ABSTRACT
The aim of the study was to evaluate the effect of Duvalia corderoyi (D. corderoyi) extracts in oxidative stress status and histopathology of liver and kidney tissues in streptozotocin (STZ)-induced diabetic rats. Fifty male Wistar albino rats were injected intraperitoneally (IP) with STZ. Rats with fasting blood glucose levels of 250 mg/dl and above were considered diabetic. Rats were subsequently fed orally with D. corderoyi extracts (100,200 mg/kg bw) for 30 days. Fasting blood glucose, insulin, and oxidative status in liver and kidney tissues were measured and histopathological analysis was performed. The blood glucose and malondialdehyde levels were decreased and the insulin, glutathione reductase, glutathione peroxidase, catalase, and superoxide dismutase levels were increased in D. corderoyi treated diabetic rats in comparison to those in untreated diabetic rats. Moreover, D. corderoyi relieved the injury in liver and kidney tissues of treated diabetic rats. Administration of D. corderoyi led to significant ameliorative effects on the oxidative stress and histopathology of liver and kidney tissues of D. corderoyi treated diabetic rats.
1. Introduction
Diabetes mellitus is one of most common chronic disease that has a significant influence on human health and the quality of life of millions of peoples. Worldwide, the number of people with diabetes will be 552 million by 2030 according to predications of International Diabetes Federation (IDF) It is characterized by hyperglycaemia that caused by defects in insulin secretion, insulin action, or both. Continuous hyperglycaemia leads to several macro and microvascular complications [Citation1].
In fact, it is believed that oxidative stress has a crucial role in the progress of vascular complications that related to diabetes especially type 2 diabetes. Oxidative stress is produced by advanced glycation products (AGEs) and the formation of the free radical caused by unsaturated lipids auto-oxidation take place in the plasma and membrane proteins [Citation2]. The formation of reactive oxygen species (ROS) occurs through physiological processes by either non-enzymatic or enzymatic pathways, those results in continuous damage to cellular proteins, lipids, and nucleic acids [Citation3]. The free radicals contribute to the development of diabetes mellitus [Citation4]. The increased oxidative stress in patients with diabetes mellitus may be a common pathway linking diverse mechanisms for the pathogenesis of complications in diabetes [Citation2]. Furthermore, diabetes-induced tissue content changes and altered the antioxidant enzyme activity [Citation5], leads to the development of complications such as altered antioxidant defences [Citation6,Citation7]. The antioxidant defence system protects cells from damages caused by increased ROS production, and its efficiency in counterbalancing ROS production determines the level of cell damage [Citation8]. The reactive oxygen species produced in the cells are scavenged by antioxidant enzymes [Citation9].
On the other hand, natural and traditional medicinal plants are of great importance source of medicine throughout the world. Many populations consider them as a potential resource used in the treating of several diseases with less side effects compared to synthetic medications. Moreover, they are more easily available, particularly in the case of inadequate accessibility or affordability to pharmaceutical medicines [Citation10–12]. Recently, there has become an urgent need to use medicinal plants as a medicine that are usually used in traditional treatment due to the side effects associated with manufactured diabetes medications and relatively low cost [Citation13]. Many studies documented the positive effects of some plant extracts, as they showed that Syzygium paniculatum Gaertn had a role in reducing oxidative stress in streptozotocin-induced diabetic rats [Citation14], as well as in Falcaria vulgaris extract had a nephroprotective property and improve renal structural and blood biomarkers in diabetic male mice [Citation15]. In addition, mentha aquatic had effect antidiabetic and against oxidative stress [Citation16], and Antidiabetic effect in diabetes rats using Duvalia corderoyi extract [Citation17]. The subfamily Asclepiadoideae comprises approximately 3000 species in 172 genera [Citation18,Citation19], including Duvalia, Orbea, Huernia, Caralluma, Hoodia, Stapelia, Echidnopsis, Edithcolea, Frerea [Citation20–22]. Gilbert divided the Caralluma group in to 4 genera based on stem and flower morphology [Citation23]. They are (1) Frerea, (2) Caralluma, (3) Pachycymbium, and (4) Duvalliandra, and some more species were moved to Duvalia and Rhytidocaulon. The species Duvalia corderoyi (D. corderoyi) or Stapelia corderoyi belongs to the genus Duvalia (Stapelieae) that follows Asclepiadaceae family. They are stem succulents distributed between the Cape and Arabia [Citation24], they are found in Arabian peninsula, Madagascar, Asia, Yemen, Saudi Arabia, Somalia, Namibia, Swaziland, and Zimbabwe, and has been used in traditional medicine as antipyretic, antidiabetic, and in the treatment of pustule and ulcerative stomatitis, it has also been used as food [Citation25,Citation26]. The current study was designed as a first-time investigation to observe the effects of D. corderoyi on oxidative stress and tissue protection activities in streptozotocin (STZ)-induced diabetic rats.
2. Materials and methods
2.1. Plant collection
D. corderoyi stem was collected from different source of Alawd area – Ibb governorate in Yemen and Kingdom of Saudi Arabia KSA during June–August 2018. Plant sample was air dehydrated for one month, milled, packaged in polyethylene bags and stored at 4°C in a dark place until later use.
2.2. Preparation of methanol extract of Duvalia corderoyi (MDC)
The powdered D. corderoyi was deeply soaked in methanol for 72 h to allow appropriate extraction at 15–25°C with agitation at intervals. The mixture was filtered with filter paper. The filtrate was concentrated and evaporated to dryness at 40°C by a rotary evaporator to get good yield. The yield was calculated, conserved in polyethylene sacs and stored at 4°C in a dark place until further use [Citation27].
2.3. Chemicals
Glimiperid was obtained from (UFC Biotechnology, BUFFALO, NY, United States), and STZ was purchased from (ThermoFisher (Kandel) GmbH, Karlsruhe, Germany) All drugs were stored under recommended temperature (−20°C). Carboxymethyl cellulose (CMC) obtained from (Loba-Chemical, Mumbai, India). Phosphate buffered saline (PBS) were brought from (Hoefer Inc, San Francisco, USA).
2.4. Animals
Fifty male Wistar albino rats that weighed (300 g ± 20) were obtained from Experimental Animal Care Centre, College of Pharmacy, King Saud University, Saudi Arabia. Rats were individually kept in stainless steel cages under controlled environmental conditions; temperature (22°C), humidity (50–55%) and light cycle 12 h dark/light. Euthanasia procedure was conducted in accordance with Guide for care and use of laboratory animals, institute for laboratory animal research, National Institute of Health (NIH publication No. 80–23; 1996).
2.5. Diabetes induction
After the acclimatization period, the rats were fasted overnight, and four groups of rats (each group consisted of 10 rats) were induced by single intraperitoneal injection (IP) with freshly dissolved STZ in citrate buffer (0.1 M, pH = 4.5) at a dose of 60 mg/kg according to the method of Poodineh et al. [Citation28], while control group were injected with an equivalent volume of citrate buffer (0.1 M, pH = 4.5), after 72 h glucose level were measured using glucometer (ACCU-CHEK Active, Germany), by loading of one drop of blood from tail vein incision to glucometer strips, and rats with 250 mg/dl or above of glucose were considered as diabetic according the method described by Sadri et al. [Citation1].
2.6. Treatments
All rats were fed a standard meal and randomly divided into five groups, each group consisted of 10 rats; the first group was control, while the STZ-induced diabetic rats were divided into 4 groups, each group consisted of 10 rats: the first group was control, the 2nd group was diabetic control (MD1), the 3rd (GBC) group was treated orally with glibenclamide (600 μg/kg bw/rat/day), and the 4th (MDC100) and 5th (MDC200) groups were fed with D. corderoyi extract at 100 mg/kg bw/rat/day and 200 mg/kg bw/rat/day concentrations respectively for 30 days.
2.7. Samples collection and preparation
At the end of the experimental period, the rats were anesthetized, sacrificed, and then blood samples were collected through cardiac puncture in a plain tube, the serum were obtained by centrifugation at 3000 rpm for 10 min, serum samples were stored at −20°C until the time of analysis. Liver and kidney were also removed from the rats and washed several times with ice cold 0.1 M phosphate buffered saline, and preserved in aluminium foil and all samples were kept at deep freezer (−80°C) until further processing. For preparation of tissue homogenates, pieces of liver and kidney weighted and homogenized in 10-fold volume of ice cold (PBS) (PH = 7.4) by homogenizer (Ultra-Turrax IKA, Deutschland, Germany) at a high speed for 5–10 s, then the homogenate was centrifuged at 10.000 × g for 15 min at 4°C, homogenates and the collected supernatant was estimated the total protein [Citation29], and then stored in a deep freezer (−80°C) until further use.
2.8. Biochemical measurements
Glutathione reductase (GSH), Glutathione Peroxidase (GSH-Px), Superoxide dismutase (SOD) and Malondehyde (MDA) were assayed by Rat ELISA Kit (MyBioSource, San Diego, California, USA) according to the manufacturer’s instructions. Catalase activity in liver and kidney tissues was evaluated by kit (Cayman Chemical Company, Ann Arbor, Michigan, USA) according to the manufacturer’s instructions.
2.9. Histology
The animals were sacrificed and livers were quickly removed under ethyl ether anaesthesia and fixed in 10% neutral buffered formalin for 72 h. The fixed specimens were processed overnight for dehydration, clearing and impregnation using an automatic tissue processor (Sakura, Japan). The specimens were embedded in paraffin blocks using embedding station (Sakura, Japan) and sections of 4-micron thickness were cut using rotary microtome (Leica-RM2245, Germany) and an Autostainer (Leica5020, Germany) was used for Hematoxylin & Eosin staining. The stained sections were observed under light microscopy Eclipse BOi (Nikon, Japan) and images were acquired with digital microscopic mounted camera (OMX1200C) from (Nikon, Japan).
2.10. Statistical analysis
Values are given as means ± standard deviation (SD). Data were analysed by one-way analysis of variance ANOVA followed by Duncan’s Multiple Range Test using SPSS version 21 (SPSS, Chicago, IL). The differences were considered as statistically significant at p ≤ 0.05.
3. Results
3.1. Effect of D. corderoyi treatment on liver GSH
Glutathione reductase (GSH) concentration was significantly higher in liver homogenates of control rats in comparison to that of all STZ-induced diabetic rat groups (p ≤ 0.05) (Figure ). However, within the diabetic rat groups, while GSH concentration were significantly increased in GBC rats in comparison to diabetic control rats (p ≤ 0.05), there was no significant differences between GSH concentrations of both D. corderoyi treated groups and GBC group (p ≤ 0.05) (Figure ). Administration of D. corderoyi 200 mg/kg.bw was more effective in controlling liver GSH levels in comparison to D. corderoyi 100 mg/kg.bw and GBC but the differences were not statistically significant (Figure ).
Figure 1. Effect of D. corderoyi extract on Glutathione reductase (GSH) and Glutathione Peroxidase (GSH-Px) levels in the liver of streptozotocin-induced diabetic rats. Data are expressed as mean ± SD. *** = p ≤ 0.001, ** = p ≤ 0.01, * = p ≤ 0.05 are p values of the significant difference in different treatment rat groups in comparison to the normal control. The letters indicate significant difference from diabetic rats.
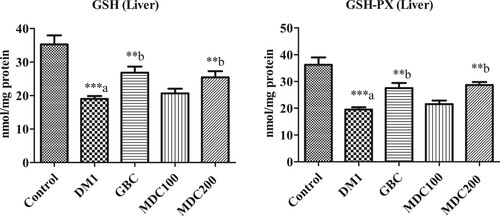
3.2. Effect of D. corderoyi treatment on liver GSH-px
GSH-Px levels also decreased significantly (p ≤ 0.05) in streptozotocin-induced diabetic rats with respect to normal controls, and there was a high significant increase in GSH-Px levels in diabetic rats that treated with 200 mg/kg b.w. (Figure ). D. corderoyi extracts and GBC in comparison to the diabetic control DM1. In contrast, there was no significant difference in liver GSH of diabetic rats treated with 100 mg/kg b.w. of D. corderoyi (MD100) compared to the diabetic control group (p ≤ 0.05) (Figure ).
3.3. Effect of D. corderoyi treatment on liver CAT
Induction of diabetes caused a significant decrease in the concentration of liver catalase CAT in rats in comparison to the non-diabetic control group (p ≤ 0.05) (Figure ). CAT levels were further higher in rats administered with GBC and 200 mg/kg D. corderoyi (MDC200) in comparison to diabetic control (DM1) rats (p ≤ 0.05 level) (Figure ). CAT level was also higher, though not statistically significant, in 100 mg/kg D. corderoyi (MDC100) rats in comparison to diabetic control rats (DM1) (Figure ).
Figure 2. Effect of D. corderoyi extract on Catalase activity and Superoxide Dismutase Enzyme (SOD) in liver of streptozotocin-induced diabetic rats. Data are expressed as mean ± SD. *** = p ≤ 0.001, ** = p ≤ 0.01, * = p ≤ 0.05 are p values of the significant differences in different treatment rat groups in comparison to normal control. The letters indicate significant difference from diabetic rats.
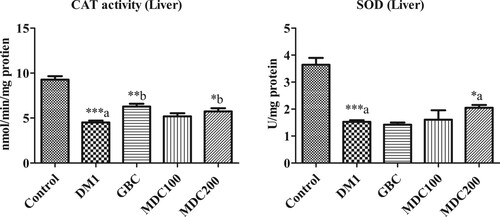
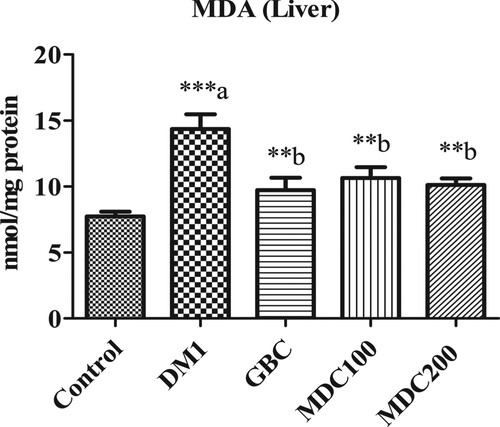
Figure 3. Effect of D. corderoyi extract on Malondialdehyde (MDA) in liver of streptozotocin-induced diabetic rats. Data are expressed as mean ± SD. *** = p ≤ 0.001, ** = p ≤ 0.01, * = p ≤ 0.05 are the p values of significant difference in comparison of different treatment groups with normal control rat groups. The letters indicate significant difference from diabetic rats.
3.4. Effect of D. corderoyi treatment on liver SOD
Superoxide Dismutase (SOD) activity was significantly elevated in diabetic control rats (DM1), GBC administered rats and MDC100 rats in comparison to diabetic rats (p ≤ 0.05) (Figure ). However, the difference, although significant, were lower in MDC200 rats in comparison to non-diabetic control rats (p ≤ 0.05) (Figure ).
3.5. Effect of D. corderoyi treatment on liver MDA
Rates of lipid oxidation, measured by assessing Malondialdehyde (MDA) levels (Figure ), were significantly higher in diabetic rats in comparison to control rats (p ≤ 0.05). The administration of D. corderoyi with both concentrations (MD100 and MD200) and GBC lead to significant diminishing of malondialdehyde levels in STZ-induced diabetic rats in comparison to diabetic control (DM1) (p ≤ 0.05). There were no significant differences in MDA levels between all other diabetic groups (GBC, MD100 and MD200).
3.6. Effect of D. corderoyi treatment on kidney GSH
Glutathione reductase (GSH) in kidney homogenate of STZ-induced diabetic rats (DM1) were decreased significantly in comparison to non-diabetic control rats (p ≤ 0.05) (Figure ). However, there were no significant differences between control and all treated (GBC, MDC100 and MDC200) groups (Figure ). D. corderoyi and GBC enhanced GSH in rats’ kidney significantly in comparison to control diabetic (DM1) rats’ group (p ≤ 0.05) (Figure ). No significant differences were found between rat kidney GSH levels amongst all treated groups (GBC, MDC100 and MDC200) (Figure ).
Figure 4. Effect of D. corderoyi extract on Glutathione reductase (GSH) and Glutathione Peroxidase (GSH-Px) in kidney of streptozotocin-induced diabetic rats. Data are expressed as mean ± SD. *** = p ≤ 0.001, ** = p ≤ 0.01, * = p ≤ 0.05 are the p values for sign.
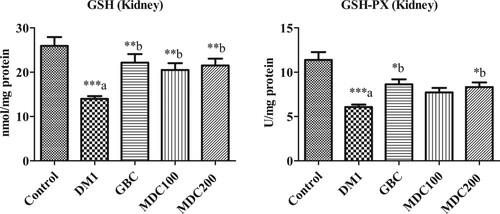
Figure 5. Effect of D. corderoyi extract on Catalase activity and Superoxide Dismutase Enzyme (SOD) in kidney of streptozotocin-induced diabetic rats. Data are expressed as mean ± SD. *** = p ≤ 0.001, ** = p ≤ 0.01, * = p ≤ 0.05 are the p values of significant difference in the different treatment groups in comparison to normal control rat groups. The letters indicate significant difference from diabetic rats.
3.7. Effect of D. corderoyi treatment on kidney GSH-px
Glutathione Peroxidase (GSH-Px) were significantly decreased in kidney of diabetes-induced rats (DM1) and rats that received different treatments (GBC, MDC100 and MDC200) in comparison to the control rat groups (p ≤ 0.05) (Figure ). Nevertheless, the administration of GBC and 200 mg/kg D. corderoyi (MDC200) increased GSH-Px in rat kidney significantly in comparison diabetic control rat that received no treatments (DM1) (p ≤ 0.05 level) (Figure ). GSH-Px activity was also higher in rats that were administered with 100 mg/kg D. corderoyi (MDC100) in comparison to diabetic control (DM1) rats though the differences were not statistically significant (Figure ).
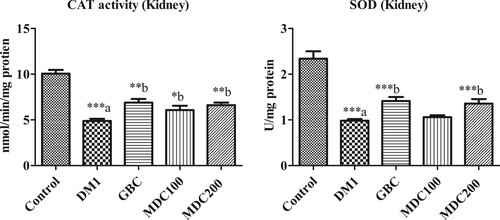
3.8. Effect of D. corderoyi treatment on kidney CAT
The catalase activity (CAT) in kidney of control rats was significantly higher than that of all streptozotocin-induced diabetic rat groups (p ≤ 0.05) (Figure ). However, the CAT was elevated in the D. corderoyi and GBC administered rat group in comparison to the diabetic control group (DM1) (p ≤ 0.05) (Figure ). There were no significant differences in CAT between using 200 mg/kg.bw of D. corderoyi (MDC200) and GBC (p ≤ 0.05), but both of these treatments had significantly elevated CAT in comparison to 100 mg/kg.bw (MDC100) (p ≤ 0.05) (Figure ).
3.9. Effect of D. corderoyi treatment on kidney SOD
Superoxide Dismutase enzyme (SOD) were significantly decreased in the kidney of all diabetic rat groups in comparison to non-diabetic control rats (p < 0.05) (Figure ). SOD levels in D. corderoyi treated diabetic rats were high in comparison to diabetic control rats, although the differences were not statistically significant (Figure ).
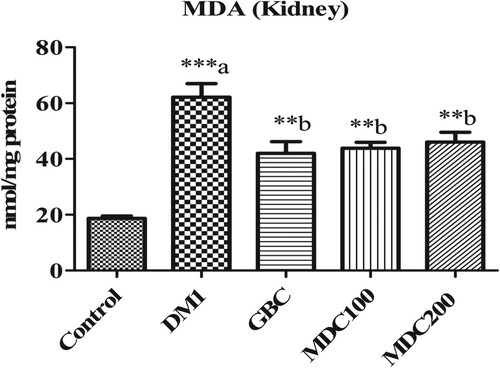
3.10. Effect of D. corderoyi treatment on kidney MDA
Malondialdehyde (MDA) was expressed at significantly higher concentration in the kidney of diabetic rats (DM1) in comparison to non-diabetic control rats (p ≤ 0.05) (Figure ). However, D. corderoyi and GBC-treated diabetic mice had significantly diminished MDA levels in comparison to control diabetic rats (DM1) (p ≤ 0.05 level) (Figure ). There were no significant differences in MDA between all the different treatment groups (GBC, MDC100 and MDC200) (Figure ).
Figure 6. Effect of D. corderoyi extract on Malondialdehyde (MDA) in kidney of streptozotocin-induced diabetic rats. Data are expressed as mean ± SD. *** = p ≤ 0.001, ** = p ≤ 0.01, * = p ≤ 0.05 are the p values of significant differences in the different treatment groups in comparison to the normal control group. The letters indicate significant difference from diabetic rats.
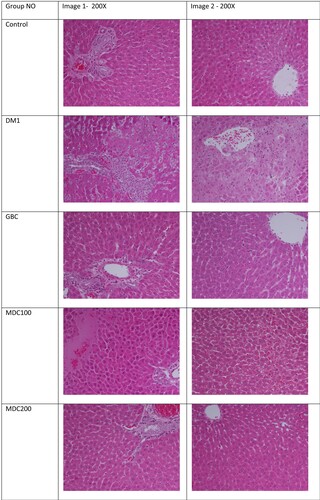
3.11. Effect of D. corderoyi treatment on liver histopathology
Histological examinations of liver were conducted to understand the effect of different treatments on liver tissue histopathology (Figure ). Based on the histological examinations, control groups had normal hepatic tissues, central vein and normal blood sinusoids, and while diabetic control rats had severe liver damages which were observable in the form of focal degeneration of hepatocytes, dilation of liver sinusoids and high inflammatory cells diffusion around portal triad areas. GBC-treated diabetic rats had lesser liver damages, little inflammatory cells diffusion and milder infiltration in comparison to control diabetic rats (DM1). MDC100 rat group also had mild degeneration of hepatic tissues with existence of nucleated hepatocytes, which as indication of remedy progress against diabetes induce liver pathology. MDC200 rats had normal hepatic architecture, although blood vessels congestion was noticed. Overall, protective effects of MD200 treatment on the rats’ liver histopathology were general better than that of MDC100 rats.
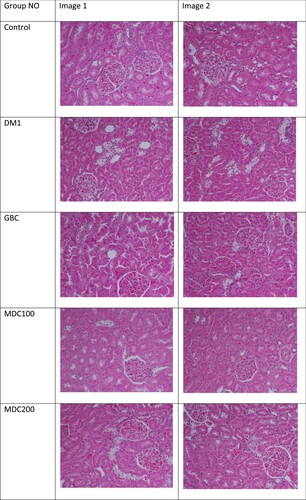
3.12. Effect of D. corderoyi treatment on kidney histopathology
Histological examinations on kidney tissues were conducted on all mice groups to understand the effect of receiving treatments such as D. corderoyi on diabetes-induced kidney histopathology (Figure ). Histological section of kidney from control rats showed the phenotype of normal renal tissues. However, histological sections of control diabetic rats (DM1) revealed severe renal injury and all cell types of the kidney such as mesangial cells, podocytes and tubulointerstitial cells were affected and glomerular and tubular hypertrophy were also noticeable in addition to tubular dilation. GBC treatment of diabetic rats reduced the diabetes-induced dilation of proximal tubules. D. corderoyi (100 mg) treated diabetic rats’ group did not reveal significant alterations in renal tissues, but D. corderoyi (200 mg) treated rats group exhibited normal tubular and glomerular integrity with insignificant cellular infiltration.
4. Discussion
The imbalanced status between the endogenous and exogenous levels of antioxidants and pre-oxidants caused by the augmentation of lipid peroxidation, hyperglycaemia, enzymatic and oxidative glycation of proteins and decrease of antioxidant enzymes such as SOD, CAT and GSH may result from generation of free radicals because of pressure situation in STZ-induced diabetic rats. There is cumulative indication that difficulties associated with diabetes may be associated to oxidative stress. Previous studies have associated oxidative stress to have an important role in the complications of diabetes, and oxidative stress that occurs because of a hyperglycaemic state caused by lipid peroxidation of the cell membrane, thus damaging the DNA [Citation9, Citation30]. Certain pathological circumstances, like hyperglycaemia, obesity and hyperlipidemia, have shown to promote oxidative stress through higher ROS formation and/or reduced antioxidant defences [Citation31]. The mechanism of enhanced oxidative stress might be due to protein glycation and inhibition of antioxidant enzyme activities (superoxide dismutase and glutathione peroxidase) [Citation32].
The antioxidant activity of D. corderoyi in this study is attributed to its high content of phenolic compounds. Phenolic compounds have been identified in the appendix volatiles and in the floral volatiles of D. corderoyi N.E.Br. and Hoodia currorii (Hook.) [Citation33]. Many species such as D. corderoyi have relatively high amounts of distinct volatile compounds such as phenol. Phenol and 2-heptanol together explained 7% of total variation and were characteristic for D. corderoyi and H. currorii, respectively [Citation34]. Aloe vera treatment resulted in a significant increase in RG, SOD, CAT, GPx and GST in the liver and kidney of diabetic rats [Citation35]. Pervious study reported that Caralluma tuberculata have antihyperglycaemic and antioxidant activities of in STZ-induced diabetic rats [Citation36]. D. corderyoi has many similarities in its odour composition with Desmidorchis (Caralluma) flava [Citation34]. Similar to the SOD and CAT activities of D. corderyoi in this study, the specific SOD and CAT activities of the Caralluma flava extract were 14.2 ± 1.18 units/mg protein and 27.2 ± 1.51 mmole of H2O2 consumed/min/mg protein, respectively. The GPx activity of the C. flava extract was found to be 34.81 ± 2.14 [Citation37].
Red blood cells of diabetic patients contain low levels of the reduced form of glutathione (GSH) and a marked reduction of the GSH/GSSG ratio, improvement of glycaemic control, e.g. by sulfonylureas, and enhanced GSH concentrations [Citation38,Citation39]. Decreased levels of liver and kidney GSH during diabetes is associated to increased oxidative stress [Citation40]. In this study, GSH increased significantly in the liver and kidney of D. corderoyi treated diabetic rats. This suggests a possibility that administration of D. corderoyi may either induce GSH biosynthesis or reduce the oxidative stress by inducing other antioxidant compounds mediated by D. corderoyi or via D. corderoyi induced increase in the glucose levels, or by a combination effect of all of these mechanisms. A significant increase in GPx and CAT activities in the liver and kidney of the rats that were treated with D. corderoyi was associated with decrease in glucose and increase in insulin levels. This study agrees with others findings, which revealed increased resistance to oxidative stress by insulin-secreting cell lines and islets of diabetic mouse models over- expressing GPx or CAT [Citation41,Citation42].
Oxidative stress is implicated in the aetiology and progression of diabetes mellitus and it damages various organs of body like brain, liver, heart, kidney, eyes and others. The present study indicates D. corderoyi as having an antioxidative effect in STZ-induced diabetic rats. In support of this, diabetic rats that received D. corderoyi extracts had greatly reduced MDA concentration in each of liver and kidney tissues in comparison to the diabetic control rats that received no other treatments. Tissue damage by peroxidation has been described in the pathogenesis and complications of both diabetes types previously. The kidneys and hearts of diabetic rats suffered from increased oxidative damage [Citation43,Citation44]. The degenerative changes in the histology of liver with abnormal localization and infiltration of hepatocytic nuclei were observed in STZ-induced diabetes [Citation44]. The seed extract of Vitis vinifera is known to prevent liver damage due to oxidative stress, which may contribute towards improved liver function and histology [Citation45].
In our study, treatment with D. corderyoi extracts and GBC reverted the increased peroxide levels to control levels, and relieved the liver and kidney tissues from diabetes-induced damage, the results of histological analysis of liver and kidney tissues showed advanced damage in diabetic control rats, and the treatment with D. corderyoi minimized the injury of liver and kidney tissues to levels similar to that of a normal control group. Results from this study suggest that antioxidant enzymes appear to be an important factor in cellular defences against oxidative damage of liver and kidney tissues of STZ-induced diabetic rats.
5. Conclusions
In conclusion, our data clearly demonstrated the protective effects of D. corderyoi against oxidative stress through increased antioxidant enzymes activities in diabetic rats and suppressed the levels of ROS in liver and kidney of STZ-induced diabetic rats. D. corderyoi also succeeded in decreasing liver and kidney injuries principally through its antidiabetic and antioxidant properties. Thus, it could be useful as a natural product in biotherapy and in the pharmaceutical industry. Further studies are necessary to determine its chemical composition, antioxidant and other potentials and are necessary towards better exploitation of the traditional uses of D. corderyoi.
Acknowledgments
This research was funded by The Deanship for Scientific Research at Princess Nourah bint Abdulrahman University (PNU), Riyadh, The Kingdom of Saudi Arabia (Grant #39-S-242).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Sadri H, Goodarzi MT, Salemi Z, et al. Antioxidant effects of Biochanin A in streptozotocin induced diabetic rats. Braz Arch Biol Technol. 2017;60:1–10. doi: 10.1590/1678-4324-2017160741
- Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405
- Radák Z, Kaneko T, Tahara S, et al. The effect of exercise training on oxidative damage of lipids, proteins, and DNA in rat skeletal muscle: evidence for beneficial outcomes. Free Radic Biol Med. 1999;27:69–74. doi: 10.1016/S0891-5849(99)00038-6
- Bambolkar S, Sainani GS. Evaluation of oxidative stress in diabetics with or without vascular complications. J Assoc Physicians India. 1995;43:10–12.
- Asayama K, Kayashibe H, Dobashi K, et al. Antioxidant enzyme status and lipid peroxidation in various tissues of diabetic and starved rats. Diabetes Res. 1989;12:85–91.
- Wohaieb SA, Godin DV. Alteration in free radical tissue defense mechanism in streptozotocin-induced diabetes in rat. Diabetes. 1987;36:1014–1018. doi: 10.2337/diab.36.9.1014
- Oberly LW. Free radicals and diabetes. Free Radic Biol Med. 1988;5:113–124. doi: 10.1016/0891-5849(88)90036-6
- Yoon GA, Park S. Antioxidant action of soy isoflavones on oxidative stress and antioxidant enzyme activities in exercised rats. Nutr Res Pract. 2014;8:618–624. doi: 10.4162/nrp.2014.8.6.618
- Genet S, Kale RK, Baquer NZ. Alterations in antioxidant enzymes and oxidative damage in experimental diabetic rat tissue; effect of vanadate and fenugreek (Trigonella faenum graecum). Mol Cell Biochem. 2002;236:7–12. doi: 10.1023/A:1016103131408
- Dorman HJD, Deans SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol. 2000;88:308–316. doi: 10.1046/j.1365-2672.2000.00969.x
- Hoet S, Opperdoes F, Brun R, et al. Natural products active against African trypanosomes: a step towards new drugs. Nat Prod Rep. 2004;21:353–364. doi: 10.1039/b311021b
- Sakkir S, Kabshawi M, Mehairbi M. Medicinal plants diversity and their conservation status in the United Arab Emirates (UAE). J Med Plants Res. 2012;6:1304–1322.
- Vishwakarma SL, Sonawane RD, Rajani M, et al. Evaluation of effect of aqueous extract of Enicostemma littorale Blume in streptozotocin-induced type 1 diabetic rats. Indian J Exp Biol. 2010;48:26–30.
- Konda PY, Dasari S, Konanki S, et al. In vivo antihyperglycemic, antihyperlipidemic, antioxidative stress and antioxidant potential activities of Syzygium paniculatum Gaertn in streptozotocininduced diabetic rats. Heliyon. 2019;5:e01373. doi: 10.1016/j.heliyon.2019.e01373
- Zangeneh M, Zangeneh A, Tahvilian R, et al. Antidiabetic, hematoprotective and nephroprotective effects of the aqueous extract of Falcaria vulgaris in diabetic male mice. Arch Biol Sci. 2018;70(00):27–27.
- Konda PY, Egi JK, Dasari S, et al. Ameliorative effects of mentha aquatica on diabetic and nephroprotective potential activities in STZ-induced renal injury. Comp Clin Path. 2020;29:189–199. doi: 10.1007/s00580-019-03042-6
- AlFaris N, Alshammari G, Alsayadi M, et al. Antidiabetic and antihyperlipidemic effect of Duvalia corderoyi in rats with streptozotocin-induced diabetes. Saudi J Biol Sci. 2020;27(3):925–934. doi: 10.1016/j.sjbs.2020.01.024
- Meve U. Species numbers and progress in asclepiad taxonomy. Kew Bull. 2002;57:459–464. doi: 10.2307/4111126
- Endress ME, Liede-Schumann S, Meve U. Advances in Apocynaceae: the enlightment, an introduction. Ann Missouri Bot Gard. 2007;94:259–267. doi: 10.3417/0026-6493(2007)94[259:AIATEA]2.0.CO;2
- Thiv M, Meve U. A phylogenetic study of Echidnopsis Hook. f. Apocynaceae-Asclepiadoideae) – taxonomic implications and the colonization of the Socotran archipelago. Plant Syst Evol. 2007;265:71–86. doi: 10.1007/s00606-007-0516-3
- Bruyns PV. A new species of Caralluma (Apocynaceae–asclepiadoideae–ceropegieae) from the Yemen. S Afr J Bot. 2010;76:249–251. doi: 10.1016/j.sajb.2009.11.001
- Meve U, Liede S. A molecular phylogeny and generic rearrangement of the stapelioid Ceropegieae (Apocynaceae-Asclepiadoideae). Plant Syst Evol. 2002;234:171–209. doi: 10.1007/s00606-002-0220-2
- Gilbert MG. A review of Caralluma R. Br. and its segregates. Bradleya. 1990;8:1–32. doi: 10.25223/brad.n8.1990.a1
- Meve U. The genus Duvalia (Asclepiadaceae) – stem-succulents between the Cape and Arabia. Plant Syst Evol Suppl. 1997;10:1–132.
- Oldfield Sara (camp.). Cactus and succulent plants – status survey and conservation action plan. IUCN/SSC Cactus and Succulent Specialist Group. IUCN, Gland, Switzerland and Cambridge, UK. 1997; IO + 212 pp.
- Szymczak G, Kwiatkowski M. Stapeliads (Asclepiadaceae) in collection of succulents in the Botanical Garden of the Maria Curie-Sklodowska University in Lublin. Biuletyn Ogrodow Botanicznych, 2003; 12: 137-140.
- Ramachandraiahgari R, Somesula S, Adi P, et al. Protective role of ethanolic extract of aloe vera antioxidant properties on liver and kidney of streptozotocin-induced diabetic rats. Dig J Nanomater Bios. 2012;7:175–184.
- Poodineh J, Khazaei FA, Nakhaee A. Antioxidant activities of Caralluma tuberculata on streptozotocin-induced diabetic rats. Drug Devel Res. 2015;76:40–47. doi: 10.1002/ddr.21239
- Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275.
- Saxena AK, Srivastava P, Kale RK, et al. Impaired antioxidant status in diabetic rat liver: effect of vanadate. Biochem Pharmacol. 1993;45:539–542. doi: 10.1016/0006-2952(93)90124-F
- Styskal J, Remmen HV, Richardson A, et al. Oxidative stress and diabetes: what can we learn insulin resistance from antioxidant mutant mouse models? Free Radic Biol Med. 2012;52:46–58. doi: 10.1016/j.freeradbiomed.2011.10.441
- Adachi T, Ohta H, Hayashi K, et al. The site of nonenzymic glycation of human extracellular-superoxide dismutase in vitro. Free Radic Biol Med. 1992;13:205–210. doi: 10.1016/0891-5849(92)90016-A
- De MC M, Demarco D. Phenolic compounds produced by secretory structures in plants: a brief review. Nat Prod Commun. 2008;3:1273–1284.
- Jürgens A, Dötterl S, Meve U. The chemical nature of fetid floral odours in stapeliads (Apocynaceae – Asclepiadoideae –ceropegieae). New Phytol. 2006;172:452–468. doi: 10.1111/j.1469-8137.2006.01845.x
- Rajasekaran S, Sivagnanam K, Subramanian S. Antioxidant effect of Aloe vera gel extract in streptozotocin-induced diabetes in rats. Pharmacol Rep. 2005;75:90–96.
- Karthishwaran K, Al Shamisi SOSO, Kurup SS, et al. Free-radical-scavenging and antioxidant capacities with special emphasis on enzyme activities and in-vitro studies in Caralluma flava N. E. Br. Biotechnol Biotechnol Equip. 2018;32:156–162. doi: 10.1080/13102818.2017.1379362
- Murakami K, Kondo T, Ohtsuka Y, et al. Impairment of glutathione metabolism in erythrocytes from patients with diabetes mellitus. Metab Clin Exp. 1989;38:753–758. doi: 10.1016/0026-0495(89)90061-9
- Sharma A, Kharb S, Chugh SN, et al. Evaluation of oxidative stress before and after control of glycemia and after vitamin E supplementation in diabetic patients. Metab Clin Exp. 2000;49:160–162. doi: 10.1016/S0026-0495(00)91117-X
- Anuradha CV, Selvam R. Effect of oral methionine on tissue lipid peroxidation and antioxidants in alloxaninduced diabetic rats. J Nutr Biochem. 1993;4:212–217. doi: 10.1016/0955-2863(93)90054-Z
- Moriscot C, Richard MJ, Favrot MC, et al. Protection of insulin-secreting INS-1 cells against oxidative stress through adenoviral-mediated glutathione peroxidase overexpression. Diabetes Metab. 2003;29:145–151. doi: 10.1016/S1262-3636(07)70021-6
- Harmon JS, Bogdani M, Parazzoli SD, et al. Beta-cell specific overexpression of glutathione peroxidase preserves in tranuclear MafA and reverses diabetes in db/db mice. Endocrinology. 2009;150:4855–4862. doi: 10.1210/en.2009-0708
- Prince PSM, Menon VP, Pari L. Hypoglycemic activity of Syzigium cumini seeds: effect on lipid peroxidation in alloxan diabetic rats. J Ethnopharmacol. 1998;61:1–7. doi: 10.1016/S0378-8741(98)00002-6
- Tatsuki R, Satoh K, Yamamoto A, et al. Lipid peroxidation in the pancreas and other organs in streptozotocin diabetic rats. Jpn J Pharmacol. 1997;75:267–273. doi: 10.1254/jjp.75.267
- Sheweita SA, Mashaly S, Newairy AA. Changes in oxidative stress and antioxidant enzyme activities in streptozotocin-induced diabetes mellitus in rats: role of Alhagi maurorum extracts. Oxid Med Cell Longev. 2016;5264064:1–8.
- Giribabu N, Kumar K, Rekha S, et al. Vitis vinifera (Muscat variety) seed ethanolic extract preserves activity levels of enzymes and histology of the liver in adult male rats with diabetes. Evid Based Complement Alternat Med. 2015;2015(542026):1–8. doi: 10.1155/2015/542026