?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The performance of oil well cement (OWC) incorporating pozzolana under diverse hydrothermal curing circumstances was inspected. OWC was partially replaced by 5 weight% of silica fume (SF) or fly ash (FA). The prepared composites were cured under different hydrothermal circumstances (60°C/0.02 MPa, 100°C/0.1 MPa and 150°C/0.48 MPa) for 7 days. The outcomes established that increasing curing temperature and pressure brought about a decline in the compressive strength magnitudes of various neat OWC pastes. This finding is correlated to the metamorphosis of CSH to the greatly crystalline phase of α-dicalcium silicate hydrate (α-C2SH). Such transformation motivates the destruction of the pore structure and promotes compressive strength decline. Admixing OWC with 5 wt.% SF or FA displayed enrichment in the mechanical aspects accompanied by the progression of highly compact microstructures. Additionally, the presence of SF or FA motivates the advancement of stable and desirable hydration products. DTG/TG, XRD and SEM analyses affirm these results.
1. Introduction
Many challenges and technical problems are highly remarkable for gas and oil industry especially in recent years [Citation1]. One of the most important challenges that faces the extraction of oil from oil wells is the large cost of the drilling process. Many attempts and investigations have been achieved in order to optimize the well cost.
Oil well cement (OWC) slurries are usually exposed to severe circumstances of pressure and temperature especially increasing the depth of the wellbore [Citation2] close to those arising under hydrothermal curing systems [Citation3–5]. G-class cement has been accepted in the oil well industry, especially for geothermal wells. It was identified by the American Petroleum Institute [Citation6]. G-class cement provided satisfactory performance at severe circumstances of pressure and temperature in geothermal wells [Citation7].
The consequence of severe curing circumstances of temperatures (150 and 200°C) and corresponding pressures (0.3 and1.2 MPa) on the hydration of OWC (G-class) has been investigated. It has been terminated that at 150°C, the major hydration product (CSH) experiences metamorphosis to the greatly crystalline phase of α-dicalcium silicate hydrate (α-C2SH) whereas at 200°C calcium silicate hydrate (CSH) experiences transformation into jaffeite (C6S2H3). Such transformation covered by the effect of high temperatures and pressures motivates undesirable characteristics as pronounced compressive strength decline, permeability development and pore structure reduction [Citation2].
It has been disclosed that CSH gel has an average value of the C/S ratio equals to 1.5 [Citation8]. And so, the key role to hinder the unsatisfactory phase transformation (amorphous CSH into crystalline α-C2SH or jaffeite) was through diminishing CaO/SiO2 (C/S) ratio to about 1 [Citation9–11]. Many researches proved that various supplements as SF [Citation12], fly ash [Citation13], silica flour [Citation14] and other silica-based ingredients [Citation15–24] motivate the advancement of stable and desirable hydration products that possess lower C/S ratio (like tobermorite) through calcium hydroxide (CH) consumption.
Many researches have been conducted regarding the utilization of low replacement applications (5–20 wt.%) of MK [Citation25–28]. It has been concluded that 15 wt.% nano-MK replacement at 250°C–800°C gives improved strength. On the other hand, the features of OWC incorporating high quantity of metakaolin (MK) have been inspected [Citation29]. Compressive strength, X-ray diffraction (XRD) and scanning electron microscopy (SEM) were investigated. Test results affirmed that specimens with 40–60 wt.% metakaolin exhibited improved strength at higher temperatures (equal to or higher than 150°C). The improved characteristics are attributed to the stable hydration products as well as the dense microstructure.
It has been reported that the advancement of calcium aluminosilicate hydrate (CASH) as a hydration product brings about more stable OWC covered by severe circumstances of pressures and temperatures. [Citation30]. Inclusion of silica fume (SF) and blast-furnace slag (BFS) has been found to boost the mechanical aspects of OWC (G-class) at elevated temperature (150°C) through the advancement of gehlenite hydrate phase (C2ASH8) [Citation31,Citation32].
OWC (G-class) was partially replaced by SF together with ground granulated BFS, and the diverse characteristics covered by hydrothermal circumstances (200°C, 1.2 MPa) have been analysed. Structure deficiency owing to (CSH – α-C2SH) and/or (CSH – C6S2H3) transformations was prevented via the application of higher than 20 wt.% substituents [Citation33]. It has been stated that curing at (165°C, 0.6 MPa) promotes the evolution of α-C2SH, whereas jaffeite phase was advanced at 220°C, 2.0 MPa. In other words, (CSH – α-C2SH) transformation occurs initially and subsequently α-C2SH is transformed into jaffeite.
Another investigation has been conducted to evaluate the partial substitution of Dyckerhoff cement with 15 wt.% of BFS, MK and SF or their combination. The phase composition and mechanical characteristics were inspected up to 28 days of hydrothermal curing at 165°C and 0.6 MPa. It was reported that depletion in compressive strength could be correlated to a large amount of α-C2SH phase formed under the specified conditions. It has been established that the combination of MK with silica flour brings about the advancement of highly stable hydration products with desirable hydraulic characters [Citation34].
Partial substitution of OWC (class G) with 5 and 10 wt.% of zeolite and fly ash has been studied, and the outcomes revealed that the application of fly ash enhanced the compressive strength of the investigated specimens [Citation35].
Other investigations studied the consequence of the substitution of OWC by fly ash to diminish the effects of cement thermal regression at elevated temperature [Citation36–39].
The current investigation attempts to establish a better comprehension of the conjugated consequences of pressure and temperature on the hydration of OWC and OWC pastes admixed with SF or fly ash at early ages. Additionally, the major target of this investigation is to achieve an improved reduction for the effect of aggressive circumstances of pressure and temperature on the mechanical aspects and microstructure of hardened OWC pastes (for application in oil-wells) via utilizing locally available raw materials (FA and SF).
2. Materials and experimental routines
2.1. Materials
High sulphate resistance OWC (class G), density of 3.15 g.cm−3, utilized in this investigation was afforded from Lafarge cement group, Egypt. The chemical analysis of the OWC in addition to its mineralogical constitution is tabulated in Table .
Table 1. Chemical oxide mineralogical phase composition of OWC.
SF, a side product of ferrosilicon alloys productions, was collected from the ferrosilicon group, Egypt 99 wt.% of SF is amorphous ball-shaped silica particles with an average radius of about 0.05 µm and a surface area of 20 × 104 cm2g−1. The-mentioned features of SF elucidate its great pozzolanic reactivity.
Low-calcium fly ash (FA), classified by ASTM C 618 as class F, used in this investigation is a product of the combustion of anthracite and bituminous coals. Its chemical analysis is given in Table .
Table 2. Major oxides composition of fly ash (mass%).
2.2. Experimental routines
2.2.1. Fabrication of the hardened cement mixes
Diverse dry mixes were made from OWC-SF and OWC-FA composites via partial substitution of OWC by 5 wt.% SF or % 5 wt.% FA, respectively. The percentage composition of the diverse OWC composites, as well as their designations, is displayed in Table . Mechanical mixing of all dry mixes was achieved utilizing a porcelain ball mill for 10 h to attain uniform dry mixes. The plasters were fabricated by blending each dry composition with a convenient amount of water (W/S ratio = 0.30). Then, each plaster was moulded using one-inch cube moulds into cubic samples and preserved overnight under a relative humidity of about 100% to reach the final setting. The cubic samples then removed from the moulds and introduced to the autoclave to be treated under diverse hydrothermal circumstances (60°C/0.02 MPa, 100°C/0.1 MPa and 150°C/0.48 MPa) for the periods of 1, 3 and 7 days. The autoclave utilized in this investigation was 2L stainless steel autoclave with a maximum operating steam pressure of 30 bar (maximum temperature, ∼235°C).
Table 3. The compositions of the different mixes and their designation.
2.2.2. Techniques
Compressive strength investigation was evaluated for the hardened pastes adopting three cubic samples at each hydration age and the average value was reported. Ton industrie-machine (West Germany, 60 tons of maximum capacity) was applied in order to accomplish the compressive strength measurements. The hydration reaction for the resultant crushed samples, after grinding, was ceased applying the regime reported in the previous research [Citation40]. The samples were left to dry for about 3 h at 90°C and left in a desiccator till reaching the interval of investigation.
Phase configuration and micro-arrangement of the developed products for specific specimens were inspected adopting XRD, differential thermogravimetric analysis (DTGA/TG) in addition to a scanning electron microscope (SEM).
3. Results and discussion
3.1. Compressive strength
The compressive strength progress is greatly relying on the rate and degree of cement hydration. Figure (a–c) exhibits the progress of the compressive strength values with the curing ages of OWC, OWC + 5 wt.%SF and OWC + 5 wt.% FA mixes cured hydrothermally at 60°C/0.02 MPa, 100°C/0.1 MPa and 150°C/0.48 MPa, respectively.
Figure 1. Compressive strength of hardened OWC pastes hydrothermally cured at (a) 60°C/0.02 MPa, (b) 100°C/0.1 MPa and (c) 150°C/0.48 MPa.
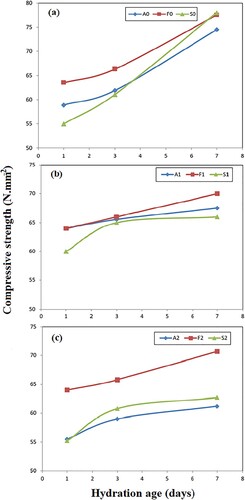
Hydrothermal curing of neat OWC pastes (Mix A0) at 0.02 MPa/60°C (Figure a) motivates a steady rise of the compressive strength magnitudes among the first 3 days of the hydrothermal reaction. Obviously, a faster rate of strength development is noted with increasing the progress of curing times (up to 7 days). This finding could be correlated to the advancement of the hydration process bringing about precipitation followed by subsequent agglomeration of the hydration yields, principally as amorphous and ill-crystalline calcium silicate hydrates (CSH), inside the pores of the hardened paste. These yields act for binding points among the unreacted particles. This action participates strongly in the compressive strength enhancement.
Mix F0, enclosing 5 wt.% fly ash as a partial substituent of OWC, exhibits a similar trend of compressive strength variation resembles that of Mix A0, but with relatively higher values at all hydration ages. The compressive strength enhancement for this mix was about 7.8, 8.0 and 4.1% relative to the values recorded for Mix A0 after 1, 3 and 7 days of hydrothermal curing, respectively. The accessed result could be correlated to the pozzolanic action of fly ash which reacts with Ca(OH)2 detached from OWC hydration, resulting in the creation of successive and secondary hydration products, mainly as calcium silicate hydrates (CSHs) in addition to calcium aluminate hydrate (CAH) and calcium aluminate silicate hydrate (CASH). These extra hydration products increase the overall contents of the binding centers in the investigated specimens as well as they fill and accumulate in the system pores, and so, observable enlargement in the compressive strength magnitudes could be achieved.
On the other hand, replacement of 5 wt.% OWC with SF, Mix S0, brings about a slight reduction in the compressive strength after 1 day of hydration. This reduction could be explained by the retardation effect of C3S hydration at early age of hydration (1 day) in the presence of SF and/or the slower speed of the pozzolanic reaction between SF and detached lime Ca(OH)2 from cement hydration [Citation32]. Such a decline is nearly recovered after 3 days of the hydrothermal reaction. However, there is late enhancement in compressive strength values by about 5% recorded for Mix S0 relative to that of control Mix (A0) after 7 days. The late enhancement in the mechanical properties observed after 7 days is related to the formation of additional amounts of CSH [Citation41].
Hydrothermal curing of neat OWC at 0.1 MPa/100°C (Mix A1) leads to compressive strength enhancement after 1 and 3 days of hydration relative to Mix A0 (Figure b). This could be related to the greater rate of the hydration reaction owing to hydrothermal curing at 0.1 MPa/100°C. However, at later ages of hydrothermal curing (7 days), a decline in the compressive strength magnitudes is recorded for Mix A1 relative to Mix A0. This observation could be explained by the enlargement in the crystallinity extent of the developed calcium silicates hydrates (CSH) due to hydrothermal curing at 0.1 MPa/100°C. Evidently, the highly crystalline CSH hydrates acquire weak hydraulic features with relatively depressed compressive strength magnitudes [Citation37,Citation42].
Covered by the hydrothermal curing conditions of 0.1 MPa/100°C, partial replacement of OWC by 5 wt.% fly ash (Mix F1) still offers a better strength notably at later hydrothermal stages in comparison with the control, Mix A1. This finding is related to the development of CSH as well as CASH as secondary hydration products bringing about more stable OWC under severe conditions of pressures and temperatures. In other words, the most important role of substitution cement by FA (class F) is to decrease the amount of Ca in the paste and at the same time aids in the development of CASH or CSH gels through the pozzolanic reaction of FA glassy phase with portlandite, whereas partial replacement of OWC with 5 wt.% SF (Mix S1) shows a minor reduction in the compressive strength magnitudes which is more notable at early hydrothermal age. Such results agree with those obtained by Ali et al [Citation43] who concluded that the addition of 5 wt.% SF reduces the compressive strength of smart cement by 26 and 24% after 7 and 28 days of curing, respectively. Actually, the decline in the compressive strength magnitudes of these mixes could be correlated with the delay in the pozzolanic reaction between SF and free lime, besides the formation of crystalline CSH as secondary hydration products, that possess weak hydraulic features [Citation37,Citation42] as mentioned previously.
Under hydrothermal curing of 0.48 MPa/150°C, deterioration of the mechanical properties of OWC paste (Mix A2) is observed (Figure c). The loss in compressive strength values of Mix A2 by increasing the curing temperature and pressure could be connected to that CSH (the main hydration product) experiences metamorphosis to the highly crystalline phase of α-dicalcium silicate hydrate (α-C2SH, Ca2SiO3(OH)2). Such transformation under the effect of high temperatures and pressures motivates undesirable characteristics as destruction of the pore structure and deficiency of microstructure promoting compressive strength decline [Citation2,Citation32].
Figure c illustrates also that the partial replacement of 5 wt.% of OWC by Fly ash or SF, Mixes F2 and S2, respectively, under the severe conditions of temperature and pressure results in an enhancement of the compressive strength magnitudes. This finding is clearly distinct for fly ash-containing mix rather than for SF-containing mix and also at later hydrothermal ages rather than at early ages. The attained outcomes are correlated with the development of secondary hydration products due to the pozzolanic reaction between fly ash (source of silica and alumina) or SF (source of silica only) with CH produced from the hydration of silicate phases (C3S and β-C2S) in OWC, generating CSH and CASH in the case of FA and CSH in the case of SF, respectively. And so, more stable hardened OWC matrices (OWC-FA and OWC-SF) with enhanced mechanical characteristics under severe conditions of pressures and temperatures are attained [Citation30]. Evidently, as previously reported by many researchers that CSH gel has an average value of C/S ratio equals to 1.5 [Citation8] and to avoid the unsatisfactory phase transformation (amorphous CSH into crystalline α-C2SH or jaffeite) under the effect of high temperatures and pressures, CaO/SiO2 (C/S) ratio has to be diminished to about 1 [Citation9–11]. In our study, using SF or FA as supplements motivates the development of stable and desirable hydration products that possess lower C/S ratio (like tobermorite, Ca5Si6(OH)2.4H2O) through calcium hydroxide consumption [Citation12,Citation13].
In conclusion, the reduction of compressive strength due to the synergetic effect of high temperature and pressure is ceased and improved with the existence of mineral additives (FA and SF) through the development of more stable hydrated products formed by the pozzolanic reaction of FA and SF with CH obtained from OWC hydration.
3.2. X-ray diffraction
The formed crystalline hydration products, as well as the remaining parts of the unhydrated phases, were diagnosed adopting the XRD investigation. The diffractograms of the hardened OWC pastes fabricated from Mixes A1, S1 and F1 after hydrothermal curing for 1and 7 days at 100°C/0.1 MPa are shown in Figures and , respectively. After 1 day of hydrothermal curing, the diffractogram of the blank OWC plaster (Mix A1) demonstrates the development of calcium silicate hydrates (CSH) and portlandite (CH) as major hydration yields. Besides, the peaks distinct for CaCO3 (C) are also recognized in the XRD pattern. The diffractogram also clarifies the peaks belonging to the remaining unhydrated segments of C3S, β-C2S as well as C4AF are also noticeable in the XRD pattern (Figure ). Obviously, after 7 days of hydrothermal hydration, an executive growth in the intensities of the patterns characterizing portlandite and CSH was observed, whereas there is a noticeable diminish in the peaks’ intensities of the identifying anhydrous phases (C3S, β-C2S and C4AF), Figure . Indeed, the XRD technique is a qualitative routine since it detects only the crystalline part of the materials, so these results will receive further support from the TG/DTG analysis.
Figure 2. XRD patterns for Mixes A1, S1 and F1 after 1 day of hydrothermal hydration at 100°C/0.1 MPa.
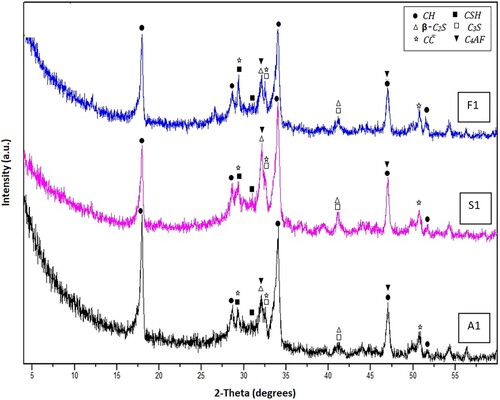
Figure 3. XRD patterns for Mixes A1, S1 and F1 after 7 days of hydrothermal hydration at 100°C/0.1 MPa.
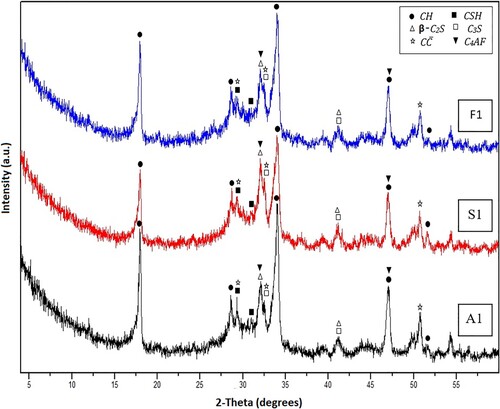
The results of XRD patterns obtained for the specimens made by replacing OWC by 5 wt.% SF or FA (by mass of OWC), Mixes S1 and F1, respectively, illustrate the same diffraction patterns as those of neat OWC hardened paste (Mix A1) with similar trends after 1 and 7 days of hydrothermal hydration at100°C/0.1 MPa, although with a little decrease in the peaks’ intensities district to portlandite (CH), specifically after 7 days of the hydrothermal reaction (Figures and ). This decline may be connected with the decrease in free CH amount (will be confirmed from TG/DTG data), owing to its utilization via the pozzolanic action with SF or FA [Citation44].
Figures and show the XRD diffractograms of hardened OWC samples fabricated from Mixes A2, S2 and F2 after 1 and 7 days of hydrothermal curing at 150°C/0.48 MPa. From the illustration, we can note that all tested mixes exhibit similar patterns and attitudes as their corresponding mixes treated at 100°C/0.1 MPa, but with a distinct decline in the intensity of the patterns connected with the presence of portlandite (CH) concerning Mixes S2 and F2. Actually, this finding is connected with the enhancement of the depletion speed of the free lime detached from OWC hydration through the pozzolanic action with SF or fly ash, and these results will receive further support from TG/DTG analysis. This pozzolanic reaction results in the development of an added quantity of ill-crystalline and approximately amorphous CSH.
Figure 4. XRD patterns for Mixes A2, S2 and F2 after 1day of hydrothermal hydration at 150°C/0.47 MPa.
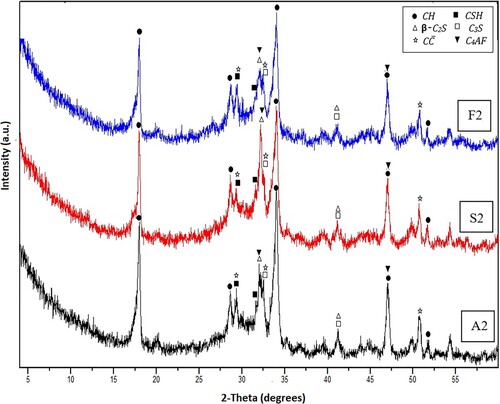
Figure 5. XRD patterns for Mixes A2, S2 and F2 after 7 days of hydrothermal hydration at 150°C/0.47 MPa.
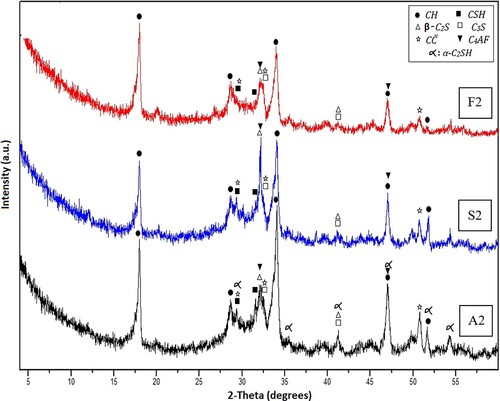
Obviously, after 7 days of hydrothermal hydration at 150°C/0.48 MPa, the specimens made from Mix A2 indicated a new peak in the XRD patterns and this peak is attributed to the formation of α-C2SH at high temperature and pressure in the specified specimen. The appearance of the peak distinct to the undesirable crystalline phases of (α-C2SH) could be attributed to the transformation of CSH to this crystalline phase (α-C2SH) [Citation32]. These findings agree with the achieved compressive strength data that exhibited a district decline in the compressive strength magnitudes for these mixes in comparison with their corresponding mixes (cured at 100°C/0.1 MPa) after 7 days of curing. The observed lowering in the compressive strength magnitudes is clarified in terms of the transformation of C–S–H to α-C2SH beneath these curing circumstances. This action leads to the formation of bulk materials that possess low hydraulic character, and so the depletion of microstructure could be reached [Citation45]. On the other hand, the absence of the expected peak corresponds to the undesired jaffeite in XRD diffractogram of Mix A2 is assigned to the non-crystalline nature of this phase when formed at 150°C/0.48 MPa.
Regarding XRD diffractograms of Mixes F2 and S2 cured 150°C/0.48 MPa for 7 days, it is clear that both diffractograms are free from the peaks distinct to crystalline α-C2SH which affirms that both constituents (FA and SF) inhibits the undesirable transformation of CSH into this phase (α-C2SH). This finding supports the attained results from the compressive strength measurements.
3.3. Differential thermogravimetric analysis (DTG/TG)
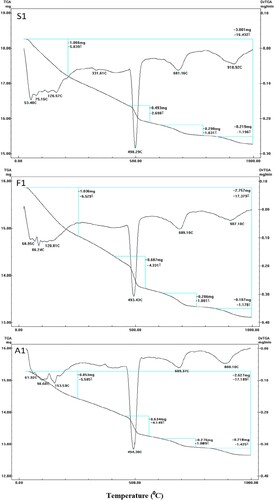
DTG/TG thermograms of the samples made from OWC, OWC blended with 5 wt.% SF or fly ash, Mixes A1, S1 and F1, respectively, after 7 days of hydrothermal treatment at 0.1 MPa/100°C are shown in Figure . The DTG/TG curves of Mix A1 showed a wide endotherm at the temperature range 60–110°C, which is related to the discharge of uncombined water and dehydration of amorphous part of CSH [Citation46]. Another endothermic peak located at 145–155°C may be related to the dehydration of sulfoaluminate hydrates (C2ASH8) [Citation2]. The main endothermic peak placed at 480–500°C is mainly attributed to the dehydration of portlandite [Citation47]. The mass failure of this endothermic peak is about 4.15% which accounts for the crystallinity degree and/or the quantity of the free portlandite exist in the cured specimen. Moreover, it is noticeable that a part of the hydrated yield has been carbonated. This confirmed by the presence of the last two endothermic peaks at 650–710°C and 860–930°C was due to the disintegration of calcium carbonate, originated from the carbonation of CH and C–S–H, of different crystallinity degrees, calcium [Citation44]. The total mass loss was 17.2%, and about 5.58% was recorded for free water and dehydration of CSH and CSAH.
DTG/TG curves for S1 and F1 Mixes after 7 days of hydrothermal treatment at 0.1 MPa/100°C exhibit similar endotherms obtained for Mix A1 with higher mass loss value for first endotherms located at 60–155°C (compared to the recorded value in the case of Mix A1) to be 5.84 and 6.53% for S1 and F1, respectively. This growth is principally connected with the development of successive amounts of the hydrothermal yields (principally as approximately amorphous and/or ill-crystalline CSH) generated from the pozzolanic action of SCMs with liberated free lime from OWC hydration. This accounts for the enhancement in the compressive strength magnitudes registered for these two mixes relative to the control (Mix A1) after 7 days [Citation48,Citation49]. Besides, a small mass loss percentage for the endotherm distinct for portlandite decomposition was registered for Mix S1 (2.698%). At the same time, an approximately comparable mass loss for portlandite composition has been recorded for F1 sample (4.33%) as compared to that of A1.
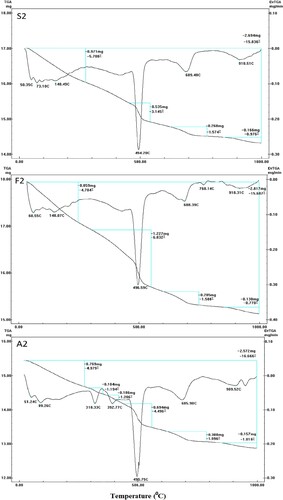
The OWC specimens treated for 7 days at 0.48 MPa/150°C (Mix A2) show two extra endotherms at 310–320°C and 380–400°C with a mass loss percentage of 1.194 and 1.203%, respectively (Figure ). These two endotherms may be related to the development and creation of crystalline α-C2SH, which possesses great permeability, owing to the transformation of calcium silicate hydrates under high pressure and temperature. These endothermic peaks do not appear in the DTG/TG curves of Mixes F1 or S1. This could be related to great silica contents (low C/S ratio) in the blended pastes that consume the free lime. This is confirmed by the low intensity of the endothermic peaks characterized by Ca(OH)2 in the case of Mix S2 (mass loss equals 3.145%). It is concluded that lower lime contents decrease the transformation of CSH to α-C2SH [Citation19,Citation20].
Besides, the presence of an endotherm with large intensity in the range of 500°C, for Mix A2 after 7 days of hydrothermal curing at 0.48 MPa/150°C, could be explained by the overlapping of the peaks correspond to portlandite decomposition with those of α-C2SH and/or Jaffeite (C6S2H3) [Citation32]. It is clear that the intensity of this endotherm decreases for the admixed OWC mixes (Mixes F2 and S2). This decline could be attributed to that the presence of FA or SF substituents decreases the C/S ratio and hinders the transformation of CSH to the undesirable phases of α-C2SH and/or Jaffeite (C6S2H3) in addition to the consumption of portlandite via the pozzolanic reaction to form additional CSH.
3.4. Morphology and microstructure
Figure exhibits SEM images of some representative hydrothermally cured OWC hardened specimens. The microstructures of the neat OWC hardened pastes after 7 days of hydrothermal treatment at 100°C/0.1 MPa and 150°C/0.48 MPa (Mixes A1 and A2) are illustrated in Figure a and 8b, respectively. The SEM micrographs of these samples reveal a structure accommodating residual unreacted phases, and the products of hydration are recognized as approximately dense homogenous structure. The SEM micrograph obtained for the specimens treated at 100°C/0.1 MPa (Mix A1) shows well-established phases like portlandite (CH) crystals admixed with limited rumpled filaments of calcium silicate hydrate (CSH). In addition, pore spaces accessible for the precipitation of additional hydrates are still observed (Figure a).
Figure 8. SEM images of hardened specimens made from (a) Mix A1, (c) Mix S1 and (e) Mix F1 after 7 days of hydrothermal curing at 100°C/0.1 MPa; (b) Mix A2, (d) Mix S2 and (f) Mix F2 after 7 days of hydrothermal curing at 150°C/0.48 MPa.
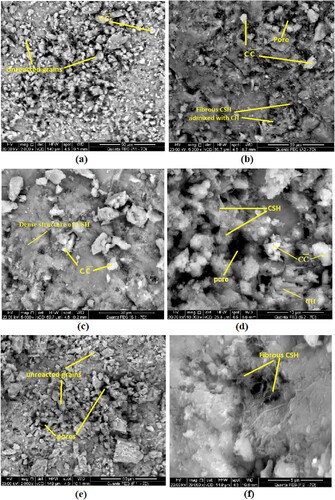
The specimens treated hydrothermally beneath the pressure of 0.48 MPa/150°C (Mix A2) exhibit a porous arrangement with well-established crystals. Evidently, the SEM micrographs of these specimens show a limited open and dense arrangement with considerable pores owing to the conjugated effects of pressure and temperature (Figure b). The progress of crystals relating to the conversion of CSH to α-C2SH under these hydrothermal conditions is the major explanation of the compressive strength reduction as well as pore structure destruction [Citation45]; these observations are in accordance with the attained compressive strength results (see Figure c).
The SEM images of the hardened specimens fabricated from OWC-SF pastes after 7 days of hydrothermal treatment at 100°C/0.1 MPa and 150°C/0.48 MPa (Mixes S1 and S2) are exhibited in Figures 8c and 8d, respectively. It is observed from the illustration of Figure c that the presence of SF as active micro-fillers (SF micro-particles fill the pores of the hardened cement paste) leads to the establishment of an ultra-dense arrangement in comparison with that of Mix A1. In addition, the dense microstructures of these specimens may be connected with the development of new quantities of CSH (with fibrous and amorphous nature) owing to the pozzolanic interaction between SF and Ca(OH)2 produced from OWC hydration. These additional amounts of CSH fill up the accessible pores and generate highly compact and dense arrangement that possesses high hydraulic characters as compared to that of Mix A1 (see Figure b). Obviously, OWC-SF specimens cured under the hydrothermal condition of 150°C/0.48 MPa (Mix S2) show a porous structure with well-developed crystals as compared to that of specimens made from Mix S1, but the microstructure is still denser and less porous than that of blank, Mix A2 (Figure d). The SEM images of these specimens (Mix S2) show a limited dense and expanded microstructure containing considerable pores as compared with those of the hardened specimens made from Mix S1 owing to the effect of severe conditions of pressure and temperature, which causes pore arrangement degradation and consequent reduction in the compressive strength values (see Figure c).
The SEM images of the OWC-FA hardened specimens after 7 days of hydrothermal hydration at 100°C/0.1 MPa and 150°C/0.48 MPa (Mixes F1 and F2) are shown in Figures 8e and 8f, respectively. The SEM micrographs of these samples reveal that the presence of FA causes the development of highly compact and dense microstructures as compared with those of all other tested specimens at both hydrothermal curing conditions. These results are attributed to the filling effect, as well as the pozzolanic activity of FA, that motivate the development of successive quantities of fibrous and microcrystalline CSH. The additional amounts of CSH could fill up the pores and the spaces between the unhydrated cement grains (Figure e). Evidently, increasing temperature and pressure up to 150°C/0.48 MPa results in limited dense and expanded microstructure with considerable pores (Figure f). This result agrees with the notable decline in the compressive strength magnitudes for these samples (see Figure c).
Conclusion
The effect of hydrothermal curing, 60°C/0.02 MPa, 100°C/0.1 MPa and 150°C/0.48 MPa, on the mechanical characteristics and microstructure of OWC blended with SF or FA was investigated in the present article.
The findings of this study could be summarized as follows:
Increasing curing temperature and pressure from 60°C/0.02 MPa to 100°C/0.1 MPa for neat OWC pastes supports the formation of crystalline CSH of weak hydraulic features with relatively depressed compressive strength magnitudes after 7 days of curing.
Hydrothermal curing of neat OWC pastes at 150°C/0.48 MPa heartens the metamorphosis of CSH (original hydration product) to the highly crystalline phase of α-dicalcium silicate hydrate (α-C2SH), this motivates undesirable characteristics as the destruction of the pore structure, deficiency of microstructure, promoting compressive strength decline.
The reduction of compressive strength values of OWC pastes due to the synergetic effect of high temperature and pressure is ceased and improved with the existence of mineral additives (FA and SF) through the development of more stable hydrated products formed via the pozzolanic action of FA and SF with CH obtained from OWC hydration.
Utilization of SF and FA as supplements motivates the development of stable and desirable hydration products that possess lower C/S ratio (like tobermorite, Ca5Si6(OH)2.4H2O) through calcium hydroxide consumption.
Fly ash exhibits better enhancement for OWC strength in comparison with that of SF. Replacing OWC by FA (class F) aids the development of CASH or CSH gels through the pozzolanic reaction of FA glassy phase with portlandite.
DTG/TG, XRD and SEM analyses affirm that the presence of both constituents (FA and SF) suppress the undesirable transformation of the amorphous CSH to the highly crystalline phase of α-dicalcium silicate hydrate (α-C2SH).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Vivek T, Nambiar S, Shah M, et al. A model on dual string drilling: on the road to deep waters. Model Earth Syst Environ. 2018;4:673–684.
- Palou MT, Zivica V, Ifka T, et al. Effect of hydrothermal curing on early hydration of G-Oil well cement. J Therm Anal Calorim. 2014;116:597–603.
- Le Saout G, Lécolier E, Rivereau A, et al. Chemical structure of cement aged at normal and elevated temperatures and pressures part I. Class G oil well cement. Cem Concr Res. 2006;36:71–78.
- Jupe AC, Wilkinson AP, Luke K, et al. Class H cement hydration at 180 °C and high pressure in the presence of added silica. Cem Concr Res. 2008;38:660–666.
- Amin MS, Habib AO, Abo-El-Enein SA. Hydrothermal characteristics of high slag cement pastes made with and without silica sand. Adv Cem Res. 2012;24:23–31.
- William CL, Gary JP. Standard Handbook of Petroleum and Natural Gas Engineering. 2nd ed. Oxford: Linacre House, Jordan Hill; 2005. (isbn:0-7506-7785-6).
- Won J, Lee D, Na K, et al. Physical properties of G-class cement for geothermal well cementing in South Korea. Renew Energy. 2015;80:123–131.
- Luke K, Taylor HFW. Equilibria and non-equilibria in the formation of xonotlite and truscottite. Cem Concr Res. 1984;14:657–662.
- Arabi N, Jauberthie R, Chelghoum N. Formation of C-S-H in calciumhydroxide-blast furnace slag-quartz-water system in autoclaving conditions. Adv Cem Res. 2015;27:153–162.
- Palou MT, Kuzielová E, Novotńy R, et al. Blended cements consisting of Portland cement-slag-silica fume-metakaolin system. J Therm Anal Calorim. 2016;125:1025–1034.
- Ray A. Hydrothermally treated cement-based building materials. Past, present, and future. Pure Appl Chem. 2002;74:2131–2135.
- Palou MT, Kuzielová E, Z˘emlic˘ka M, et al. The effect of curing temperature on the hydration of binary Portland cement: slag and Portland cement-metakaolin-blended cements. J Therm Anal Calorim. 2016;125:1301–1310.
- Hope BB. Autoclaved concrete containing fly ash. Cem Concr Res. 1981;11:227–233.
- Morsy MS, Alsayed SH, Aqel M. Effect of elevated temperature on mechanical properties and microstructure of silica flour concrete. Int J Civil Environ Eng. 2010;10:1–5.
- El-Gamal SMA, Hashem FS, Amin MS. Influence of carbon nanotubes, nanosilica and nanometakaolin on some morphological-mechanical properties of oil well cement pastes subjected to elevated water curing temperature and regular room air curing temperature. Constr Build Mater. 2017;146:531–546.
- Bjönström J, Martenelli A, Matic A, et al. Accelerating effects of colloidal nano-silica for beneficial calcium-silicate-hydrate formation in cement. Chem Phys Lett. 2004;392:242–248.
- Byung-Wan J, Chang-Hyun K, Jae-Hoon L. Characteristics of cement mortar with nano-SiO2 particles. ACI Mater. 2007;104-M45:404–407.
- Jeng-Ywan S, Chang T-P, Hsiao T-C. Effect of nano silica on characterization of Portland cement composite. Mater Sci Eng A. 2006;424:266–274.
- Li H, Xiao HG, Ou JP. A study on mechanical and pressure-sensitive properties of cement mortar with nano phase materials. Cem Concr Res. 2004;34:435–438.
- Li H, Xiao HG, Yuan J, et al. Microstructure of cement mortar with nanoparticles. Compos Part B. 2004;35:185–189.
- Amin MS, Hashem FS. Hydration characteristics of hydrothermal treated cement kiln dust–sludge–silica fume pastes. Constr Build Mater. 2011;25:1870–1876.
- Abo-El-Enein SA, Hashem FS, Amin MS, et al. Physicochemical characteristics of cementitious building materials derived from industrial solid wastes. Constr Build Mater. 2016;126:983–990.
- Amin MS, El-Gamal SMA, Abo-El-Enein SA, et al. Physico-chemical characteristics of blended cement pastes containing electric arc furnace slag with and without silica fume. HBRC. 2015;11:321–327.
- El-Gamal SMA, El-Hosiny FI, Amin MS, et al. Ceramic waste as an efficient material for enhancing the fire resistance and mechanical properties of hardened Portland cement pastes. Constr Build Mater. 2017;154:1062–1078.
- Shatat MR. Hydration behavior and mechanical properties of blended cement containing various amounts of rice husk ash in presence of metakaolin. Arab J Chem. 2016;9:S1869–S1874.
- Morsy MS, Al-Salloum YA, Abbas H, et al. Behavior of blended cement mortars containing nano-metakaolin at elevated temperatures. Constr Build Mater. 2012;35:900–905.
- Nadeem A, Memon SA, Lo TY. The performance of fly ash and metakaolin concrete at elevated temperatures. Constr Build Mater. 2014;62:67–76.
- Nadeem A, Memon SA, Lo TY. Evaluation of fly ash and metakaolin concrete at elevated temperatures through stiffness damage test. Constr Build Mater. 2013;38:1058–1065.
- Bu Y, Du J, Guo S, et al. Properties of oil well cement with high dosage of metakaolin. Constr Build Mater. 2016;112:39–48.
- Meller N, Kyritsis K, Hall C. The mineralogy of the CaO–Al2O3–SiO2–H2O (CASH) hydro ceramic system from 200 to 350°C. Cem Concr Res. 2009;39:45–53.
- Jez˘o L’, Palou MT, Kozánková J, et al. Determination of activation effect of Ca(OH)2 upon the hydration of BFS and related heat by isothermal calorimeter. J Therm Anal Calorim. 2010;101:585–593.
- Palou MT, S˘oukal F, Bohác˘ M, et al. Performance of G-Oil well cement exposed to elevated hydrothermal curing conditions. J Therm Anal Calorim. 2014;118:865–874.
- Kuzielova E, Žemlička M, Masilko J, et al. Pore structure development of blended G-oil well cement submitted to hydrothermal curing conditions. Geothermics. 2017;68:86–93.
- Kuzielová E, Žemlička M, Másilko J, et al. Development of G-oil well cement phase composition during long therm hydrothermal curing. Geothermics. 2019;80:129–137.
- Ledesma RB, Lopes NF, Georg Bacca K, et al. Zeolite and fly ash in the composition of oil well cement: Evaluation of degradation by CO2 under geological storage condition. Petrol. doi:10.1016/j.petrol.2019.106656.
- Kutchko BG, Strazisar BR, Lowry GV, et al. Rate of CO2 attack on hydrated class H well cement under geologic sequestration conditions. Environ Sci Technol. 2008;42:6237–6242.
- Luke K. Phase studies of pozzolanic stabilized calcium silicate hydrates at 180°C. Cem Concr Res. 2004;34:1725–1732.
- Pernites RB, Santra AK. Portland cement solutions for ultra-high temperature wellbore applications. Cem Concr Compos. 2016;72:89–103.
- Zhang L, Dzombak DA, Nakles DV, et al. Characterization of pozzolan-amended wellbore cement exposed to CO2 and H2S gas mixtures under geologic carbon storage conditions. Int J Green Gas Control. 2013;19:358–368.
- Abo-El-Enein SA, Daimon M, Ohsawa S, et al. Hydration of low porosity slag–lime pastes. Cem Concr Res. 1972;4:4299–4312.
- Senff L, Hotza D, Repette WL, et al. Effect of nanosilica and microsilica on microstructure and hardened properties of cement pastes and mortars. Adv Appl Ceram. 2010;109:104–110.
- Glasser FP, Hong SY. Thermal treatment of C–S–H gel at 1 bar H2O pressure up to 200°C. Cem Concr Res. 2003;33:405–416.
- Ali K, Vipulanandan C, Head B. Effect of silica fume on the fluid loss and piezoresistive properties of smart oil well cement. Proceedings CIGMAT- Conference & Exhibition. 2015.
- El-Gamal SMA, Amin MS, Ramandan M. Hydration characteristics and compressive strength of hardened cement pastes containing nano metakaolin. HBRC. 2017;13:114–121.
- Palou MT, Zivica V, Bagel L, et al. Influence of hydrothermal curing on G-oil well cement properties. Build Res J. 2012;60:223–230.
- Hashem FS, Amin MS, El-Gamal SMA. Improvement of acid resistance of Portland cement pastes using rice husk ash and cement kiln dust as additives. J Therm Anal Calorim. 2013;111:1391–1398.
- Heikal M, El-Didamony H. Pozzolanic activity of Homra with lime. Mansoura Sci Bull. 1999;26:79–95.
- Chen W, Brouwers HJP. The hydration of slag, part 1: reaction models for alkali-activated slag. J Mat Sci. 2007;42:428–443.
- Connan H, Klimesch D, Ray A, et al. Thermal characterization of autoclaved cement made with alumina-silica rich industrial waste. J Therm Anal Calorim. 2006;84:521–525.