?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Coconut (Cocos nucifera) shell was chemically treated with sulfuric acid (H2SO4) to produce acid- factionalized biosorbent for methylene blue (MB) dye removal from aqueous environment. Various analytical techniques were utilized to investigate the surface area, surface morphology, crystallinity, elemental composition, and functional group of the sulfuric acid-treated coconut shell (SATCS). The adsorption parameters such as adsorbent dosage (0.02–0.20 g), solution pH (3–10), contact time (0–360 min), and initial MB dye concentration (25–200 mg/L) were studied. The adsorption results were illustrated by pseudo-second order kinetic and Freundlich isotherm models. It was found that SATCS has a maximum adsorption capacity (qmax) of 50.6 mg/g at 303 K. The adsorption mechanism of MB dye on the SATCS surface can be assigned to the various types of interactions such as electrostatic attractions, H-bonding interaction, and π-π interaction. This work shows SATCS as promising acid- factionalized biosorbent for removal MB dye.
1. Introduction
Textile dyes are frequently utilized in unlimited industrial applications such as textile, printing, paint, food, and cosmetics as a colouring agent [Citation1]. The discharge of dye contaminated water is one of the most important environmental problems due to its risks on aquatic life and human health [Citation2]. Methylene blue (MB) dye is widely used in the textile industry for dyeing cotton, wood, and leather, in addition to the pharmaceutical industries [Citation3]. MB dye causes harmful effects on human health such as nausea, vomiting, heart rate increasing and eye/skin irritation [Citation4,Citation5]. Several methods were applied to remove textile dyes prior to discharge into water such as adsorption [Citation6], photocatalysis [Citation7], oxidation [Citation8], and coagulation [Citation9]. Adsorption is a prime wastewater treatment method for dye removal due to its simplicity of design, non-generation of toxic materials, low cost and high efficiency [Citation10,Citation11]. Activated carbon is the most common adsorbent utilized for the treatment of a wide range of water pollutants [Citation12]. However, activated carbon requires a high cost for preparation and regeneration. Therefore, the researchers shifted towards natural biopolymers, biomass, and waste materials as an economical alternative and environment-friendly adsorbents [Citation13–16].
In recent years, various types of biomass, such as coconut husk [Citation17], loofah sponge [Citation18], algae [Citation19], and soy waste [Citation20], were utilized for dyes and metal ion removal from aqueous environment. Generally, various biomass and agricultural wastes are preferred for preparing carbonaceous adsorbents due to its multiple advantages such as renewability, low-cost precursor, and environment friendliness [Citation21]. Biomass materials and agricultural wastes were widely utilized as low-cost biosorbents for the removal of cationic dyes dye such as Citrus limetta peel waste [Citation22], Cucumis sativus peel waste [Citation23], Punica granatum peel [Citation24], sulphuric acid-treated orange peel [Citation25], fallen leaves [Citation26], Tectona grandis sawdust [Citation27], and Luffa aegyptiaca peel [Citation28]. The adsorption capacity and surface property of the adsorbent prepared from biomass depend on the type of chemical activator, and the source of the precursor, in addition to the activation process [Citation29].
Chemical activation of biomass is one the most significant methods frequently applied for developing oxygenated and hydrophilic carbonaceous adsorbents that contain different types of functional groups such as carboxyl (-COOH) and hydroxyl (-OH) groups. Sulphuric acid (H2SO4) is one of the super-oxidizing agents that are frequently utilized as a chemical activator for enhancing the surface functionality of biomass materials with oxygenated-acidic functional groups [Citation30]. Previous studies indicate that the concentration of the chemical activators is responsible for enhancing the oxygenated-functional group content on the surface of treated biomass [Citation31,Citation32]. Therefore, H2SO4 acid was selected to be an oxidizing activator in this research due to its many desirable properties such as super oxidation power, cost-effectiveness, relatively low activation temperature, and the ability to tune the amorphous domains of cellulosic materials into aromatize form of carbon framework [Citation33,Citation34].
Therefore, the main aim of this study is to develop acid-functionalized biosorbent from renewable biomass waste, namely coconut shell (CS) by the chemical treatment method with sulphuric acid (H2SO4) to obtain sulphuric acid-treated coconut shell (SATCS) as a low-cost biosorbent. The effectiveness of the developed SATCS was tested for the removal of MB dye from the aqueous environment under optimum adsorption operational parameters such as adsorbent dosage, solution pH, contact time and MB dye concentration. Moreover, the adsorption mechanism of MB on the surface of SATCS was discussed.
2. Experimental procedure
2.1. Materials
Methylene blue (MB) dye (MW: 319.86 g/mol, assay: ∼ 99%, λmax = 661 nm) and sulphuric acid (H2SO4, 95–98%) were purchased from R&M Chemicals. The other reagents utilized throughout this study were of analytical grade. All experiments of this research were performed using ultrapure water.
2.2. Preparation of sulphuric acid-treated coconut shell (SATCS)
Coconut shell (CS) was collected from a local market in Shah Alam, Selangor, Malaysia. CS was fully rinsed by using tap water, then by distilled water to eliminate soluble impurities, and then crushed and sieved to a certain mesh size of ≤ 1 mm. The impregnating process with concentrated sulphuric acid (H2SO4, 95– 98%) was carried out following the published activation procedure by Garg et al. [Citation35], with a mixing ratio of 1 g coconut shell powder and 1 mL of sulphuric acid. The obtained sulphuric acid-treated coconut shell (SATCS) was washed many times in boiled and distilled water in order to reach a neutral pH value. After that, SATCS powder is left for 24 h for the drying process inside an oven at 100°C. The final dried powder form of SATCS was sieved to the desired mesh size of ≤ 0.25 mm for further applications.
2.3. Characterization of SATCS
The characterization of SATCS was performed by various analytical methods and techniques. The point of zero charge (pHpzc) for SATCS was determined based on the reported method [Citation36]. The porosity and pore volume of the SATCS was investigated by Micromeritics ASAP 2060 analyser at 77 K. Scanning electron microscope (SEM) (Hitachi, TM3030Plus, Tabletop Microscope) was utilized to determine the morphological characteristics of SATCS before and after the adsorption of MB molecules. The X-Ray diffraction (XRD, X’Pert PRO, PAnalytical) was used to determine the crystallinity of SATCS. Fourier Transform Infrared (FTIR) spectroscopy was performed by using Perkin-Elmer, Spectrum RX I to identify the available functional group on the surface of SATCS before and after adsorption. The CHNS analysis (Flash 2000, Organic Elemental Analyser) was performed to determine the elemental composition of SATCS.
2.4. Batch adsorption experiments
The adsorption experiments of MB dye on SATCS were investigated in a batch mode. The experiments were carried out in a series of Erlenmeyer flask (250 mL) containing MB dye solution (100 mL) with different initial MB dye concentrations (25–200 mg/L). The different dosages of SATCS (0.02–0.2 g) were added to the 100 mL of MB dye solution with different levels of pH (3–10), and agitated at a fixed shaking speed of 110 strokes/min at 303 K by using a water bath (WNB7-45, Memmert, Germany). After the adsorption process, the adsorbents was separated from aqueous solutions using a syringe filter (0.20 µm), and the MB dye uptake was calculated by UV-Vis spectroscopy (HACH DR 2800) at 661 nm. Equilibrium isotherms were performed at optimum conditions (temperature = 303 K, adsorbent dosage = 0.1 g/100 mL, and pH = 8) by using initial MB concentrations from 25 to 200 mg/L. The MB dye removal (DR%) and adsorbed amount of MB dye at equilibrium, qe (mg/g) were determined by the following Equations (1) and (2), respectively.
(1)
(1)
(2)
(2) where Co (mg/L) is the MB initial concentration while Ce (mg/L) is the equilibrium concentration of MB. V (L) is the volume of MB solution, and W (g) is the weight of SATCS.
3. Results and discussion
3.1. Characterization
3.1.1. Physicochemical properties of SATCS
The textural properties and elemental analysis of SATCS are recorded in Table . It is observed that the SATCS has a relatively high content of C (51.90) with a high content of O (44.63%), while the contents of H (2.97%) and N (0.37%) are relatively low. This observation can be attributed to the releasing of volatile compounds during carbonization to eliminate non-carbon species and enrich carbon. The high oxygen content and detectable amount of sulphur can be attributed to the role of sulphuric acid as a chemical activator. However, the low surface area (1.137 m²/g) of SATCS can be attributed to a high concentration of H2SO4 acid which is responsible for reducing the pore volume on the surface of SATCS [Citation32].
Table 1. The physicochemical properties of the SATCS.
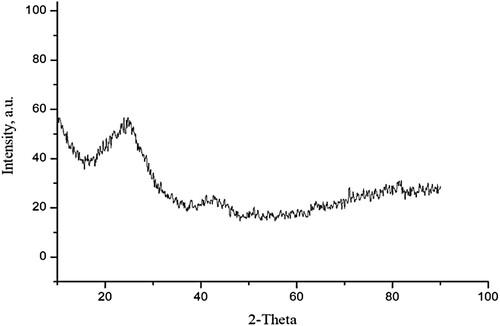
3.1.2. XRD analysis
The crystallinity and amorphous nature of SATCS is investigated by the XRD analysis. Figure presents the XRD pattern of the SATCS. As can be seen, two broad diffraction peaks display at 2θ = 24° (002) and 2θ = 42° (100), indicating the SATCS an amorphous structure and composed of crystallographic planes of carbonaceous materials [Citation37,Citation38].
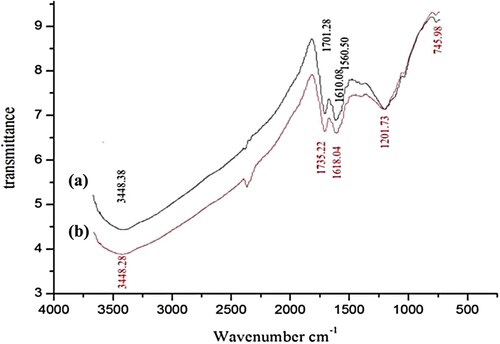
3.1.3. FTIR analysis
The surface functional groups of SATCS are identified by the FTIR spectral analysis. Figure (a) displays the FTIR spectrum of SATCS before MB dye adsorption. It is observed from Figure (a) that characteristic peaks show at 3400 and 1700 cm−1 corresponding to the (v-O−H groups) and (v-C = O of RCOOH or RCOOR), while peaks at 1610 and 1560 cm−1 attributed to the asymmetric and symmetric of v–COO [Citation39], respectively. The bands at ∼1610 and 1200 cm−1 indicate the (v-C = C of aromatic rings) [Citation40] and (v-C–O–C and/or v-C–O of Ar-OH, ROH, and H2SO4 acid) [Citation14], respectively. The FTIR spectrum of SATCS after MB dye adsorption is shown in Figure (b). It was observed from Figure (b), some bands are shifted to higher wavenumbers, indicating the interactions between adsorption functional groups of SATCS and MB dye molecules that are loaded on the surface of the SATCS.
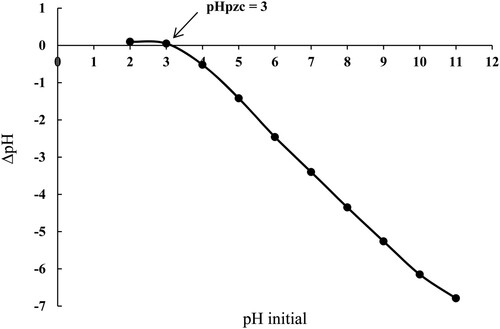
3.1.4. pHPZC analysis
pHPZC analysis was performed to determine the surface charge of the SATCS. The value of pHPZC for the SATCS was found to be 3.0, as presented in Figure . This result indicates the acidic characteristic of the SATCS surface which might be attributed to the existence of some acidic functional groups such as COO- and SO3- on the SATCS surface. Generally, the surface of the SATCS can be obtained a negative charge at pH value above the pHpzc, and as a result, the adsorption of cationic species such as MB dye would be preferable. A similar observation was noticed by other researchers for the pHpzc values after various biomass materials treated with H2SO4 such as coconut leaf (pHpzc = 3.2) [Citation14] and mango peel (pHpzc = 4.6) [Citation41].
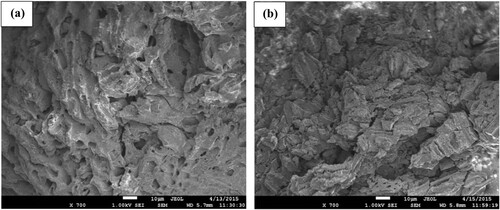
3.1.5. SEM analysis
The surface morphology of SATCS before and after MB dye adsorption is observed by the SEM analysis. Figure (a,b) displays the SEM images of the SATCS before and after MB dye adsorption, respectively. From Figure (a), it is observed that the surface of the SATCS appears as a rough, porous surface with visible cracks and irregular shape pores. The generated pores on the surface of the SATCS could be ascribed to the evaporative loss of the chemical activator during the activation process. The presence of these pores within the structure of the SATCS exhibits an essential role in the adsorption process of MB dye molecules. The surface of the SATCS after the adsorption of MB dye (Figure (b)) turned to be less porous with the evanescence of crevices on the SATCS surface, indicating successful loading of MB dye molecules on the SATCS surface.
3.2. Adsorption study
3.2.1. Effect of SATCS dosage
The influence of SATCS dosage on the removal of MB dye by the SATCS was tested by varying the adsorbent dosage from 0.02 to 0.2 g with fixed volume and initial concentration of MB dye, solution pH, temperature, contact time, and shaking speed at 100 mL and 200 mg/L, 5.6, 303 K, 480 min, and 110 strokes/min, respectively. As shown in Figure , the removal of MB dye increased from 9.3% to 83% with increasing adsorbent dosage from 0.02 g/100 mL to 0.1 g/100 mL. The highest MB dye removal (83%) was obtained at 0.1 g/100 mL, which can be assigned to higher surface area and/or the adsorption active sites that are available for the adsorption of MB molecules. Further increase in adsorbent dosage above 0.1 g did not show any further improvement in MB removal. Therefore, 0.1 g/100 mL was chosen to be an optimum adsorbent dosage for further investigations.
3.2.2. Effect of solution pH
The effect of initial solution pH on the adsorption capacity of MB dye onto the SATCS was performed at different pH levels (pH 3–10) with fixing others adsorption parameters (adsorbent dosage 0.1 g/100 mL, concentration 200 mg/L, and temperature 303 K). It is observed from Figure , with the increase in solution pH from 3 to 8 the adsorption capacity, qt (mg/g) rises from 76.3 mg/g to 82.3 mg/g. The highest adsorption capacity of the MB was recorded at solution pH 8, and a gradual decrease in adsorption capacity could be noticed at acidic medium. The pHpzc value of the SATCS was found to be 3, as shown in Figure , indicating the surface of the SATCS is positively charged. On the other hand, the surface of the SATCS can be converted to negative charge at basic pH environment, and therefore a strong electrostatic attraction can occurs between the negatively charged functional group on the SATCS surface and positively charged group of MB and [Citation42]. Therefore, solution pH 8 is determined to be the best solution pH for further applications.
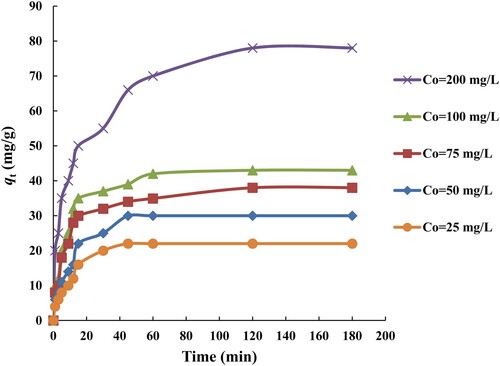
3.2.3. Effect of initial MB concentration and contact time
The influence of initial MB dye concentration and contact time on adsorption equilibrium was investigated. The adsorption capacity, against time at several initial MB dye concentrations of 25, 50, 75, 100 and 200 mg/L is investigated and depicted in Figure , while the other optimum factors like adsorbent dose = 0.1 g, pH of solution = 8, and temperature = 303 K are kept constant. The results showed that the MB dye uptake on the surface of the SATCS in the first 15 min is very high (as shown in Figure ) in all concentrations and reached equilibrium within 60 min. It is observed from Figure , the quantity of MB dye molecules uptake onto the surface of SATCS increased from 22 to 78 mg/g by increasing the initial MB dye concentrations from 25 to 200 mg/L. This can be assigned to the higher concentration gradient which provides a driving force to move the MB dye molecules towards active adsorption sites [Citation43]. A similar observation is noticed by other researchers for the MB adsorption by sulphuric acid-treated coconut leaf [Citation14] and mango peel [Citation41].
3.3. Adsorption kinetic
The non-linear pseudo-first-order (PFO) and non-linear pseudo-second-order (PSO) kinetic models are utilized to analyse the experimental data of the MB dye adsorption on the SATCS surface at different initial MB concentration. The non-linear equations of the kinetic models PFO [Citation44] and PSO [Citation45] are expressed in Equations (3) and (4) as follows:
(3)
(3)
(4)
(4) where qe (mg/g) is the amount of MB dye adsorbed at equilibrium, and qt (mg/g) is the amount of MB dye adsorbed at the time (t). k1 (1/min) is the rate constant of PFO, and k2 (g/mg min) is the rate constant of PSO. The kinetic data are also analysed by the coefficient of determination (R2), and non-linear average relative error (ARE). The equations of R2 and ARE functions are presented as follows:
(5)
(5)
(6)
(6) where qt.meas (mg/g) and qt.cal. (mg/g) are measured and calculated adsorption capacities of MB dye at time t, while n is the number of observations.
The non-linear plots of PFO and PSO models are displayed in Figure . The parameters of kinetic models, R2 and ARE values are recorded in Table . According to the experimental data (Table ), it can be concluded that the adsorption of MB dye molecules by the SATCS adsorbent follows PSO model due to the higher correlation coefficient (R2) values, and the lower ARE values, in addition to the calculated qe (qe,cal) values fitted well with the experimental qe (qe, exp) values. This result exhibits that the adsorption of MB dye molecules by the SATCS is the chemisorption process involving the electrostatic interaction between the negatively charged available on the SATCS surface and the MB dye cations [Citation46].
Figure 8. Non-linear plots of PFO and PSO models for MB adsorption on the SATCS surface (V = 100 mL, SATCS dose = 0.1 g, solution pH 8, shaking speed = 110 strokes/min, and 303 K).
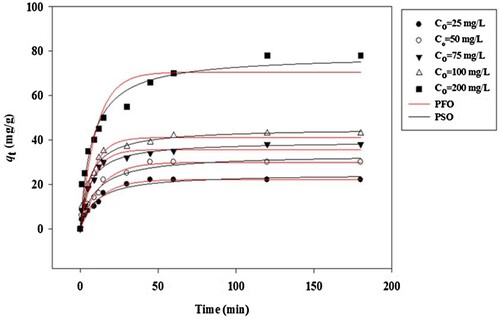
Table 2. Kinetic parameters for the adsorption of MB by SATCS at 303 K.
3.4. Adsorption isotherms
Non-linear equilibrium models of Langmuir, Freundlich, and Temkin are utilized to fit the experimental data. The non-linear equations of the equilibrium models Langmuir [Citation47], Freundlich [Citation48], and Temkin [Citation49] are presented in Equations (7)–(9), respectively.
(7)
(7)
(8)
(8)
(9)
(9) where qe (mg/g) is the amount of MB dye adsorbed at equilibrium, Ce (mg/L) is the concentration of MB dye at equilibrium, qmax (mg/g) is the maximum adsorption capacity, and Ka (L/mg) is the Langmuir constant. Kf ((mg/g) (L/mg)1/n) is the Freundlich constant, n is the dimensionless constant that indicates the adsorption intensity. KT (L/mg) is the Temkin constant, T (K) is the temperature, R (8.314 J/molK) is the universal gas constant, and bT (J/mol) is the heat of adsorption. The non-linear curves of the equilibrium models are shown in Figure . The parameters of equilibrium models are recorded in Table . According to the R2 values (Table ) obtained from the isotherm models, Freundlich isotherm shows the highest correlation (R2 0.97) which indicates the MB dye adsorption occurred on the heterogeneous surface [Citation50]. The value of 1/n is less than 1, indicating the strong adsorption bond between MB dye molecules onto SATCS [Citation51]. The maximum adsorption capacity (qmax) of SATCS for MB dye is 50.6 mg/g at 303 K. The qmax of SATCS for MB dye is compared with other adsorbents of biomass materials treated with H2SO4 and presented in Table which indicates the desirable adsorption property of the SATCS for the removal of cationic dye compared to other available biomass-based adsorbents.
3.5. Adsorption mechanism
There are three functional groups available on the surface SATCS can be involved in the adsorption mechanism of MB dye such as hydroxyl (OH), carboxyl (COOH), and carbonyl (C = O) as confirmed previously by FTIR spectral analysis. The sulphonation process is responsible for creating SO3 acidic group on the surface of SATCS, which can play an important role in the adsorption process of MB dye through electrostatic attractions with the positively charged MB dye molecules. Generally, the adsorption mechanism of MB dye on the SATCS surface by different types of interactions is shown in Figure . An electrostatic attraction (Figure (a)) is considered from the most interaction that can occur between MB dye and the SATCS surface. This mechanism involves the electrostatic interaction between MB dye cations with negatively charged available on the SATCS surface. Adsorption mechanism also includes H-bonding interactions (Figure (b)) that occurred between H atom available on the surface of the SATCS, and N atoms in the MB dye structure. Finally, π-π interaction (Figure (c)) can occur between the hexagonal skeleton of SATCS and aromatic rings of MB dye. According to the above discussion, it can be concluded that these interactions are effective in enhancing the adsorption process of MB dye on the surface of the SATCS. Similar results were reported by other studies for the MB dye adsorption on multi-wall carbon nanotube [Citation52], chemically treated carbon microspheres [Citation53], wrapping carbon nanotube [Citation54], and coal activated carbon [Citation37].
Figure 9. Isotherm models plots of Langmuir, Freundlich, and Tempkin, for the adsorption of MB on the SATCS at 303 K.
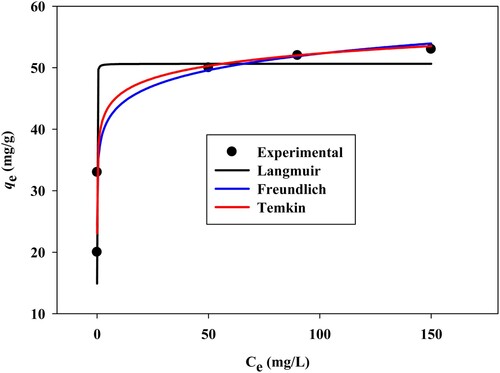
Figure 10. Illustration of the possible interaction between the SATCS and MB: (a) electrostatic attraction, (b) H-bonding interaction, and (c) π-π interactions.
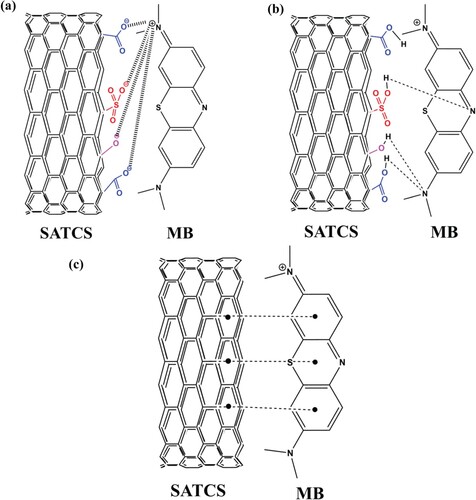
Table 3. Adsorption isotherms for MB on SATCS at 303 K.
Table 4. Comparative of adsorption capacities for MB on different biomass materials treated with H2SO4.
4. Conclusion
The sulphuric acid-treated coconut shell (SATCS) was synthesized and applied for the removal of MB dye from the aqueous environment. The optimum conditions of adsorption process parameters were adsorbent dosage (0.1 g/100 mL), solution pH 8, and temperature (303 K). The kinetics and isotherms models data revealed that the adsorption was affected by chemisorption and heterogeneous mode of adsorption. The adsorption capacity of the SATCS was 50.6 mg/g at 303 K for MB dye. The adsorption mechanism included electrostatic attraction and H-bonding interaction, and π-π interactions. The adsorption results revealed that the SATCS is a feasible and low-cost biosorbent for the removal of MB dye.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Odoemelam SA, Emeh UN, Eddy NO. Experimental and computational chemistry studies on the removal of methylene blue and malachite green dyes from aqueous solution by neem (Azadirachta indica) leaves. J Taibah Uni Sci. 2018;12(3):255–265. doi: 10.1080/16583655.2018.1465725
- Abdulhameed AS, Mohammad AT, Jawad AH. Modeling and mechanism of reactive orange 16 dye adsorption by chitosan-glyoxal/TiO2 nanocomposite: application of response surface methodology. Desalin Water Treat. 2019;164:346–360. doi: 10.5004/dwt.2019.24384
- Brião GV, Jahn SL, Foletto EL, et al. Highly efficient and reusable mesoporous zeolite synthetized from a biopolymer for cationic dyes adsorption. Coll Surf. 2018;556:43–50. doi: 10.1016/j.colsurfa.2018.08.019
- Jawad AH, Sabar S, Ishak MAM, et al. Microwave-assisted preparation of mesoporous activated carbon from coconut (Cocos nucifera) leaf by H3PO4-activation for methylene blue adsorption. Chem Eng Commun. 2017;204:1143–1156. doi: 10.1080/00986445.2017.1347565
- Jawad AH, Abdulhameed AS. Mesoporous Iraqi red kaolin clay as an efficient adsorbent for methylene blue dye: adsorption kinetic, isotherm and mechanism study. Surf Interface. 2020;18: 100422.
- Abdulhameed AS, Mohammad AT, Jawad AH. Application of response surface methodology for enhanced synthesis of chitosan tripolyphosphate/TiO2 nanocomposite and adsorption of reactive orange 16 dye. J Clean Prod. 2019;232:43–56. doi: 10.1016/j.jclepro.2019.05.291
- Jawad AH, Alkarkhi AFM, Mubarak NSA. Photocatalytic decolorization of methylene blue by an immobilized TiO2 film under visible light irradiation: optimization using response surface methodology (RSM). Desalin Water Treat. 2015;56:161–172. doi: 10.1080/19443994.2014.934736
- Nidheesh PV, Zhou M, Oturan MA. An overview on the removal of synthetic dyes from water by electrochemical advanced oxidation processes. Chemosphere. 2018;197:210–227. doi: 10.1016/j.chemosphere.2017.12.195
- Beluci NDCL, Mateus GAP, Miyashiro CS, et al. Hybrid treatment of coagulation/flocculation process followed by ultrafiltration in TiO2-modified membranes to improve the removal of reactive black 5 dye. Sci Total Environ. 2019;664:222–229. doi: 10.1016/j.scitotenv.2019.01.199
- Mu B, Wang A. Adsorption of dyes onto palygorskite and its composites: a review. J Environ Chem Eng. 2016;4(1):1274–1294. doi: 10.1016/j.jece.2016.01.036
- Abdulhameed AS, Jawad AH, Mohammad AT. Synthesis of chitosan-ethylene glycol diglycidyl ether/TiO2 nanoparticles for adsorption of reactive orange 16 dye using a response surface methodology approach. Bioresour Technol. 2019;293: 122071. doi: 10.1016/j.biortech.2019.122071
- Bhatnagar A, Hogland W, Marques M, et al. An overview of the modification methods of activated carbon for its water treatment applications. Chem Eng J. 2013;219:499–511. doi: 10.1016/j.cej.2012.12.038
- Mohammad AT, Abdulhameed AS, Jawad AH. Box-Behnken design to optimize the synthesis of new crosslinked chitosan-glyoxal/TiO2 nanocomposite: Methyl orange adsorption and mechanism studies. Int J Biol Macromol. 2019;129:98–109. doi: 10.1016/j.ijbiomac.2019.02.025
- Jawad AH, Rashid RA, Ishak MAM, et al. Adsorption of methylene blue onto activated carbon developed from biomass waste by H2SO4 activation: kinetic, equilibrium and thermodynamic studies. Desalin Water Treat. 2016;57:25194–25206. doi: 10.1080/19443994.2016.1144534
- Jawad AH, Mehdi ZS, Ishak MAM, et al. Large surface area activated carbon from low-rank coal via microwave-assisted KOH activation for methylene blue adsorption. Desalin Water Treat. 2018;110:239–249. doi: 10.5004/dwt.2018.22226
- Jawad AH, Mubarak NSA, Abdulhameed AS. Hybrid crosslinked chitosan epichlorohydrin/TiO2 nanocomposite for reactive red 120 dye adsorption: kinetic, isotherm, thermodynamic, and mechanism study. J Polym Environ. 2020;28:624–637. doi: 10.1007/s10924-019-01631-8
- Aljeboree AM, Alkaim AF, Al-Dujaili AH. Adsorption isotherm, kinetic modeling and thermodynamics of crystal violet dye on coconut husk-based activated carbon. Desalin Water Treat. 2015;53(13):3656–3667. doi: 10.1080/19443994.2013.877854
- Li Z, Wang G, Zhai K, et al. Guo, methylene blue adsorption from aqueous solution by loofah sponge-based porous carbons. Colloids Surf A Physicochem Eng Asp. 2018;538:28–35. doi: 10.1016/j.colsurfa.2017.10.046
- Ouasfi N, Bouzekri S, Zbair M, et al. Carbonaceous material prepared by ultrasonic assisted pyrolysis from algae (Bifurcaria bifurcata): response surface modeling of aspirin removal. Surf Interfac. 2019;14:61–71. doi: 10.1016/j.surfin.2018.11.008
- Bulgariu L, Bulgariu D. Functionalized soy waste biomass-a novel environmental-friendly biosorbent for the removal of heavy metals from aqueous solution. J Clean Prod. 2018;197:875–885. doi: 10.1016/j.jclepro.2018.06.261
- Khaniabadi YO, Heydari R, Nourmoradi H, et al. Low-cost sorbent for the removal of aniline and methyl orange from liquid-phase: aloe vera leaves wastes. J Taiwan Inst Chem Eng. 2016;68:90–98. doi: 10.1016/j.jtice.2016.09.025
- Shakoor S, Nasar A. Removal of methylene blue dye from artificially contaminated water using citrus limetta peel waste as a very low cost adsorbent. J Taiwan Inst Chem Eng. 2016;66:154–163. doi: 10.1016/j.jtice.2016.06.009
- Shakoor S, Nasar A. Adsorptive treatment of hazardous methylene blue dye from artificially contaminated water using cucumis sativus peel waste as a low-cost adsorbent. Groundwater Sustain Develop. 2017;5:152–159. doi: 10.1016/j.gsd.2017.06.005
- Shakoor S, Nasar A. Utilization of Punica granatum peel as an eco-friendly biosorbent for the removal of methylene blue dye from aqueous solution. J Appl Biotechnol Bioeng. 2018;5:242–249.
- Senthil Kumar P, Fernando PSA, Ahmed RT, et al. Effect of temperature on the adsorption of methylene blue dye onto sulfuric acid–treated orange peel. Chem Eng Commun. 2014;201(11):1526–1547. doi: 10.1080/00986445.2013.819352
- Kong L, Gong L, Wang J. Removal of methylene blue from wastewater using fallen leaves as an adsorbent. Desalin Water Treat. 2015;53(9):2489–2500. doi: 10.1080/19443994.2013.863738
- Mashkoor F, Nasar A, Asiri AM. Exploring the reusability of synthetically contaminated wastewater containing crystal violet dye using Tectona grandis sawdust as a very low-cost adsorbent. Sci Reports. 2018;8(1):8314.
- Mashkoor F, Nasar A. Preparation, characterization and adsorption studies of the chemically modified Luffa aegyptica peel as a potential adsorbent for the removal of malachite green from aqueous solution. J Mol Liq. 2019;274:315–327. doi: 10.1016/j.molliq.2018.10.119
- Liu QS, Zheng T, Li N, et al. Modification of bamboo-based activated carbon using microwave radiation and its effects on the adsorption of methylene blue. Appl Surface Sci. 2016;256:3309–3315. doi: 10.1016/j.apsusc.2009.12.025
- Zhang Y, Li M, Li J, et al. Surface modified leaves with high efficiency for the removal of aqueous Cr (VI). Appl Surface Sci. 2019;484:189–196. doi: 10.1016/j.apsusc.2019.04.088
- Gokce Y, Aktas Z. Nitric acid modification of activated carbon produced from waste tea and adsorption of methylene blue and phenol. Appl Surface Sci. 2014;313:352–359. doi: 10.1016/j.apsusc.2014.05.214
- Valdes H, Sanchez-Polo M, Rivera-Utrilla J, et al. Effect of ozone treatment on surface properties of activated carbon. Langmuir. 2002;18:2111–2116. doi: 10.1021/la010920a
- Gerçel Ö, Özcan A, Özcan AS, et al. Preparation of activated carbon from a renewable bio-plant of Euphorbia rigida by H2SO4 activation and its adsorption behavior in aqueous solutions. Appl Surf Sci. 2007;253:4843–4852. doi: 10.1016/j.apsusc.2006.10.053
- Georgin1 J, Marques BS, Salla JS, et al. Removal of Procion Red dye from colored effluents using H2SO4-/HNO3- treated avocado shells (Persea americana) as adsorbent. Environ Sci Pollut Res Int. 2018;25(7):6429–6442. doi: 10.1007/s11356-017-0975-1
- Garg VK, Kumar R, Gupta R. Removal of malachite green dye from aqueous solution by adsorption using agro-industry waste: a case study of Prosopis cineraria. Dyes Pigm. 2004;62:1–10. doi: 10.1016/j.dyepig.2003.10.016
- Lopez-Ramon MV, Stoeckli F, Moreno-Castilla C, et al. On the characterization of acidic and basic surface sites on carbons by various techniques. Carbon N Y. 1999;37:1215–1221. doi: 10.1016/S0008-6223(98)00317-0
- Jawad AH, Ismail K, Ishak MAM, et al. Conversion of Malaysian low-rank coal to mesoporous activated carbon: structure characterization and adsorption properties, Chinese. J Chem Eng. 2019;27(7):1716–1727.
- Barpanda P, Fanchini G, Amatucci GG. Structure, surface morphology and electrochemical properties of brominated activated carbons. Carbon N Y. 2011;49:2538–2548. doi: 10.1016/j.carbon.2011.02.028
- Jawad AH, Ngoh YS, Radzun KA. Utilization of watermelon (Citrullus lanatus) rinds as a natural low-cost biosorbent for adsorption of methylene blue: kinetic, equilibrium and thermodynamic studies. J Taibah Uni Sci. 2018;12(4):371–381. doi: 10.1080/16583655.2018.1476206
- Chen B, Chen Z, Lv S. A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Bioresour Technol. 2011;102:716–723. doi: 10.1016/j.biortech.2010.08.067
- Jawad AH, Mamat NFH, Abdullah MF, et al. Adsorption of methylene blue onto acid-treated mango peels: kinetic, equilibrium and thermodynamic. Desalin Water Treat. 2017;59:210–219. doi: 10.5004/dwt.2017.0097
- Bedin KC, Martins AC, Cazetta AL, et al. KOH-activated carbon prepared from sucrose spherical carbon: adsorption equilibrium, kinetic and thermodynamic studies for methylene blue removal. Chem Eng J. 2015;286:476–484. doi: 10.1016/j.cej.2015.10.099
- Jawad AH, Mubarak NSA, Abdulhameed AS. Tunable Schiff’s base-cross-linked chitosan composite for the removal of reactive red 120 dye: adsorption and mechanism study. Int J Biol Macromol. 2020;142:732–741. doi: 10.1016/j.ijbiomac.2019.10.014
- Lagergren S. Zur theorie der sogenannten adsorption geloster stoffe, K. Sven Vetenskapsakad. Handl. 1898;24:1–39.
- Ho YS, McKay G. Sorption of dye from aqueous solution by peat. Chem Eng J. 1998;70:115–124. doi: 10.1016/S0923-0467(98)00076-1
- Iqbal A, Sabar S, Mun-Yee MK, et al. Pseudomonas aeruginosa USM-AR2/SiO2 biosorbent for the adsorption of methylene blue. J Environ Chem Eng. 2018;6(4):4908–4916. doi: 10.1016/j.jece.2018.07.025
- Langmuir I. The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc. 1918;40(9):1361–1403. doi: 10.1021/ja02242a004
- Freundlich HMF. Over the adsorption in solution. J Phys Chem. 1906;57:385–471.
- Temkin MI, Pyzhev V. Kinetics of ammonia synthesis on promoted iron catalyst. Acta Phys Chim USSR. 1940;12(1):217–222.
- Malek NNA, Jawad AH, Abdulhameed AS, et al. New magnetic Schiff’s base-chitosan-glyoxal/fly ash/Fe3O4 biocomposite for the removal of anionic azo dye: an optimized process. Int J Biol Macromol. 2020;146:530–539. doi: 10.1016/j.ijbiomac.2020.01.020
- Duong D. Adsorption analysis equilibria and kinetics 2. London: Imperial College; 1998.
- Aia L, Zhang C, Liao F, et al. Removal of methylene blue from aqueous solution with magnetite loaded multi-wall carbon nanotube: kinetic, isotherm and mechanism analysis. J Hazard Mater. 2011;198:282–290. doi: 10.1016/j.jhazmat.2011.10.041
- Jia Z, Li Z, Li S, et al. Adsorption performance and mechanism of methylene blue on chemically activated carbon spheres derived from hydrothermally-prepared poly(vinyl alcohol) microspheres. J Mol Liq. 2016;220:56–62. doi: 10.1016/j.molliq.2016.04.063
- Zhang Z, Xu X. Wrapping carbon nanotubes with poly (sodium 4-styrenesulfonate) for enhanced adsorption of methylene blue and its mechanism. Chem Eng J. 2014;256:85–92. doi: 10.1016/j.cej.2014.06.020
- Jawad AH, Mallah SH, Mastuli MS. Adsorption behavior of methylene blue on acid-treated rubber (Hevea brasiliensis) leaf. Desalin Water Treat. 2018;124:297–307. doi: 10.5004/dwt.2018.22915
- Jawad AH, Rashid RA, Ishak MAM, et al. Adsorptive removal of methylene blue by chemically treated cellulosic waste banana (Musa sapientum) peels. J Taibah Univ Sci. 2018;12(6):809–819. doi: 10.1080/16583655.2018.1519893
- Jawad AH, Mohammed SA, Mastuli MS, et al. Carbonization of corn (Zea mays) cob agricultural residue by one-step activation with sulfuric acid for methylene blue adsorption. Desalin Water Treat. 2018;118:342–351. doi: 10.5004/dwt.2018.22680
- Low LW, Teng TT, Ahmad A, et al. A novel pretreatment method of lignocellulosic material as adsorbent and kinetic study of dye waste adsorption. Water Air and Soil Pollut. 2011;218:293–306. doi: 10.1007/s11270-010-0642-3
- Pathania D, Sharma S, Singh P. Removal of methylene blue by adsorption onto activated carbon developed from Ficus carica bast. Arab J Chem. 2017;10:S1445–S1451. doi: 10.1016/j.arabjc.2013.04.021
- Lata H, Garg VK, Gupta RK. Removal of a basic dye from aqueous solution by adsorption using Parthenium hysterophorus: an agricultural waste. Dyes Pigm. 2007;74:653–658. doi: 10.1016/j.dyepig.2006.04.007
- Ho YS, Malaryvizhi R, Sulochana N. Equilibrium isotherm studies of methylene blue adsorption onto activated carbon prepared from Delonix regia pods. J Environ Prot Sci. 2009;3:1–6.
- Mahadeva Swamy M, Nagabhushana BM, Hari Krishna R, et al. Fast adsorptive removal of methylene blue dye from aqueous solution onto a wild carrot flower activated carbon: isotherms and kinetics studies. Desalin Water Treat. 2017;71:399–405. doi: 10.5004/dwt.2017.20520
- Karagöz S, Tay T, Ucar S, et al. Activated carbons from waste biomass by sulfuric acid activation and their use on methylene blue adsorption. Bioresour Technol. 2008;99:6214–6222. doi: 10.1016/j.biortech.2007.12.019

![Figure 5. Influence of SATCS dosage on MB dye removal (V: 100 mL; [MB] = 200 mgL−1; solution pH: 5.6, T = 303 K).](/cms/asset/99710c14-0823-4a1a-8c0f-38bed6c693a0/tusc_a_1736767_f0005_ob.jpg)
![Figure 6. Influence of pH on MB dye uptake using SATCS (V: 100 mL; [MB] = 200 mgL−1; SATCS dosage: 0.1 g/100 mL, T = 303 K).](/cms/asset/6dd61085-cc24-4e76-a079-9e14ed60b23a/tusc_a_1736767_f0006_ob.jpg)