?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This work sought to draw a comparison between the photocatalytic proficiency of NiO nanoparticles (NPs) for the degradation of Methylene blue (MB) and Rhodamine B (Rh B) dye. The NiO NPS were synthesized by a facile straightforward chemical route by utilizing NiCl2 and NaHCO3 as a precursor. The morphological characteristics of the synthesized NiO NPs have been characterized by using SEM. In addition XRD, FTIR and EDX analysis were also carried out to investigate the crystalline size, functional group and elemental composition of the NPs. The NiO NPs were subsequently used for the photocatalytic degradation of the MB and Rh B in the presence of the visible light irradiation. For evaluation of photocatalytic proficiency, the influence of various key parameters viz. pH of the dye solution, initial dye concentration, Irradiation time and amount of the catalyst were investigated. The extent of photocatalytic degradation of MB and Rh B was found to be 98.7 and 80.33% at pH 2 and 10 respectively. The kinetic investigation of photocatalytic degradation followed a pseudo-first-order rate kinetics for MB and Rh B dye with rate constant 4.6 × 10−1 and 3.6 × 10−2. Additionally, the synthesized NiO NPs was used for the antimicrobial activity against the few gram-positive and gram-negative microorganisms.
Highlights
The NiO nanocrystalline semiconducting photocatalyst was successfully synthesized by a facile straightforward chemical route by utilizing NiCl2 and NaHCO3 as a precursor.
Novel simple chemical route synthesis at room temperature.
First time report a combine study of photocatalytic degradation of Methylene blue and Rhodamine B dye by using NiO as a photocatalyst.
Also, Antimicrobial study was examined which clearly indicate the biocompatibility of synthesized NiO nanoparticles.
GRAPHICAL ABSTRACT
1. Introduction
At present many environmental issues have been arising due to the effect of different natural as well as artificial factors on the earth's crust. Generally, contamination of any unnecessary and unacceptable change in the environment because of various activities of human beings are known as environmental pollution. The direct or indirect changes in the biological, chemical and physical properties of the natural water streams which leads to harmful effect not only on human beings but also on aquatic ecosystems. There are different types of the factor responsible for the polluting the natural water bodies such as population explosion, urbanization, fast industrialization, etc. Among all the factors the fast industrialization mainly textile, food processing, dyeing, paper, and dye manufacturing industries are the major sources of water pollution. The effluent of such industries containing different types of dyes and organic pollutants. An organic dye contamination assumed to play an active role in polluting aquatic ecosystems. Near about 10%–15% of the total world production of the dye is lost to the effluent during the various processes [Citation1]. The dyes in wastewater don't allow the sunlight penetration into the water stream, consequently, reduce the photosynthetic reactions, and a few dyes are lethal and even malignant neoplastic disease that could be a severe health threat to human beings and animals [Citation1,Citation2]. The MB and Rh B dye contamination in the industrial effluent is a major concern and show the harmful effect on both types of the ecosystem. The increasing water scarcity and open mindfulness toward the threats imposed by industrial effluents, International environmental standards are taking a lot of rigorous across the world, leading to the development of innovative frameworks and techniques to remove dyes and other organic pollutants from the effluent before discharge [Citation3].
In recent years, nanotechnology has been gained a marvelous impetus in this rapidly emerging technology era by creating an abundance of scientific ideas to compete with the daily challenges of growing technology [Citation4,Citation5]. The Nanomaterials have been exploring the various scientific and technologies interest with their innumerable applications and specific properties [Citation6–13]. These properties are conferred because of their characteristics size, shape and surface area [Citation14–20]. Nanostructure semiconductor oxide such as TiO2, ZnO, SnO2 and so on is reported to be a proficient photocatalyst for the degradation of organic pollutants [Citation21–28]. The greater part of the semiconducting oxides used for water purification are n-type among this nanostructure TiO2 is the most intensively studied [Citation29,Citation30]. Recent studies on the photocatalytic activity of many p-type semiconducting transition metal oxides such as NiO, Cu2O, FeO, etc. have appeared in the literature [Citation31–33]. The studies on the use of nanostructure NiO as a photocatalyst for the degradation of organic dyes such as MB, Rh B, Methyle Orange, Acid red 1 have been reported [Citation34–36]. These reports indicate the potential applications of nanocrystalline NiO as a photocatalyst for many more reactions, including the degradation of organic pollutants for water purification. And also, antimicrobial activity against some gram-positive and gram-negative bacteria [Citation37–39]. So it is needed that some comparative studies regarding the photocatalytic and antimicrobial properties of NiO NPs.
NiO NPs are chemically stable and show a very high electro-optical proficiency with a wide band gap (3.6–4.0 eV). NiO is a P-type semiconductor that has been widely used in numerous chemical and physical applications like catalysis, Solar cell and gas sensing [Citation40–43]. According to the literature, there are several methods for the preparation of NiO NPs has been reported as thermal decomposition [Citation44], combustion [Citation45], sol–gel [Citation46] co-precipitation [Citation47], spray pyrolysis [Citation48], anodic arc plasma method [Citation49]. The present study reports the facile straightforward chemical synthesis, it has an advantage over other methods as it is so simple, rapid and energy-saving [Citation50]. The obtained product shows the best photocatalytic activity against MB and Rh B dyes.
In the present study, we report the synthesis and characterization of nanocrystalline NiO with the large surface area through facile straightforward chemical synthesis. The comparative study of the photocatalytic proficiency of NiO NPs for the degradation of MB and Rh B dyes was investigated by optimizing the various parameters viz. effect of pH, irradiation time, initial dye concentration. Best of author knowledge, this is the first report for a comparative study of the photocatalytic proficiency of NiO NPs. In addition to this, the antimicrobial property was examined.
2. Experimental
2.1. Materials and methods
For the synthesis of NiO NPs all chemicals and reagents are of the A.R grade. Nickel chloride (NiCl2), Sodium bicarbonate (NaHCO3), Methylene blue (C16H18ClN3S) and Rhodamine B (C28H31ClN2O3) were purchased from S. D. Fine Chemicals, India Ltd. Mumbai. The gram-positive (Staphylococcus aureus, Bacillus subtilis) and gram-negative (Pseudomonas aeruginosa, Escherichia coli) bacterial strains were purchased from National Collection of Industrial Microorganisms (NCIM), National chemical Laboratory (NCL), Pune-411008 (India).
2.2. Preparation of dye solution
The stock solution (100 mg/L) of MB and Rh B was prepared using deionized water. The desired concentration of dye solutions (5, 10, 15, 20 and 25 mg/L) was prepared by further dilution of the stock solution with deionized water.
2.3. Synthesis of nano crystalline NiO
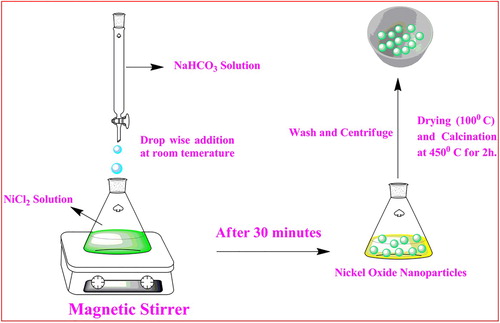
The synthesis of NiO NPs was done by the simple chemical route. NiCl2 and NaHCO3 were taken in 1:0.5 ratios and dissolved in 10 ml distilled water in a separate container. The NiCl2 solution was stirred by a magnetic stirrer for 25 min. Then the NaHCO3 solution was added to the NiCl2 solution dropwise with the constant stirring. After 30 min the products were collected wash with distilled water and alcohol, dried at 100°C. Then the dried sample was calcinated at 450°C for 2 h. The NiO NPs synthesis procedure is as shown in Figure .
2.4. Characterizations
The synthesized NiO NPs was characterized by scanning electron microscopy (SEM Hitachi S-4800 Japan), X-ray diffraction (XRD- Bruker D 8 Advance X-ray diffractometer Germany), electron dispersive X-ray spectroscopy (EDX- Bruker X Flash 5030), Fourier-transform infrared spectroscopy (Shimadzu FTIR-8400) techniques and the photocatalytic activity was performed by a UV–Vis spectrophotometer (Systronics 2203 India).
2.5. Adsorption study
To investigate the adsorption of MB and Rh B on the NiO NPs surface, the MB and Rh B dyes solution were stirred with NiO NPs in dark for the 30 min to ensure the adsorption–desorption equilibrium. To examine the change in concentration of MB and Rh B solutions were determined with the help of a UV-Visible double spectrophotometer at the wavelength of absorbance maximum of dyes (for MB λmax = 663 nm and Rh B λmax = 543 nm). The amount of MB and Rh B adsorbed on the NiO NPs surface was calculated by the equation 1.
(1)
(1) where Co (mg/L) is the initial dye concentration and Ct (mg/L) is the dye concentration after time t, qt (mg/g) adsorption capacity at time t; V (l) is the initial volume of dye solution and W (g) is the amount of catalyst.
2.6. Photocatalytic degradation experiments
In a typical experiment, 1 mg of the synthesized NiO NPs were added in 50 ml of an aqueous solution of MB and Rh B. Then the solution is kept in the photocatalytic reactor (Photocatalytic reactor, Lelesil Innovative System, Mumbai) with continuous stirring to ensure that the suspensions of the catalyst were uniform over the span of reaction. During this adequate amount of dye solution was removed and centrifuged for 10 min to separate NiO NPs after that the supernatant solution was analyzed with the help of UV-Visible double beam spectrophotometer at the wavelength of absorbance maximum of dye to obtain the concentration of MB and Rh B in solution. The percentage of degradation of dye has been calculated by using equation 2.
(2)
(2)
2.7. Antimicrobial activity
The antimicrobial activity of NiO NPs was investigated by using the agar diffusion method. The DMSO (dimethyl sulfoxide) was used as a solvent for the preparation of stock solutions. The sterile Petri dishes containing nutrient agar medium aseptically inoculated with desired microbial strains. By using a sterile cork borer of a size 6 mm, well were made in petri-dish. The accurately 100 µg/ well, NiO NPs were used to examine the antimicrobial activity. At the same time the standard antibiotic, Chloramphenicol i.e. 10 µg/disc (Hi-media, Mumbai, India) was used against the selected bacterial strains. Then, the petri-dish allow to incubated 37°C for 24 h. After the incubation period, the zone of inhibition of well was measured by using a vernier caliper to get average values.
3. Results and discussion
3.1. XRD analysis
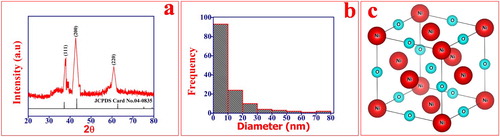
The crystalline structures of the NiO NPs were characterized by XRD with a monochromatic high intensity Cu Kα radiation (1.5418 Å) as shown in Figure (a). The average crystalline size of NiO NPs was found to be 6.36 nm which was estimated from the FWHM (Full-width half maxima) and peak position of an XRD pattern by Scherer's formula (equation 3) [Citation51]. Further confirmed with the help of PSA (Particle size analyzers) results as shown in Figure (b). the particle size analysis has been carried out using Scanning electron micrographs. The obtained particle size was found to be in the range of 5–30 nm which is in good agreement with XRD results.
(3)
(3) where D is the average crystalline size, λ is a wavelength in Å, β is the FWHM in radian and θ is diffraction angle in degree. The major diffraction peaks appear at 2θ, 38.0, 42.7 and 60.86, which can be indexed as (111), (200), and (220) planes. All these diffraction peaks can be perfectly indexed to the Face-centered cubic (FCC) Figure (c). The crystalline structure of NiO NPs is in accordance with JCPDS card No. 04-0835. The high intensity of peaks indicates the crystalline nature of NiO NPs and the structural parameters are shown in Table . The XRD pattern of NiO shows that the NiO NPs are single phase and no any other impurity diffraction peak except the NiO peak FCC was detected.
Table 1. The structural parameter of NiO NPs.
3.2. SEM and EDX analysis
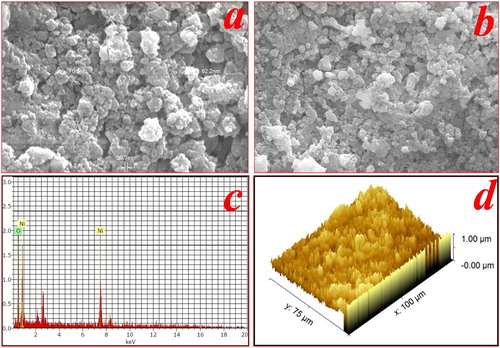
The EDX of NiO NPs is as shown in Figure (c). It is clearly indicated that the synthesized NPs are made up of Ni and O. It confirms the purity of NiO NPs and no other impurity was detected in the sample spectrum. The exact atomic percentage of elements Ni and O is 25% and 75% respectively. The SEM images of NiO NPs are shown in Figure (a,b). The SEM images exhibited irregular spherical morphology with the different sized particles due to agglomeration. It shows a large surface area which is beneficial for the photocatalytic activity [Citation12,Citation52]. Figure (d) shows the 3D view of the SEM image of NiO NPs has several nanosized growth sites distributed on the surface of a catalyst. Also, many well-outlined crystallites in the form of a cone with a pointed apex were observed.
3.3. FTIR analysis
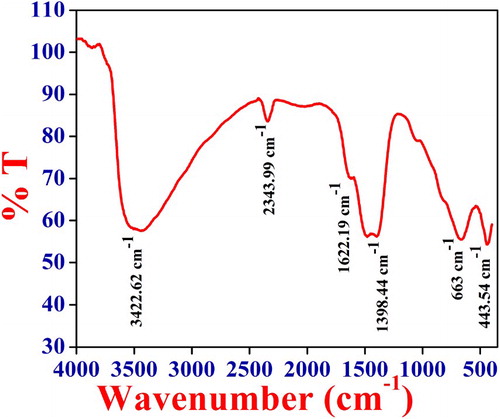
To understand the dominant functional group present in the catalyst FTIR was recorded at room temperature. The FTIR spectra of NiO NPs are as shown in Figure . From the FTIR spectra peak around the 443.54 and 663 cm−1 is because of the nickel oxygen bond stretching vibrations. The broadness of a peak indicates that the NiO catalyst is crystalline in nature. The absorption band at 2343.99 cm−1 is due to the symmetric and the asymmetric stretching mode of vibrations of the CO2 molecule absorbed from the air [Citation53]. Also, the broad peak at 3422.62 and 1398.44, 1622.19 cm−1 is may be because of stretching and bending vibrations of– OH group absorbed on catalyst surface from the atmosphere when FTIR analysis was carried out [Citation54].
3.4. Photocatalytic studies
3.3.1. Effect of pH of solution
The pH of reaction media has been recognized as the one that decides the photocatalytic proficiency of the catalyst. The photocatalytic proficiency of synthesized NiO NPs was analyzed against the different pH of MB and Rh B solution ranging from 2 to 10. The pH of the solution was adjusted by using 0.1 M NaOH and 0.1 M H2SO4. The experiment was carried out in the presence of NiO NPs for irradiation time 120 min at 10 mg/L dye concentration as shown in Figure (a,b). The Photocatalytic proficiency of the synthesized NiO NPs is directly proportional to the pH of the dye solution. Therefore, as the pH of the dye solution increases from 2 to 10, the degradation of MB dye also increases. This is because of the variation of pH alters the surface properties of NiO NPs. The pHPZC (point of zero charge) of NiO NPs was estimated at 8.19 using the reported method [Citation52,Citation55]. At pHPZC NiO has net-zero charges and at pH < pHPZC the surface of a catalyst becomes positively charged. Whereas at pH < pHPZC the surface is negatively charged. MB is a cationic dye, hence at pH < pHPZC displayed repulsive behaviour due to a positively charged surface of the catalyst. Therefore degradation proficiency decreases. When pH > pHPZC the catalyst surface has a negative charge which attracts the MB dye molecules to a greater extent. At pH 10 per hydroxyl, radicals are formed, leading to the formation of hydrogen peroxide which offers ascend to hydroxyl radicals on a large scale. This enhances the photocatalytic proficiency of NiO NPs under visible light irradiation and the degradation of MB dye reaches up to 97%.
Figure 5. Effect of pH on photocatalytic degradation of (a) MB conditions: pH = 2–10, dye concentration 10 mg/L and catalyst dose 1 gm/L for the irradiation time 120 min. (b) Rh B, conditions: pH = 2–10, dye concentration. 10 mg/L and catalyst dose 1 gm/L for the irradiation time 120 min.
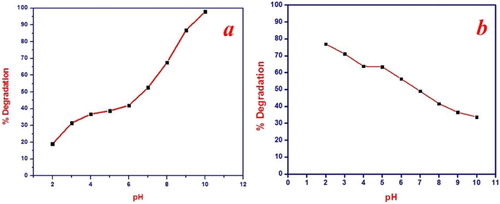
For Rh B maximum degradation was obtained at pH 2. At this pH the surface of NiO NPs is positively charged and Rh B forms a zwitterionic conformation in a polar solvent. Hence, there is an attraction between the catalyst surface and Rh B dye molecule. The positive holes are prominent oxidizing species at low pH [Citation56]. The photogenerated valence band hole is sufficiently positive to produce hydroxyl radicals. These hydroxyl radicals are responsible for the degradation of Rh B. But as the pH of the solution increases the zwitterion formation also increases. Which leads to the aggregation of Rh B and formation of dimer molecule. These dimer molecules are incapable to enter into the pore structure of the NiO NPs. Hence the degradation proficiency of the catalyst decreases at higher pH [Citation1].
3.3.2. Effect of initial dye concentration
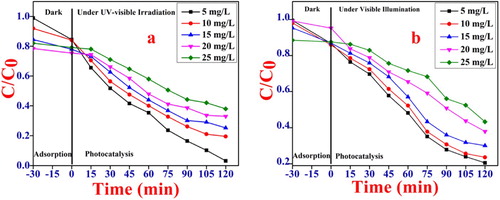
For determining the impact of dye concentration on the degradation proficiency of the catalyst. The experiment was carried out an optimized pH and irradiation time 120 min by changing the initial dye concentration from 5 to 25 mg/L. The MB and Rh B solutions containing NiO NPs were kept in dark for 30 min to attained the adsorption–desorption equilibrium. The percent removal of MB and Rh B solutions ranging from 5 to 25 mg/L was found to be around 10%–15% after the 30 min adsorption in dark. Then it was followed by photocatalytic degradation under the UV-visible light irradiation by assuming the time t = 0. Figure (a,b), it clearly indicates the degradation proficiency of the catalyst is inversely proportional to the initial dye concentration of MB and Rh B dye. As the initial dye concentration of MB and Rh B increases, the time needed for complete degradation is increasing and photocatalytic proficiency decreases. At higher concentration of a dye maximum number of dye molecules adsorbed on the catalyst surface resulting in reduced light penetration. Due to this lesser number of hydroxyl and superoxide radicals and hence photocatalytic activity diminished [Citation52,Citation57].
Figure 6. Effect of initial dye concentration on the photocatalytic degradation (a) MB, conditions pH = 10, dye concentration 5, 10, 15, 20, 25 mg/L and catalyst dose 1 gm/L for irradiation time 120 min. (b) Rh B, conditions: pH = 2 dye concentration 5, 10, 15, 20, 25 mg/L and catalyst dose 1 gm/L for irradiation time 120 min.
3.3.3. Effect of irradiation time
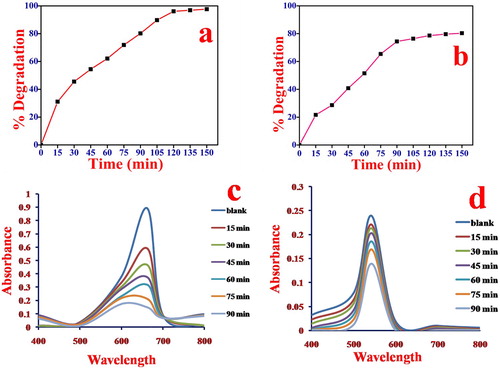
The relationship between degradation proficiency of catalyst for MB and Rh B dye degradation and contact time were investigated at optimized condition (for MB at pH 10 and for Rh B at pH 2) dye concentration 5 mg/L and catalyst dose 1 gm/L. The results are shown in Figure (a–d). The degradation of MB has shown regular reduction with increasing the irradiation time under Visible-light. The decolorization of the dye solution took place within 150 min of irradiation. The corresponding degradation proficiency of MB was found to be more 98.7%. For Rh B degradation gradually increasing with irradiation time and attains equilibrium. This is because of the formation of zwitterions that occupy the active sites of the catalyst. Therefore, the degradation of Rh B was found to be only 80.33% within 150 min irradiation time [Citation58].
Figure 7. Effect of irradiation time on photocatalytic degradation of (a) MB, conditions pH = 10, dye concentration 5 mg/L and catalyst dose 1 gm/L irradiation time 150 min. (b) Rh B, conditions: pH = 2 dye concentration 5 mg/L and catalyst dose 1 gm/L irradiation time 150 min. (c) UV-Visible spectra of MB dye solution at 663 nm (d) UV-Visible spectra of Rh B dye solution at 543 nm.
The data has been further utilized for the kinetic study of MB and Rh B dye in the presence of NiO NPs (Figure (a,b)). It is found that the degradation of MB and Rh B dye is pseudo-first-order reaction. The rate constant (k) was calculated by employing the following equation (4) where k is the apparent rate constant, Co is the initial concentration of MB and Rh B solution and Ct is the concentration of MB and Rh B at time t [Citation59]. The slope of the ln Co/Ct Vs time plot gives the value of the rate constant k in min−1.
(4)
(4) The photocatalytic activity can be compared to k value and the linear regression coefficient (R2) for MB and Rh B solution with different initial concentrations are summarized in Table . The k values, which are obtained by the linear fitting from Table are 0.6102, 0.4836, 0.4099 min−1 for MB and 0.0483, 0.0437, 0.0371 for Rh B respectively.
Figure 8. Pseudo first order kinetics for photocatalytic degradation of (a) MB, conditions pH = 10, dye concentration. 5, 10, 15 mg/L and catalyst dose 1 gm/L irradiation time 120 min. (b) Rh B, conditions: pH = 2 dye concentration. 5, 10, 15 mg/L and catalyst dose 1 gm/L irradiation time 120 min.
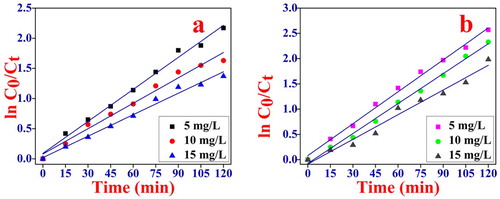
Table 2. Pseudo first order rate constant of MB and Rh B for photocatalytic degradation.
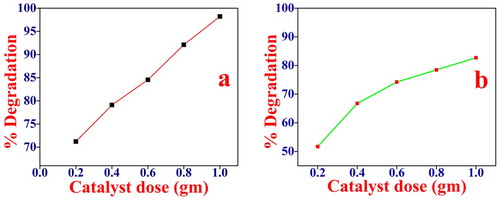
3.3.4. Effect of catalyst weight
The effect of photocatalyst weight was studied on dye degradation keeping other experimental conditions constant. The percentage degradation of MB and Rh B by NiO photocatalyst at different catalyst doses 0.2–1 gm/L for 5 mg/L of dye concentration was studied. It is observed that the initial rate increases with the increase in catalyst concentration, and becomes maximum as shown in Figure (a,b). The number of photons absorbed and the number of dye molecules degraded is increased with the increase in catalyst concentration, thereby enhancing the rate of degradation. The photocatalytic degradation mechanism can be explained by the following equations.
(5)
(5)
(6)
(6)
(7)
(7)
(8)
(8)
(9)
(9)
(10)
(10)
(11)
(11)
(12)
(12)
Figure 9. Effect of catalyst weight on the photocatalytic degradation of (a) MB, conditions pH = 10, dye concentration 5 mg/L and catalyst dose 0.2–1 gm/L irradiation time 120 min. (b) Rh B, conditions: pH = 2 dye concentration 5 mg/L and catalyst dose 0.2–1 gm/L irradiation time 120 min.
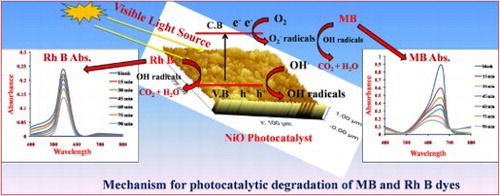
When the light incident on the NiO semiconducting photocatalyst then the electron gets excited. These excited electrons can be jumped from the valence band to the conduction band leads to the formation of the positive hole (h+) in the valence band (equation 5). The hydroxyl ion (OH−) from the water molecule capture by the positive hole (h+) present on the photocatalyst surface which results in the formation of the hydroxyl radicals () (equation 6,7). The excited electron in the conduction band reacts with the O2 molecule to generate the .O2 radicals (equation 8) and helps to produce a large number of hydroxyl radicals (
) (equation 9–11). When these highly oxidized species (
) react with the MB and Rh B dye molecule, it oxidized to these dye molecules into the CO2 and H2O.
3.3.5. Antimicrobial study of NiO NPs
The antimicrobial activity of synthesized NiO NPs was investigated by the Agar diffusion method [Citation60,Citation61]. The bacterial action of NiO NPs against gram-positive (Staphylococcus aureus, Bacillus subtilis) and gram-negative (Pseudomonas aeruginosa, Escherichia coli) as shown in Figure and the results are tabulated in Table . The synthesized NiO NPs do not show antimicrobial activity against both grams positive and gram-negative microorganisms. These results pointed out the biocompatibility of the NiO NPs. It was also found that the as-prepared NPs were not harmful as they do not inhibit the growth of microorganisms at a low concentration while it shows a toxic effect and inhibits the growth of the microorganism at higher concentrations reported in the literature are listed in Table . Therefore obtained NPs were biocompatible, non-toxic and used for environmental remediation.
Figure 10. Antimicrobial activity test of NiO NPs against the (a) Pseudomonas aeruginosa (b) Bacillus subtilis (c) Staphylococcus aureus, (d) Escherichia coli.
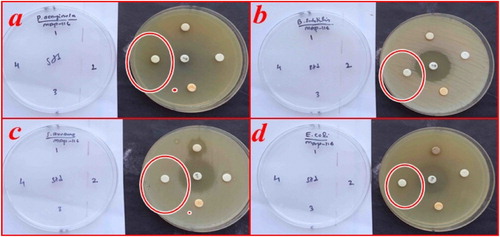
Table 3. Zone of inhibition (mm) of NiO NPs for different type of microorganisms.
Table 4. Antimicrobial activity of NiO NPs for different type of microorganisms reported in the literature with the variable amount of NiO NPs.
3.3.6. Recycle performance of the NiO
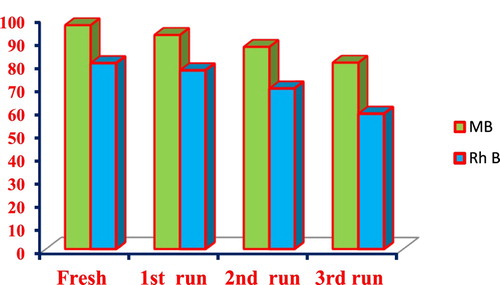
To study its recyclability, the optimized catalyst dose (1gm) of NiO NPs was allowed to settle by gravity after the photocatalytic degradation. The recovered nanocomposite was then collected and reused 3 times under the same photodegradation conditions. The Figure . Shows that the removal of the MB and Rh B by the NiO photocatalyst after the 1st run achieved up to 92.47% and 77.25% respectively. After the 3rd run, removal of the MB and Rh B decreases down to 80.54 and 58.62%. The decrease in the removal of the MB and Rh B is probably due to the loss of the recycled catalyst during sampling. The Figure shows that NiO NPs has excellent stability and does not suffer from photo-corrosion during degradation.
4. Conclusions
The face-centered cubic NiO NPs with superior stability and the recyclability for removal of hazardous water contamination caused by the dyes were successfully synthesized via facile straightforward chemical synthesis. The synthesized NiO NPs were characterized by different analysis methods such as XRD, SEM, EDX FTIR, and PSA. The synthesized NiO NPs have a spherical shape with crystalline size 6.36 nm calculated from XRD and confirmed by the PSA analysis. The synthesized NiO NPs were found to be useful for the removal of these dyes from their aqueous suspensions. The rate of photocatalytic degradation was increased with increases in the pH of the MB solution while it decreases with increases in the pH of the Rh B solution. The extent of photocatalytic degradation of MB dye was found to be 98.7% and for Rh B 80.33%. The photocatalytic proficiency of the NiO NPs was found to decrease with the increasing initial dye concentration of both MB and Rh B dyes. The results clearly indicate that the synthesized NiO NPs show good photocatalytic activity for the removal of MB dye from their aqueous suspension than the Rh B dye. The NiO NPs were also used for the antimicrobial activity against the gram-positive and gram-negative microorganisms. Results indicate that the NiO NPs don't show any antimicrobial activity against the gram-positive and gram-negative micro-organisms at the lower concentration. Therefore, the synthesized NiO NPs are biocompatible and non-toxic.
Acknowledgments
The author Subhash Khairnar gratefully acknowledges to Kavayitri Bahinabai Chaudhari North Maharashtra University, Jalgaon for providing financial support under the scheme of Shree G. H. Raisoni fellowship for doctoral research. The authors are thankful to DST-FIST providing the necessary grant for instruments.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Subhash Dharmraj Khairnar http://orcid.org/0000-0002-6732-366X
Vinod Shankar Shrivastava http://orcid.org/0000-0002-2065-1068
References
- Zollinger H. Color chemistry: syntheses, properties and applications of organic dyes and pigments. Weinheim: VCH; 1991.
- Motahari F, Mozdianfard MR, Soofivand F, et al. NiO nanostructures: synthesis, characterization and photocatalyst application in dye wastewater treatment. RSC Adv. 2014;4(53):27654–27660. doi: 10.1039/c4ra02697g
- Lin SH, Peng CF. Continuous treatment of textile wastewater by combined coagulation, electrochemical oxidation and activated sludge. Water Res. 1996;30(3):587–592. doi: 10.1016/0043-1354(95)00210-3
- Din MI, Nabi AG, Rani A, et al. Single step green synthesis of stable nickel and nickel oxide nanoparticles from Calotropis gigantea: catalytic and antimicrobial potentials. Environ Nanotechnol Monit Manage. 2018;9:29–36.
- Ahmed S, Ahmad M, Swami BL, et al. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J Adv Res. 2016;7(1):17–28. doi: 10.1016/j.jare.2015.02.007
- Khan MSJ, Kamal T, Ali F, et al. Chitosan-coated polyurethane sponge supported metal nanoparticles for catalytic reduction of organic pollutants. Int J Biol Macromol. 2019;132:772–783. doi: 10.1016/j.ijbiomac.2019.03.205
- Ali F, Khan SB, Kamal T, et al. Synthesis and characterization of metal nanoparticles templated chitosan-SiO2 catalyst for the reduction of nitrophenols and dyes. Carbohydr Polym. 2018;192:217–230. doi: 10.1016/j.carbpol.2018.03.029
- Ali N, Awais, Kamal T, et al. Chitosan-coated cotton cloth supported copper nanoparticles for toxic dye reduction. Int J Biol Macromol. 2018;111:832–838. doi: 10.1016/j.ijbiomac.2018.01.092
- Haider A, Haider S, Kang I-K, et al. A novel use of cellulose based filter paper containing silver nanoparticles for its potential application as wound dressing agent. Int J Biol Macromol. 2018;108:455–461. doi: 10.1016/j.ijbiomac.2017.12.022
- Kamal T, Khan SB, Haider S, et al. Thin layer chitosan-coated cellulose filter paper as substrate for immobilization of catalytic cobalt nanoparticles. Int J Biol Macromol. 2017;104:56–62. doi: 10.1016/j.ijbiomac.2017.05.157
- Kreyling WG, Semmler-Behnke M, Chaudhry Q. A complementary definition of nanomaterial. Nano Today. 2010;5(3):165–168. doi: 10.1016/j.nantod.2010.03.004
- Maniammal K, Madhu G, Biju V. Nanostructured mesoporous NiO as an efficient photocatalyst for degradation of methylene blue: structure, properties and performance. Nano-Structures Nano-Objects. 2018;16:266–275. doi: 10.1016/j.nanoso.2018.07.007
- Wang Z, Liu Y, Huang B, et al. Progress on extending the light absorption spectra of photocatalysts. Phys Chem Chem Phys. 2014;16(7):2758–2774. doi: 10.1039/c3cp53817f
- Houas A, Lachheb H, Ksibi M, et al. Photocatalytic degradation pathway of methylene blue in water. Appl Catal B. 2001;31(2):145–157. doi: 10.1016/S0926-3373(00)00276-9
- Zhang H, Chen G, Bahnemann DW. Photoelectrocatalytic materials for environmental applications. J Mater Chem. 2009;19(29):5089–5121. doi: 10.1039/b821991e
- Ansari SA, Khan MM, Kalathil S, et al. Oxygen vacancy induced band gap narrowing of ZnO nanostructures by an electrochemically active biofilm. Nanoscale. 2013;5(19):9238–9246. doi: 10.1039/c3nr02678g
- Venkata Subba Rao K, Rachel A, Subrahmanyam M, et al. Immobilization of TiO2 on pumice stone for the photocatalytic degradation of dyes and dye industry pollutants. Appl Catal B. 2003;46(1):77–85. doi: 10.1016/S0926-3373(03)00199-1
- Pillai SC, Periyat P, George R, et al. Synthesis of high-temperature stable anatase TiO2 photocatalyst. J Phys Chem C. 2007;111(4):1605–1611. doi: 10.1021/jp065933h
- Lang X, Li-Ping J, Jun-Jie Z. Sonochemical synthesis and photocatalysis of porous Cu2O nanospheres with controllable structures. Nanotechnology. 2009;20(4):045605. doi: 10.1088/0957-4484/20/4/045605
- Wang C, Wang X, Xu B-Q, et al. Enhanced photocatalytic performance of nanosized coupled ZnO/SnO2 photocatalysts for methyl orange degradation. J Photochem Photobiol A. 2004;168(1):47–52. doi: 10.1016/j.jphotochem.2004.05.014
- Kaizra S, Louafi Y, Bellal B, et al. Electrochemical growth of tin(II) oxide films: application in photocatalytic degradation of methylene blue. Mater Sci Semicond Process. 2015;30:554–560. doi: 10.1016/j.mssp.2014.10.045
- Khan FU, Asimullah, Khan SB, et al. Novel combination of zero-valent Cu and Ag nanoparticles @ cellulose acetate nanocomposite for the reduction of 4-nitro phenol. Int J Biol Macromol. 2017;102:868–877. doi: 10.1016/j.ijbiomac.2017.04.062
- Kamal T, Ul-Islam M, Khan SB, et al. Adsorption and photocatalyst assisted dye removal and bactericidal performance of ZnO/chitosan coating layer. Int J Biol Macromol. 2015;81:584–590. doi: 10.1016/j.ijbiomac.2015.08.060
- Ali F, Khan SB, Kamal T, et al. Chitosan coated cotton cloth supported zero-valent nanoparticles: simple but economically viable, efficient and easily retrievable catalysts. Sci Rep. 2017;7(1):16957. doi: 10.1038/s41598-017-16815-2
- Kamal T, Ahmad I, Khan SB, et al. Synthesis and catalytic properties of silver nanoparticles supported on porous cellulose acetate sheets and wet-spun fibers. Carbohydr Polym. 2017;157:294–302. doi: 10.1016/j.carbpol.2016.09.078
- Kamal T, Anwar Y, Khan SB, et al. Dye adsorption and bactericidal properties of TiO2/chitosan coating layer. Carbohydr Polym. 2016;148:153–160. doi: 10.1016/j.carbpol.2016.04.042
- Ali F, Khan SB, Kamal T, et al. Chitosan-titanium oxide fibers supported zero-valent nanoparticles: highly efficient and easily retrievable catalyst for the removal of organic pollutants. Sci Rep. 2018;8(1):6260. doi: 10.1038/s41598-018-24311-4
- Kamal T, Khan SB, Asiri AM. Synthesis of zero-valent Cu nanoparticles in the chitosan coating layer on cellulose microfibers: evaluation of azo dyes catalytic reduction. Cellulose. 2016;23(3):1911–1923. doi: 10.1007/s10570-016-0919-9
- Singh S, Joshi M, Panthari P, et al. Citrulline rich structurally stable zinc oxide nanostructures for superior photo catalytic and optoelectronic applications: a green synthesis approach. Nano-Structures Nano-Objects. 2017;11:1–6. doi: 10.1016/j.nanoso.2017.05.006
- Iole V, Nadia B, Maria Vittoria R, et al. Electrodeposited ZnO with squaraine sentisizers as photoactive anode of DSCs. Mater Res Express. 2014;1(1):015040. doi: 10.1088/2053-1591/1/1/015040
- Min S, Wang F, Jin Z, et al. Cu2O nanoparticles decorated BiVO4 as an effective visible-light-driven p-n heterojunction photocatalyst for methylene blue degradation. Superlattices Microstruct. 2014;74:294–307. doi: 10.1016/j.spmi.2014.07.003
- Hu W, Chu D, Wang L, et al. Ultrasound-assisted synthesis of hexagonal cone-like Cu2O architectures with enhanced photocatalytic activity. Nano-Structures Nano-Objects. 2017;12:220–228. doi: 10.1016/j.nanoso.2017.09.018
- Li J, Liu Z, Wang D, et al. Visible-light responsive carbon–anatase–hematite core–shell microspheres for methylene blue photodegradation. Mater Sci Semicond Process. 2014;27:950–957. doi: 10.1016/j.mssp.2014.08.038
- Qing Z, Haixia L, Huali L, et al. Solvothermal synthesis and photocatalytic properties of NiO ultrathin nanosheets with porous structure. Appl Surf Sci. 2015;328:525–530. doi: 10.1016/j.apsusc.2014.12.077
- Anandan K, Rajendran V. Effects of Mn on the magnetic and optical properties and photocatalytic activities of NiO nanoparticles synthesized via the simple precipitation process. Mater Sci Eng B. 2015;199:48–56. doi: 10.1016/j.mseb.2015.04.015
- Wang Y, Zhang F, Wei L, et al. Facet-dependent photocatalytic performance of NiO oriented thin films prepared by pulsed laser deposition. Phys B. 2015;457:194–197. doi: 10.1016/j.physb.2014.10.014
- Rakshit S, Ghosh S, Chall S, et al. Controlled synthesis of spin glass nickel oxide nanoparticles and evaluation of their potential antimicrobial activity: a cost effective and eco friendly approach. RSC Adv. 2013;3(42):19348–19356. doi: 10.1039/c3ra42628a
- Feng Y, Liu L, Zhang J, et al. Photoactive antimicrobial nanomaterials. J Mater Chem B. 2017;5(44):8631–8652. doi: 10.1039/C7TB01860F
- Raghunath A, Perumal E. Metal oxide nanoparticles as antimicrobial agents: a promise for the future. Int J Antimicrob Agents. 2017;49(2):137–152. doi: 10.1016/j.ijantimicag.2016.11.011
- Fazlali F, Mahjoub AR, Abazari R. A new route for synthesis of spherical NiO nanoparticles via emulsion nano-reactors with enhanced photocatalytic activity. Solid State Sci. 2015;48:263–269. doi: 10.1016/j.solidstatesciences.2015.08.022
- Behnajady MA, Bimeghdar S. Synthesis of mesoporous NiO nanoparticles and their application in the adsorption of Cr(VI). Chem Eng J. 2014;239:105–113. doi: 10.1016/j.cej.2013.10.102
- Huang J, Zhu N, Yang T, et al. Nickel oxide and carbon nanotube composite (NiO/CNT) as a novel cathode non-precious metal catalyst in microbial fuel cells. Biosens Bioelectron. 2015;72:332–339. doi: 10.1016/j.bios.2015.05.035
- Ren L, Zeng Y-P, Jiang D. The improved photocatalytic properties of P-type NiO loaded porous TiO2 sheets prepared via freeze tape-casting. Solid State Sci. 2010;12(1):138–143. doi: 10.1016/j.solidstatesciences.2009.09.021
- Davar F, Fereshteh Z, Salavati-Niasari M. Nanoparticles Ni and NiO: synthesis, characterization and magnetic properties. J Alloys Compd. 2009;476(1):797–801. doi: 10.1016/j.jallcom.2008.09.121
- Song X, Gao L. Facile synthesis of polycrystalline NiO nanorods assisted by microwave heating. J Am Ceram Soc. 2008;91(10):3465–3468. doi: 10.1111/j.1551-2916.2008.02667.x
- Li Q, Wang L-S, Hu B-Y, et al. Preparation and characterization of NiO nanoparticles through calcination of malate gel. Mater Lett. 2007;61(8):1615–1618. doi: 10.1016/j.matlet.2006.07.113
- Li J, Yan R, Xiao B, et al. Preparation of nano-NiO particles and evaluation of their catalytic activity in pyrolyzing biomass components. Energy Fuels. 2008;22(1):16–23. doi: 10.1021/ef700283j
- Wang W-N, Itoh Y, Lenggoro IW, et al. Nickel and nickel oxide nanoparticles prepared from nickel nitrate hexahydrate by a low pressure spray pyrolysis. Mater Sci Eng B. 2004;111(1):69–76. doi: 10.1016/j.mseb.2004.03.024
- Wei Z, Xia T, Bai L, et al. Efficient preparation for Ni nanopowders by anodic arc plasma. Mater Lett. 2006;60(6):766–770. doi: 10.1016/j.matlet.2005.10.008
- Chakrabarty S, Chatterjee K. Synthesis and characterization of nano-dimensional nickelous oxide (NiO) semiconductor. J Phys Sci. 2009;13:245–250.
- Khairnar SD, Patil MR, Shrivastava VS. Hydrothermally synthesized nanocrystalline Nb2O5 and its visible-light photocatalytic activity for the degradation of Congo red and methylene blue. Iranian J Catal. 2018;8(2):143–150.
- Patil SB, Ravishankar T, Lingaraju K, et al. Multiple applications of combustion derived nickel oxide nanoparticles. J Mater Sci Mater Electron. 2018;29(1):277–287. doi: 10.1007/s10854-017-7914-2
- Jayakumar G, Albert Irudayaraj A, Dhayal Raj A. Photocatalytic degradation of methylene blue by nickel oxide nanoparticles. Mater Today Proc. 2017;4(11, Part 3):11690–11695. doi: 10.1016/j.matpr.2017.09.083
- Wongsaprom K, Maensiri S. Synthesis and room temperature magnetic behavior of nickel oxide nanocrystallites. Ciang Mai J Sci. 2013;40(1):99–108.
- Nezamzadeh-Ejhieh A, Bahrami M. Investigation of the photocatalytic activity of supported ZnO–TiO2 on clinoptilolite nano-particles towards photodegradation of wastewater-contained phenol. Desalin Water Treat. 2015;55(4):1096–1104. doi: 10.1080/19443994.2014.922443
- Akpan UG, Hameed BH. Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: a review. J Hazard Mater. 2009;170(2):520–529. doi: 10.1016/j.jhazmat.2009.05.039
- Wang C-C, Lee C-K, Lyu M-D, et al. Photocatalytic degradation of C.I. basic violet 10 using TiO2 catalysts supported by Y zeolite: an investigation of the effects of operational parameters. Dyes Pigm. 2008;76(3):817–824. doi: 10.1016/j.dyepig.2007.02.004
- Marathe YV, Ramanna MMV, Shrivastava VS. Synthesis and characterization of nanocrystalline CdS thin films grown by chemical bath deposition at different molarities for removal of methylene blue. Desalin Water Treat. 2013;51(28-30):5813–5820. doi: 10.1080/19443994.2013.769720
- Patil MR, Khairnar SD, Shrivastava VS. Synthesis, characterisation of polyaniline–Fe3O4 magnetic nanocomposite and its application for removal of an acid violet 19 dye. Appl Nanosci. 2016;6(4):495–502. doi: 10.1007/s13204-015-0465-z
- Jorgensen JH. Susceptibility test methods: dilution and disk diffusion methods. In: Murray PR, Baron EJ, editors. Manual of clinical microbiology. Vol. 2. Washington (DC): ASM Press; 2007. p. 1152–1173.
- Ingroff E, Pfaller MA. Susceptibility test methods: yeasts and filamentous fungi. In: Murray PR, Baron EJ, editors. Manual of clinical microbiology. Vol. 2. Washington (DC): ASM Press; 2007. p. 1972–1986.