300 V, the potential of complete passivation, correspondently, − 0.280 V and –0.150 V. The critical current density of passivation current of Steel 5 and Steel 15 is up to 6·105 A/m2. In nitrophosphate solution, the potential of early passivation of Steel 5 is 0.370 V, and Steel 0.020–0.150 V. Thus, in mineral fertilizers under study, the passivation of high-quality steels starts at less negative potentials compared to standard steels; and the current indexes of corrosion rates of standard steels are higher in comparison with high-quality steels. Corrosion currents are the highest in nitrophosphate (saturated solution), satisfactorily coinciding with gravimetric data. As current indexes are instantaneous, their values should better correlate with the corrosion rates obtained in a period of one day by a weight method. According to (3), corrosion currents satisfactory correlate with the corrosion rates obtained by a gravimetric method, provided that Fe0 oxidizes to Fe3+(Table ).
Thus, nitrophosphate and ammonium sulphate together with water are highly corrosion active towards the investigated materials.
It was proved that the rate of corrosion of steels of standard quality (St3, St5) and of high-quality steels (Steel 10, Steel 15, Steel 20, Steel 25) in mineral fertilizer solutions are maximum during the first day and gradually decrease with the increase of exposure time, in particular, in nitrophosphate solution they become dimensional with the rate of corrosion in distilled water. It was determined that saturated solutions of ammonium sulphate and nitrophosphates, in contrast to their concentrates in a crystalline form, cause intense corrosion damage of the studied steels, whose corrosion rates reach 0.29–0.33 mm/year, which is 2.2–2.5 times higher than speeds in the rainwater environment. It was proved that this type of dependence of the corrosion rate on the time of exposure is conditioned by the formation of protective layers of the passivation nature on the surface of the steel. For the studied materials corrosion in saturated solutions of nitrophosphate and ammonium sulphate has a local character. After removal of corrosion products, chemical elements (components of mineral fertilizers) were found on the surface of the samples. In distilled water, the average values of corrosion rates of quality steels are lower by 5%. In the saturated solutions of the investigated mineral fertilizers, the difference is much higher and is 45–50%. Thus, when designing agricultural machines it is recommended to prefer the use of high-quality steels.
The polarization curves of standard quality steels in fertilizer solutions in comparison with distilled water, lie in the region of negative potentials, the potential for the inhibition of the anode dissolution of standard quality steels is more negative than that of high-quality steels. The Tafel constants of both groups of steels in distilled water and solutions of nitrophosphate and ammonium sulphate are insignificant, indicating a similar nature of the cathode reaction in the field of stationary potential. The Tafel constants are the lowest in nitrophosphate solution, which indicates the lowest polarization of the anode process, and the corrosion currents are the highest, this is consistent with the massometric velocities obtained during the first day.
6. Conclusions
The corrosion rate of agricultural semi-trailers metal structures materials (Steel 10, Steel 15, Steel 20, Steel 25, Steel 3, Steel 5) in solutions of mineral fertilizers was determined to be maximum during exposure for the first day, then it reduces to values comparable with the corrosion rate in rainwater model. The determined nature of corrosion rate depending on the exposure time is caused by the formation of protective layers of passivation (nitrophosphate) or salt (ammonium sulphate) deposits on the materials surface.
Ammonium sulphate and nitrophosphate are proved to cause more intensive corrosion damages to high-quality steels and standard steels when they are in saturated solutions rather than in crystalline state. The corrosion rate of steels reaches 0.35 mm/year that is above 2.5 times higher than in standard model rainwater solution.
Corrosion of groups of steels under study in saturated solutions of nitrophosphate and ammonium sulphate is local. After the removal of corrosion products, the elements that are a part of environments under study were revealed on the samples surface.
According to electro-chemical study corrosion currents in high-quality steels and standard steels were determined to be the highest in nitrophosphate solution. This statement is proved by gasometrical indicators of rates obtained during the first day of exposure. Steady-state potentials of both groups of steels assume the most negative values in ammonium sulphate solution, indicating a lower thermodynamic stability of materials. Polarization curves of standard steels in solutions of fertilizers, in comparison with standard rainwater model assume more negative potentials. The potential of early passivation is more negative compared to high-quality steel. Tafel constants of cathodic reaction in both groups of steels in distilled water, nitrophosphate solution and ammonium sulphate slightly differ, indicating the similarity of cathode reactions in the steady-state potential. Tafel constants of anode reaction are the lowest in nitrophosphate solution because of the lowest polarization of anodic process.
The influence of such aggressive agricultural environments as mineral fertilizers causes intensive damages to metal structures materials. Consequently, the performance reliability decreases, resulting in performance degradation of trailers.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
P. Popovych http://orcid.org/0000-0001-5516-852X
O. Shevchuk http://orcid.org/0000-0001-8283-4620
L. Poberezhna http://orcid.org/0000-0002-4944-0192
M. Mazur http://orcid.org/0000-0002-5746-9876
References
- Gaydar SM. Protection of agricultural equipment from corrosion and wear with the use of nanotechnology – Doctoral thesis in tech. sci.- Moscow, 2011. 442p.
- Severnev MM. Wear and corrosion of agricultural. In: MM Severnev, et al., Minsk: Bielarusscience; 2011. p. 333.
- Maruschak P, Poberezhny L, Pyrig T. Fatigue and brittle fracture of carbon steel of gas and oil pipelines. Transport. 2013;28(3):270–275. DOI:10.3846/16484142.2013.829782
- Yavorskyi AV, Karpash MO, Zhovtulia LY, et al. Safe operation of engineering structures in the oil and gas industry. J Nat Gas Sci Eng. 2017;46:289–295. DOI:10.1007/s11003-014-9719-210.1016/j.jngse.2017.07.026
- Popovych PV, Slobodyan ZB. Corrosion and electrochemical Behaviors of 20 Steel and St. 3 Steel in ammonium sulfate and Nitrophoska. Mater Sci. 2014;49(6):819–826. DOI:10.1007/s11003-014-9679-6
- Popovych PV, Mahlatyuk LA, Kupovych RB. Influence of organic fertilizers on the corrosion-electrochemical characteristics of low-carbon steels. Mater Sci. 2014;50(2):284–289. DOI:10.1007/s11003-014-9719-2
- Popovych P, Shevchuk O, Dzyura V, et al. Assessment of the influence of corrosive aggressive cargo transportation on vehicle reliability. Int J Eng Res Afr. 2018;38:17–25. DOI:10.4028/www.scientific.net/JERA.38.17.
- Popovych PV, Lyashuk OL, Murovanyi IS, et al. The service life evaluation of fertilizer spreaders undercarriages. INMATEH – Agric Eng. 2016;50(3):39–46.
- Popovych PV, Lyashuk OL, Shevchuk OS, et al. Influence of organic operation environment on corrosion properties of metal structure materials of vehicles. INMATEH – Agric Eng. 2017;52(2):113–118.
- Gerasymenko AA. Protection against corrosion, aging and biodegradation of machinery, equipment and structures directory. M.: Mech Engineering. 1987;11:784.
- Shchurin KV. Predicting and improving fatigue life of bearing systems of agricultural vehicles [thesis for the degree of Doctor Sciences]. Orenburg: OP, 1994. 423p.
- Uhlig HH. Uhlig’s corrosion handbook. Vol. 51. Pennington: Wiley; 2011.
- Anonymous. Corrosion control of agricultural equipment and buildings, http://www.npl.co.uk/ncs/docs.
- Oki M, Anawe PA. A review of corrosion in agricultural industries. Phys Sci Int J. 2015;5(4):216–222. DOI:10.9734/PSIJ/2015/14847
- Eker B, Yuksel E. Solutions to corrosion caused by agricultural chemicals. Trakia J Sci. 2005;3(7):1–6.
- Popovych PV. Comprehensive analysis of the reliability of tractor trailers in their operation. Bulletin KhNTUA. – Kharkiv, 2010. Ed. No. 93. p. 411–414.
- Popovych PV. Areas of research of corrosion – fatigue durability of metal structures used for fertilization. Bulletin KhNTUA. Kharkiv, 2013. Ed. No. 134. p. 227–233.