Abstract
In vitro inhibition of cell migration/wound closure and cellular apoptosis was studied of four chloroform extract fractions of clove (Syzygium aromaticum L.) on human A549 and H1299 cancer cell lines. The flow cytometry analysis was carried out with A549 and H1299 cancer cell lines. The results indicated that chloroform extract fractions of clove showed inhibition of wound closure/cell migration of A549 and H1299 and induced apoptosis of H1299. This was demonstrated by calculating low wound closure percentage in cells; treated with these fractions as compared to control (70%). The morphological features of the nuclei of the cells treated with fractions indicated chromatin compression, nuclear shrinkage and apoptotic bodies formation, which pointed out towards death by apoptosis. The flow cytometer analysis of the treated cancer cells H1299 also revealed death due to the apoptosis. These results confirmed that the chloroform extract of clove bud may be used to treat lung cancer.
1. Introduction
The scientists all over the world are struggling to find the cure against the greatly nasty human enemy called “cancer”. This struggle has been going on for a very long period of time and human beings are often finding themselves at the losing end [Citation1–5]. Cancer is often linked to age and it is observed that the numbers of new cancer cases just keep on rising during the passage of time and do not seem to go down [Citation5–10]. It is now recognized as one of the foremost reasons for death globally. The probability of death caused by cancer is 1 in 8 deaths worldwide, and a huge wave of new cases amounting to 12.7 million was diagnosed in 2008. As per GLOBOCAN estimation 18.1 million fresh cases and 9.6 million mortality were occurred globally in 2018 owing to cancer only [Citation11]. Cancer is considered the broad classification for more than 80 diverse diseases of the human body initiated by normal cell growth which goes wild. This abnormal cell growth results in irregular cell division and these abnormal cells in turn spreads in or invades the body and ultimately cause death when not controlled.
In the contemporary world, the figure of cancer cases and deaths happening is increasing each passing year. The total number of cancer cases is expected to grow rapidly [Citation12]. Nowadays, the treatment modalities available for cancer consist chemotherapy, surgery or radiotherapy [Citation13–18]. However, cancer chemotherapy is commonly known to lead to serious side effects in treating patients [Citation19–23]. Some of these deleterious effects of chemotherapy include to ulcerative stomatitis, hepatotoxicity, fibrosis and cirrhosis of liver, diarrhoea, encephalopathy, myelosuppression, infertility, skin reactions, leukopoenia, alopecia, neutropenia, anaemia, thrombocytopenia, GI distress, anorexia, cardiac arrest, pulmonary fibrosis, capillary leak syndrome, infection, low RBCs, etc. Drug resistance restricts the efficacy of chemotherapeutics, which then leads to cancer recurrence and, subsequently, to key cancer complications. The drug resistance is also responsible for a poor prognosis of the disease of cancer [Citation24]. Therefore, it has become imperative and necessary to search for new and novel molecules for the treatment of different cancers. Besides, it is also important to find new drugs that can be used to augment the anticancer properties of common chemotherapeutic drugs; presently being employed for cancer treatment [Citation25]. In recent years, substantial attention has been paid towards identifying naturally occurring chemo-preventive agents having property of preventing, hindering, or reversing the process of multi-phased carcinogenesis [Citation26–28].
It is well-known that different kinds of spices possess the capabilities to prevent carcinogenic bioactivation, reduce free radical concentration, cell division delay and encourage apoptosis in malignant cells. These properties of spices add to their cancer inhibition potentials. It has been established that eating of about 1 g/day of herbs/spices being the best foundation of antioxidants considerably adds to total adequate antioxidant intake (>1 mmol) [Citation29–32]. The consumption of spices by human beings may be predominantly essential in reducing oxidative injury to cells due to environmental stress. This oxidative stress can lead to degenerative illnesses with cancer. A number of spices have been recognized/epidemiologically proved to impact and influence the risk of cancer. Moreover, the mechanism by which the spices facilitate anti-carcinogenic effects is also fetching progressively apparent.
Syzygium aromaticum L. is commonly known as clove and is an evergreen tropical plant, which has been used for cooking and therapeutic properties throughout the world [Citation33]. Cloves are the dry flower buds of family Myrtaceae tree. Syzygium species are found to show antibacterial [Citation34], and anti-inflammatory activities [Citation35]. It has been described that the buds of Syzygium aromaticum were famous in traditional system of medicines for odontalgic, diuretic, tonicardiac, stomachic, aromat properties and carminative and stimulant activity [Citation36]. The successful role of Syzygium aromaticum by oral administration for asthma and various allergic disorders has been documented [Citation37]. It is also utilized as rubefacient, carminative, and served as preserving in herbal formulae; signifying likely antibacterial actions [Citation38]. The different species of Syzygium contain a wide range spectrum of important chemical compounds such as sesquiterpenes [Citation39], tannins [Citation40], triterpenoids [Citation41] and a phenolic compound called eugenol (4-allyl- 2-methoxyphenol). Eugenol seems to act as an antioxidant, carminative, antispasmodic, antiseptic and antimicrobial agent [Citation42,Citation43]; and also has antimutagenic property [Citation44]. In earlier studies, it was verified that oral admin of aqueous clove (100 μL/mouse/day for 21 weeks) by oral route, reduced the frequency of tumour growth by >50% in a mouse of benzo[a]pyrene (BP) initiated lung carcer and that the chemo-preventive effect of clove might be owing to prevention of expression of anti-apoptotic gene with that of Bcl-2, VEGFA and CD44 [Citation45]. It is worth mentioning that earlier research work also has shown that clove extracts were able to induce apoptosis through mitochondrial pathways in some cancer cell lines [Citation46]. Keeping these factors into consideration, the efforts are made to extract clove (Syzygium aromaticum) dried flower buds and used as anticancer agents.
2. Experimental
2.1. Materials and methods
All the solvents, reagents and chemicals used were of analytical grade and put to use without more purification in original form. The chemicals used were hexane, ethanol, methanol ethyl acetate and chloroform of HPLC grades. These were purchased from Merck, India.
2.2. Instruments used
The equipments used were pH metre, sonicator, centrifuge machine and weighing balance, water bath, biosafety cabinet class 2, laminar air flow, CO2 incubator, Elisa plate reader and microscope fitted with camera.
2.3. Preparation of clove bud powder
The fresh flower buds of E. caryophyllus were procured from the nearby market, Khariboale, New Delhi, India. These were carefully dried under shed for 10 days. After drying, these buds were crushed to fine small particles by means of a blender. The fine powder produced was stored in a desiccator at ambient temperature for the extraction purpose.
2.4. Extraction and fractionation of clove bud powder
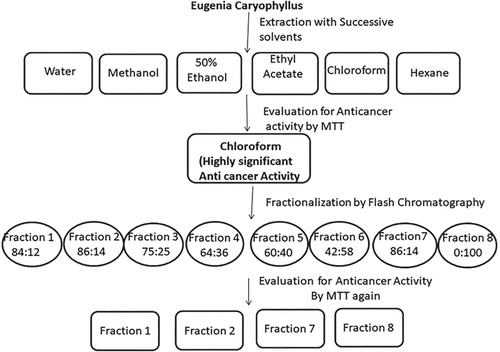
The extraction of clove was accomplished in the different successive solvents of different polarity by suturing separately 50 g clove fine powder in 200 mL of double-distilled water, methanol, 50% ethanol, ethyl acetate, chloroform and hexane in separate round bottom flasks and extracted in Soxhlet apparatus. This method of extraction was continued for many cycles and during each new cycle fresh clove powder and fresh solvents were used. The extraction protocol is shown in Figure . All the extracts were allowed to stand for 24 h before filtration with Whatman no. 1 filter paper. The filtrates were evaporated using a rotary vacuum evaporator. The solid residues obtained were used to make the final solution before being transferred to a water bath for further drying. The stock solutions of 100 mg/mL of each extract were prepared for all 6 extracts and evaluated for anticancer activity.
As already described in our previous work that after checking anticancer potential of the plant extracts, fractionation of these extracts was carried out by flash chromatography; to find out the number of components and their activities. The flash chromatography with binary and quaternary solvent delivery system, UV-Vis/Dual variable wavelength detector, Cheeta purification software (Bonna-Agela Technology, China) was used. Hexane-ethyl acetate of varied ratio was utilized eluent with a flow rate of 30 mL/minute. The column used was of silica (20 g, 40–60 µm). 500 mg Crude extract was mixed with silica to make it in the form of slurry and loaded into silica column and eluted with the above-mentioned solvent in increasing polarity ratio. The fractions (30 mL) were obtained with increase in polarity of mobile phase i.e. with the increased volume of ethyl acetate. The purity of each fraction was checked by TLC using MeOH-CHCl3 (1:9, v/v) as eluent. The spots were detected and identified in long UV cabinet. Besides, TLC plates were also identified using iodine chamber. The fractions having same Rf were pooled together to obtain the individual fraction. After fractionation, cytotoxic activity of each fraction was also checked.
2.5. Cell culture
Two lung cancer cell lines i.e. A549 and H1299 were put into use to carry out anticancer activity during this study. These cell lines were acquired from National centre for cell sciences, Pune. These were sustained in cell growth media, known DMEM media (Dulbecco’s Modified Eagle’s Medium). This cell culture media comprises also 10% FBS (Foetal Bovine serum) and 1% penicillin/streptomycin. The cells were cultivated and harvested in the incubator at 37 ± 0.5°C and 5% CO2 and 95% humidity, respectively. The cells were grown as adherent monolayer for use in the study.
2.6. Cell viability assay
The cell viability assay of the plant extracts was assessed by performing cell line studies by colorimetric assays (MTT). This assay is widely used for the assessment of cell viability and proliferation and cytotoxicity studies in cell biology. MTT assay provides a yellowish aqueous solution, that on reduction by mitochondrial succinate dehydrogenase; existing in active cells; gives a water insoluble violet-blue formazan crystal. The lipid soluble formazan product may be extracted with organic solvents (e.g. DMSO, iso-propanol) and assessed by ELISA reader in the range of 500–600 nm. Eight plant extracts were tried for their cytotoxic activities against two cell line i.e. A549 and H1299 at different concentrations using MTT assay according to the method of Mosmann. The anticancer activities of the plant’s extracts were performed with different concentrations. The cancer cell lines were planted in 96-well plates with 2 × 103 cells/well density. These were incubated at 37°C in humidified atmosphere with 5% CO2 for 24 h before analyse. The cells were incubated in media having different amounts of the extracts. After 24 h, the medium was removed and washed with Phosphate Buffered Saline (PBS). Approximately 20 μL of MTT solution was added to each well following 4 h incubation at 37°C. Later, the medium was removed following an addition of 200 μL DMSO. Later, gradually shaking (twice) for 5 s, absorbance of each well was estimated at 570 nm. The cell viability (%) was estimated as the ratio of the number of living cells with test compounds and blank.
2.7. Wound healing assay
In order to carry out the wound healing assay, two non-small cell lung cancer cell lines i.e. A549 and H1299 were seeded in a six-well plate separately and permitted to grow as monolayer till 100% confluence in the growth of the cells was achieved in each six-well plate containing these two cell types. Then a sterile 20–200 μL pipette tip was held upright to scratch a line in each well and in this way a wound was produced in the monolayer of these cell lines at the central in each of the six-well plates. The detached cells produced; due to the scratching; were detached by washing twice with 500 μL PBS and shaking at 500 rpm for 5 min. The two cell lines A459 and H1299 were cultured in 500 μL of fresh medium containing 5% FBS with or without fraction samples was added after and incubated for 24 h. The process of wound healing; due to the migration of A549 and H1299 cells into the created denuded zone; was supervised and visualized using a 40× magnification microscope. The plate was washed with 500 μL pre-warmed PBS and mildly shaken for 30 s before the image capturing then after addition of pre-warmed medium the pictures of the open wound were taken. The scratch closure was checked and imaged in 24 h intervals using a KeyenceBZ-9000 microscope (Keyence, Neu-Isenburg, Germany) at 40 × magnification and 1/3700 s contact time. The experiments were performed in triplicate to avoid bias.
2.8. Analysis of open wound area
The examination of the scratch generated open wound area images were accomplished using the Image J Version 1.31 software [Citation47,Citation48]. This software was used to calculate the scratch area (= open wound area) for each image. The open wound area percentage was graphed with time for each concentration. The data are described as mean ± SD. Three replicates were comprised in the analysis and an unpaired Student’s t-test was carried out.
2.9. Apoptosis analysis of A549 and H1299 cells by DAPI staining
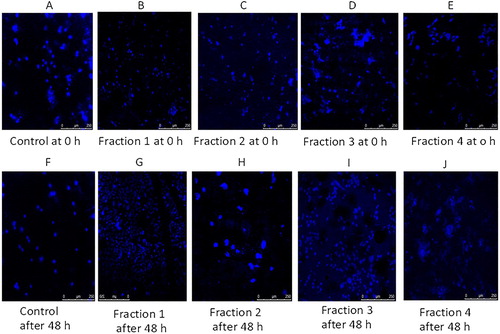
DAPI (4′, 6-diamidino-2-phenylindole dihydrochloride) staining was carried out to observe the morphology of the nuclei of cancer cell lines after treatment with clove bud fractions to compare it with nuclei of the cells in control. For this experiment, two cell lines i.e. A549 and H1299 were seeded into the 6-well plates. After reaching approximately 90% confluency in 6-well plates, the cells were treated with IC50 concentrations of dissimilar fractions and were incubated for 48 h. The cells were seen after 48 h to see changes in morphology in cells suffering from death. The cells were eroded twice with 1× PBS and formaldehyde (0.01%) was added and mixed mildly for 1 h. Later, the cells were again eroded twice with PBS and DAPI was added and put for 10 min in dark. Lastly, the cells were eroded twice with PBS and suspended in 500 μL of fresh PBS and the nuclei morphology was seen. The morphology of the cells was imagined and photographed by DMR fluorescence microscope at ×400 magnification (Leica Microsystems GmbH, Wetzlar, Germany) utilizing fluorescence excitation at 340 nm [Fluorescence microscopy (Olympus 1 × 81 Inverted Research Microscope)].
2.10. Flow cytometric analysis of cell apoptosis
During the study, cell death the rate was assessed by apoptosis or necrosis by treating H1299 cells with one time IC50 concentrations of the fractions of clove and then stained with both propidium iodide (PI) and annexin V-fluorescein isothiocyanate (FITC) using an Annexin V-FITC apoptosis detection kit (cat. no. 559763BD Pharmingen; BD Biosciences USA) in accordance with the manufacturer’s instructor. Further analysis was carried out by flow cytometry. The brief procedure followed during this experiment include that H1299 cells at 80–90% confluence were seeded in 6-well plates at a concentration/density of 4 × 105 cells/well. These cells were cultured for 24 h in complete media. The cells were treated with IC50 concentrations of four fractions of clove or vehicle control for 48 h. After complete treatment, the cells were reaped by cryogenic centrifugation at 4°C, 1,500 × g for 5 min and washed twice with 4°C PBS. The concentration of 1 × 106 cells/mL of H1299 cell line was used to re-suspended in 1X binding buffer. A total of 100 μL of the solution containing (1 × 105 cells) was shifted to a 5-mL culture tube. This was following by successive adding of 5 μL annexin V-FITC and 5 μL PI to the cells in culture tube and incubated at room temperature in dark for 15 min. The amount of stained cells apoptosis or necrosis was assessed utilizing a flow cytometer (FACS Calibur; BD Biosciences, Franklin Lakes, NJ, USA).
3. Results and discussion
3.1. Cell viability assay
In our previous experiment the cell viability was estimated by MTT assay as described earlier [Citation49]. Briefly, during our previous experiment, we calculated IC50 values of six clove extracts. These values confirmed that chloroform extract had more activity (IC50 = 118.0 μg/mL) in comparison to the other extracts. Therefore, during our previous experiment, this chloroform extract was selected for further studies by subjecting it to fractionalization on flash chromatography. The fractions obtained by flash chromatography were 8 in number. These fractions were further tested for their anticancer activities against two cell lines i.e. A549 and H1299. It was found that the IC50 values for all these fractions ranged from 142.5 to 2879.8 mg. Furthermore, only four fractions out of eight exhibited significant dose-related cytotoxicity against two cell lines A549 and H1299. These fractions were called as active ones and were 1, 2, 7 and 8, respectively. Therefore, these four fractions were further evaluated for cell migration assay and apoptotic studies.
3.2. Wound healing assay
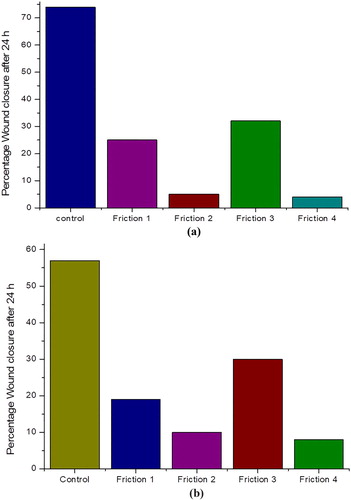
The wound healing method was carried out to ascertain and determine the interference in cell migration by fractions of clove. The migration of the cells in the mechanically generated scratch open wound was observed in the presence or absence of clove fractions. The images of mechanical scratch areas wound were taken at different time intervals, which include from the time points 0 h and after 24 h. These images are illustrated in Figure (a,b). The representative control is shown at Figure (a,b) at 0 and 24 h time point, respectively; showing that the scratch was partially locked within 24 h. The cell migration inhibition was more pronounced in H1299 cancer cell line. The effects of all the four fractions of clove on wound migration of these two cell lines were quantified and compared with control by determining total the open wound area after 24 h (Table ). The open wound area percentage after 24 h was also determined. Our experimental data evidently showed that treatment with fractions of clove; obtained by flash chromatography; produced a substantial inhibition of cell migration in IC50 value concentration. Compared with other fractions, the fraction 4, 2 and 1 repressed cell migration even stronger; and considerably checked the scratch closure. The migration of the cells was also impaired prominently by these fractions at applied concentration of IC50 value. The cell migration inhibition profile was more predominant in H1299 cell line by fraction 4 and fraction 1. All the four fractions can be arranged for percentage of wound closure in decreasing order as 4 > 2 > 1 > 3.
Figure 2. Inhibition of cell migration by cytotoxic fractions of Syzygium aromaticum on (a) H1299 and(b) A549 cell lines.

Table 1. IC50 value of chloroform fractions with A549 and H1299 cell lines.
3.3. Analysis of open wound area
The effects of all the four fractions of clove on wound migration of these two cell lines were quantified and compared with control by determining total open wound area at 0 h and after 24 h (Table ). The open wound area percentage after 24 h was also determined. Our experimental data evidently showed that treatment with fractions of clove obtained on flash chromatography produced a substantial inhibition of cell migration in IC50 value concentration as compared to control (70%). Compared with other fractions, the fraction 4, 2 and 1 eroded suppressed cell migration stronger and considerably checked the scratch closure. A close look at percentage closure graph (Figure (a,b)) reveals that cell migration inhibition is more prominent in fraction 4 (2%) followed by fraction 3 (10%) and fraction 1 (20%). The migration of cells was also impaired prominently by these fractions at an applied concentration of IC50 value. The cell migration inhibition profile was more predominant in H1299 cell line by fraction 4 and fraction 1. All the four fractions can be arranged for percentage of wound closure in increasing order as 4 > 2 > 1 > 3.
Table 2. Open scratch area of H1299 and A549 cells treated with four fractions of clove obtained by flash Chromatography for elucidation of cell migration inhibition.
3.4. Apoptosis analysis of A549 and H1299 cells by DAPI staining
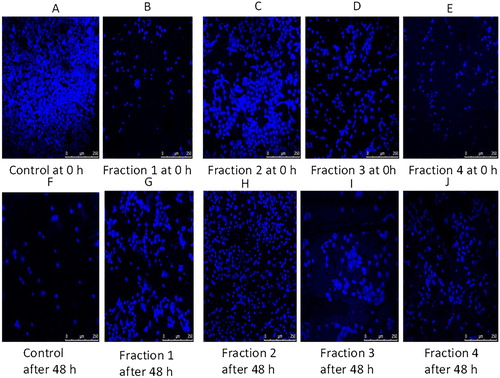
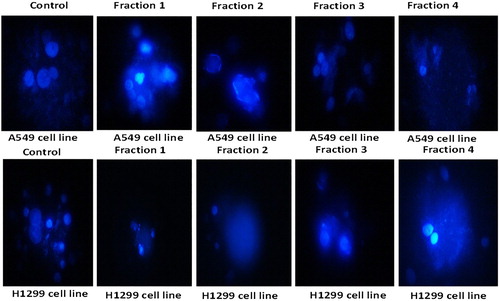
Apoptotic cell death is one of the mechanisms by which cell growth is checked. The DAPI staining is a fluorescent nuclear dye, which binds strongly to DNA. This DAPI dye is not completely permeable. Once it overpasses the cell membrane of normal cells, the blue Florence is observed by fluorescent microscope. DAPI staining is in practice to illustrate the consequence of phytochemical and plant extract incited apoptosis in cancer cells. DAPI is a fluorescent stain that is meant to focus on the nuclear morphorgical alterations taking place during apoptosis and also to evaluate the proportion of apoptotic cells with condensed and fragmented chromatin [Citation50]. The range of nuclear variations taking place throughout apoptosis in cancer cells, as measured by DAPI staining in the control and treated groups, are presented in Figures and . It is clear from the results of fluorescent microscope photographs that the fractions of clove exhibited apoptosis in H1299 cells as demonstrated by their augmented permeability to DAPI; with the attendance of nuclear apoptotic bodies and chromatin condensation. The apoptosis initiation by an ethyl acetate extract of the stem bark of Cudrania tricuspidata in HL-60 human leukaemia cells was established by DAPI staining [Citation51]. The results of our experiment are also in complete promise with literature, where during DAPI staining the morphological variations are linked to designated cellular apoptosis. The results showed good correlation with cell morphology study, which confirmed the efficiency of fractions of clove in inducing apoptosis. As illustrated in Figure , the cells showed characteristic apoptotic morphological structures, counting chromatin condensation, nuclear shrinkage and the formation of apoptotic bodies with the treatment of four clove fractions with their IC50 concentration for 48 h. Apoptosis is an important anticancer mechanism and numerous anticancer drugs executed their anticancer activities via apoptosis induction [Citation52].
Figure 4. Morphological features of nuclei of H1299 cells treated with four fractions of Syzygium aromaticum for 8 h.
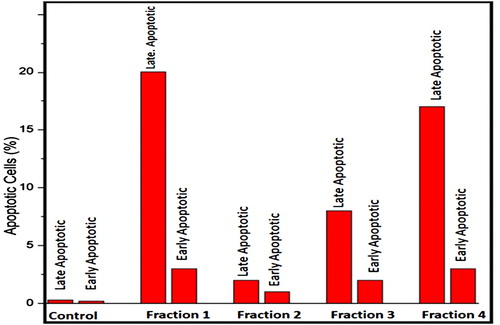
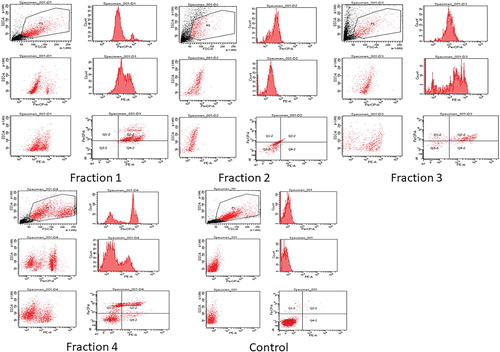
3.5. Flow cytometric analysis of cell apoptosis
Apoptosis (programmed cell death) is an extremely controlled procedure and serves as a crucial role in cancer treatment [Citation53–55]. Annexin V is a sensitive method for finding apoptosis of cancer cells by utilizing fluorescein (FITC) as fluorescent probe [Citation56]. To conclude whether the anti-proliferation of the fractions of clove are involved in the initiation of apoptosis, H1299 cells were treated with the above four clove fractions, and then stained with both propidium iodide (PI) and Annexin V-fluorescein isothiocyanate (FITC), and investigated by flow cytometry. The investigation of flow cytometric outcomes exhibited that the percentages of apoptotic cells treated with clove fraction 1 and fraction 4 were 23% and 20% (Figure ), respectively. With respect to other two fractions, it can be observed that no significant process of apoptosis had taken place when H1299 cells were treated with them and the percentages of apoptotic cells treated with these clove fractions including fraction 2 and fraction 3 were 3% and 10% (Figure ), respectively. This indicated that the two fractions 1 and fraction 4 prompted cell death through early and late apoptosis. Apoptosis is categorized by discrete morphological structures with the chromatic condensation, cell and nuclear shrinkage, membrane blabbing and oligonucleosomal DNA fragmentation. Consequently, the apoptosis rate of H1299 cells was examined by flow cytometric analysis using Annexin V-FITC/PI double staining. It was concluded that the anti-proliferative action of two clove fractions was owing to the initiation of apoptosis as shown by the annexin-V flow cytometric method. The outcomes showed that the apoptotic cells’ percentage increased when H1299 cells were treated with fractions of clove as compared with control.
Figure 8. Analysis of apoptotsis by flow cytometry induced in H1299 cells, including control and treated cells.
The statistical analysis is carried out by repeating experiments three times (n = 3). The values of the standard deviation, correlation coefficients and confidence levels were in the ranges of 0.80–0.90, 0.9992–0.9995 and 94.4–96.1, respectively, which indicated good efficiency and precision of the experimental results. The considerably extraordinary presence of phenolic and flavonoids in the clove flower buds might be accountable for the anti-proliferation properties on A549 and H1299 cell lines. The results of our study are in agreement with the earlier work of other researchers [Citation57–59] that have revealed that Terminalia species are rich in the hydroxy group and, hence, understood to be accountable for the anti-proliferation activity. The phenolic compounds found in these plants including flavonoids, gallic acid, caffeic acid and derivatives synthesized from them are known to exhibit diverse pharmacological activities like free radical scavenging, antioxidant, pro-oxidant toxicity and apoptosis [Citation60]. In this study eugenol is the main ingredient responsible for anticancer activities [Citation49,Citation61].
4. Conclusion
Keeping under consideration the low toxicity and anti-proliferative efficiency of naturally occurring medicinal plants, the studied fractions of clove might be a bright prospective for development as a fresh anticancer drug. Our data may offer scientific justification for refining the current thoughtful of the anticancer mechanism of clove fractions and to further develop these fractions as a favourable novel anticancer agent to be used alone or in combination with other chemotherapeutic drugs for the treatment of lung cancer. This study also advocates that the fractions of clove may be employed in amalgamation with conventional chemotherapeutic agents to attain enhanced favourable results in the treatment of lung cancer. In the end, we conclude that the data of our experiments indicated that two fractions (1 and 4) of clove were able to induce typical apoptosis in human non-small cell lung cancer cell line H1299. A number of points should be deliberated in future studies. These results confirmed that the chloroform extract of clove bud may be used to treat lung cancer.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Imran Ali http://orcid.org/0000-0001-6511-8374
Mohd Farooq Naqshbandi http://orcid.org/0000-0001-6264-0669
Mohammad Husain http://orcid.org/0000-0003-3876-8181
References
- Ali I, Haque A, Saleem K, et al. Curcumin-I Knoevenagel’s condensates and their Schiff’s bases as anticancer agents: synthesis, pharmacological and simulation studies. Bioorg Med Chem. 2013;21:3808–3820. doi: 10.1016/j.bmc.2013.04.018
- Aboul-Enein HY, Ali I. Determination of tadalafil in pharmaceutical preparation by HPLC using monolithic silica column. Talanta. 2004;65:276–228. doi: 10.1016/j.talanta.2004.06.012
- Ali I, Gaitonde VD, Aboul-Enein HY, et al. Chiral separation of β-adrenergic blockers on cellucoat column by HPLC. Talanta. 2009;78:458–463. doi: 10.1016/j.talanta.2008.11.043
- Ali I, Saleem K, Hussain I, et al. Polysaccharides chiral stationary phases in liquid chromatography. Sep Purif Rev. 2009;38:97–147. doi: 10.1080/15422110802589916
- Ali I, Gupta VK, Aboul-Enein HY, et al. Role of racemization in optically active drug development. Chirality. 2007;19:453–463. doi: 10.1002/chir.20397
- Ali I, Lone MN, Al-Othman ZA, et al. Heterocyclic scaffolds: centrality in anticancer drug development. Curr Drug Target. 2015;16:711–734. doi: 10.2174/1389450116666150309115922
- Ali I, Sanagi MM, Aboul-Enein HY. Advances in chiral separations by non-aqueous capillary electrophoresis in pharmaceutical and biomedical analysis. Electrophoresis. 2014;35:926–936. doi: 10.1002/elps.201300222
- Ali I, Aboul-Enein HY. Impact of immobilized polysaccharide chiral stationary phases on enantiomeric separations. J Sep Sci. 2006;29:762–769. doi: 10.1002/jssc.200500372
- Aboul-Enein HY, Ali I. A comparative study of the enantiomeric resolution of econazole, miconazole and sulconazole by HPLC on various cellulose chiral columns in normal phase mode. J Pharm Biomed Anal. 2002;27:441–446. doi: 10.1016/S0731-7085(01)00575-1
- Ali I, Aboul-Enein HY. Enantioseparation of some clinically used drugs by HPLC using cellulose tris-(3,5-dichlorophenylcarbamate) chiral stationary phase. Biomed Chromatogr. 2003;17:113–117. doi: 10.1002/bmc.220
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Can J Clin. 2018;68:394–424. doi: 10.3322/caac.21492
- Alharbi OML, Basheer AA, Khattab RA, et al. Health and environmental effects of persistent organic pollutants. J Mol Liq. 2018;263:442–453. doi: 10.1016/j.molliq.2018.05.029
- Basheer AA. Chemical chiral pollution: impact on the society and science, and need of the regulations in the 21st century. Chiralty. 2018;30:402–406. doi: 10.1002/chir.22808
- Ali I. Nano anti-cancer drugs: pros and cons and future perspectives. Cur Can Drug Targ. 2011;11:131–134. doi: 10.2174/156800911794328457
- Aboul-Enein HY, Ali I. Studies on the effect of alcohols on the chiral discrimination mechanisms of amylose stationary phase on the enantioseparation of nebivolol by HPLC. J Biochem Biophys Meth. 2001;48:175–188. doi: 10.1016/S0165-022X(01)00148-8
- Aboul-Enein HY, Ali I. Optimization strategies for HPLC enantioseparation of racemic drugs using polysaccharides and macrocyclic glycopeptide antibiotic chiral stationary phases. IL Farmaco. 2002;57:513–529. doi: 10.1016/S0014-827X(02)01242-9
- Ali I, Naim L, Ghanem A, et al. Chiral separations of piperidine-2,6-dione analogues on Chiralpak IA and Chiralpak IB columns by using HPLC. Talanta. 2006;69:1013–1017. doi: 10.1016/j.talanta.2005.12.004
- Aboul-Enein HY, Ali I. HPLC Enantiomeric resolution of nebivolol on normal and reversed amylose based chiral phases. Pharmazie. 2001;56:214–216.
- Ali I, Aboul-Enein HY, Ghanem A. Enantioselective toxicities and carcinogenesis. Cur Pharmaceut Anal. 2005;1:109–125. doi: 10.2174/1573412052953328
- Al-Othman ZA, Al-Warthan A, Ali I. Advances in enantiomeric resolution on chiral monolithic phases in liquid chromatography and electrochromatography. J Sepn Sci. 2014;37:1033–1057. doi: 10.1002/jssc.201301326
- Ali I, Saleem K, Wesselinova D, et al. Synthesis, DNA binding, hemolytic and anticancer assays of curcumin I based ligands and their ruthenium (III) complexes. Med Chem Res. 2013;22:1386–1398. doi: 10.1007/s00044-012-0133-8
- Ali I, Rahis-ud-din, Saleem K, et al. Social aspects of cancer genesis. Can Ther. 2011;8:6–14.
- Aboul-Enein HY, Ali I, Simons C, et al. Enantiomeric resolution of the novel aromatase inhibitors by HPLC on cellulose and amylose based reversed and chiral stationary phases. Chirality. 2000;12:727–733. doi: 10.1002/1520-636X(2000)12:10<727::AID-CHIR5>3.0.CO;2-T
- Erenpreisa J, Cragg M. Cancer: a matter of life cycle? Cell Biol Int. 2007;31:1507–1510. doi: 10.1016/j.cellbi.2007.08.013
- Ajani JA. Evolving chemotherapy for advanced gastric cancer. Oncologist. 2005;10:49–58. doi: 10.1634/theoncologist.10-90003-49
- Ali I, AL-Othman ZA, Hussain A, et al. Chiral separation of β-adrenergic blockers in human plasma by SPE-HPLC. Chromatographia. 2011;73:251–256. doi: 10.1007/s10337-010-1891-4
- Ali I, Singh P, Aboul-Enein HY, et al. Chiral analysis of ibuprofen residues in water and sediment. Anal Lett. 2009;42:1747–1760. doi: 10.1080/00032710903060768
- Aboul-Enein HY, Ali I. A comparison of chiral resolution of econazole, miconazole and sulconazole by HPLC using normal phase amylose CSPs. Fresenius J Anal Chem. 2001;370:951–955. doi: 10.1007/s002160100884
- Basheer AA, Ali I. Stereoselective uptake and degradation of (±)-o,p-DDD pesticide stereomers in water-sediment system. Chirality. 2018;30:1088–1095. doi: 10.1002/chir.22989
- Ali I, Waseem A, Wani A, et al. Synthesis and synergistic antifungal activities of a pyrazoline based ligand and its Copper (II) and Nickel (II) complexes with conventional antifungals. Microb Pathogen. 2012;53:66–73. doi: 10.1016/j.micpath.2012.04.005
- Ali I, Wani WA, Khan A, et al. Thalidomide: a banned drug resurged into future anticancer drug. Cur Drug Ther. 2012;7:13–23. doi: 10.2174/157488512800389164
- Ali I, Wani WA, Saleem K, et al. Syntheses, DNA binding and anticancer profiles of L-Glutamic acid ligand and its copper(II) and ruthenium(III) complexes. Med Chem. 2013;9:11–21. doi: 10.2174/157340613804488297
- Ahmad T, Latif S, Qasmi A. Effect of 50% ethanolic extract of Syzygium aromaticum (L.) Merr. & Perry. (clove) on sexual behaviour of normal male rats. BMC Complement Altern Med. 2004;5:4–17.
- Letelier ME, Molina-Berríos A, Cortés-Troncoso J, et al. DPPH and oxygen free radicals as pro-oxidant of biomolecules. Toxicol In Vitro. 2008;22:279–286. doi: 10.1016/j.tiv.2007.08.002
- Boulos L. Medicinal plants of North Africa. Algonac: Reference Publications; 1983, 110.
- Odugbemi TO. Outlines and pictures of medicinal plants from Nigeria. Lagos: University of Lagos Press; 2006.
- Skehan P, Storeng R, Scudiero D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Can Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107
- Joyeux M, Lobstein A, Anton R, et al. Comparative antilipoperoxidant, antinecrotic and scavenging properties of terpenes and biflavones from Ginkgo and some flavonoids. Plant Med. 1995;61:126–129. doi: 10.1055/s-2006-958030
- Zheng GQ, Kenney PM, Lam LK. Sesquiterpenes from clove (Eugenia caryophyllata) as potential anticarcinogenic agents. J Nat Prod. 1992;55:999–1003. doi: 10.1021/np50085a029
- Tanaka T, Orii Y, Nonaka G, et al. Tannins and related compounds. CXXIII. Chromone, acetophenone and phenylpropanoid glycosides and their galloyl and/or hexahydroxydiphenoyl esters from the leaves of Syzygium aromaticum MERR. Et PERRY. Chem Pharmaceut Bull. 1993;41:1232–1237. doi: 10.1248/cpb.41.1232
- Umehara K, Takagi R, Kuroyanagi M, et al. Studies on differentiation-inducing activities of triterpenes. Chem Pharmaceut Bull. 1992;40:401–405. doi: 10.1248/cpb.40.401
- Farag R, Badei A, El Baroty G. Influence of thyme and clove essential oils on cottonseed oil oxidation. J Am Oil Chem Soc. 1989;66:800–804. doi: 10.1007/BF02653671
- Farag R, Badei A, Hewedi F, et al. Antioxidant activity of some spice essential oils on linoleic acid oxidation in aqueous media. J Am Oil Chem Soci. 1989;66:792–799. doi: 10.1007/BF02653670
- Miyazawa M, Hisama M. Antimutagenic activity of phenylpropanoids from clove (Syzygium aromaticum). J Agric Food Chem. 2003;51:6413–6422. doi: 10.1021/jf030247q
- Banerjee S, Panda CK, Das S. Clove (Syzygium aromaticum L.), a potential chemopreventive agent for lung cancer. Carcinogenesis. 2006;27:1645–1654. doi: 10.1093/carcin/bgi372
- Liu H, Schmitz JC, Wei J, et al. Clove extract inhibits tumor growth and promotes cell cycle arrest and apoptosis. Oncol Res. 2014;21:247–259. doi: 10.3727/096504014X13946388748910
- Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Meth. 2012;9:671–675. doi: 10.1038/nmeth.2089
- Collins TJ. ImageJ for microscopy. Bio Tech. July 2007;43(1 Suppl):25–30.
- Ali I, Naqshabndi MF, Husain M. In vitro anticancer, antioxidant and DNA binding studies of Eugenia caryophyllus plant bud extracts. Paper submitted for publication, Lett. Drug Design & Discov. 2019, forthcoming.
- Choi BY, Kim HY, Lee KH, et al. Clofilium, a potassium channel blocker, induces apoptosis of human promyelocytic leukemia (HL-60)cells via Bcl-2 insensitive activation of caspase-3. Can Lett. 1999;147:85–93. doi: 10.1016/S0304-3835(99)00280-3
- Seo WG, Pae HO, Oh GS, et al. Ethyl acetate extract of the stem bark of Cudrania tricuspidata induces apoptosis in human leukemia HL-60 cells. Am J Chin Med. 2001;29:313–320. doi: 10.1142/S0192415X01000332
- Pistritto G, Trisciuoglio D, Ceci C, et al. Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging (Albany NY). 2016;8:603–619. doi: 10.18632/aging.100934
- Ameisen JC. On the origin, evolution, and nature of programmed cell death: a timeline of four billion years. Cell Death Differ. 2002;9:367–393. doi: 10.1038/sj.cdd.4400950
- Gerl R, Vaux DL. Apoptosis in the development and treatment of cancer. Carcinogenesis. 2004;26:263–270. doi: 10.1093/carcin/bgh283
- Elmore S. Apoptosis. A review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337
- Wlodkowic D, Telford W, Skommer J, et al. Apoptosis and beyond: cytometry in studies of programmed celldeath. Meth Cell Biol. 2011;103:55–98. doi: 10.1016/B978-0-12-385493-3.00004-8
- Rice-Evans C, Miller N, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–159. doi: 10.1016/S1360-1385(97)01018-2
- Saleem A, Husheem M, Härkönen P, et al. Inhibition of cancer cell growth by crude extract and the phenolics of Terminalia chebula retz. fruit. J Ethnopharmacol. 2002;81:327–336. doi: 10.1016/S0378-8741(02)00099-5
- Ali I, Wani WA, Saleem K, et al. Design and synthesis of thalidomide based dithiocarbamate Cu(II), Ni(II) and Ru(III) complexes as anticancer agents. Polyhedron. 2013;56:134–143. doi: 10.1016/j.poly.2013.03.056
- Miliauskas G, Venskutonis P, Van Beek T. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004;85:231–237. doi: 10.1016/j.foodchem.2003.05.007
- Kähkönen MP, Hopia AI, Vuorela HJ, et al. Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem. 1999;47:3954–3962. doi: 10.1021/jf990146l