?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Barium hexaferrite nanoparticles are synthesized through co-precipitation technique at different annealing temperature. Series of BaFe12-xHgxO19 nanoparticles, 0.00 ≤ x ≤ 0.30, are prepared under the best verified conditions (annealed at 1000°C) to investigate the effect of partial substitution of Hg2+ ions on the physical properties of BaFe12O19 nanoparticles. The hysteresis loops at room temperature show the behavior of hard ferromagnetic. The estimated values of saturation magnetization , remnant magnetization
and magnetic moment
rise as Hg2+ ions content increases till x = 0.10, beyond which they reduce. A reverse trend is obtained for intrinsic coercivity Hi and coercivity Hc versus Hg2+ ions content. BaFe12-xHgxO19 nanoparticles are investigated through the measurements of electron paramagnetic resonance (EPR). The calculated EPR parameters show an enhancement for BaFe12-xHgxO19 nanoparticles up to x = 0.10. The obtained results reveal that samples under investigation can be suitable candidate for different industrial applications.
1. Introduction
One type of magneto-plumbite group of oxides is M-type hexagonal ferrites with formula MFe12O19 (M = Ba, Sr). Specific attention for barium hexaferrite BaFe12O19 is always increasing as a result of its large saturation magnetization, strong uniaxial magnetic anisotropy, high Curie temperature, better coercivity, good chemical stability and perfect corrosion resistivity, as well as its low price for production [Citation1]. Therefore, it plays an important role in the magnetic media as the production of permanent magnetic materials, microwave devices and magnetic recording media [Citation2–5].
The structural and magnetic properties for BaFe12O19 can be achieved either by the substitution with different ions or by using different preparation techniques. The magnetic properties of barium hexaferrite can be enhanced by the partially ions substitution, especially at Fe3+ site either with di-, tri- or tetravalent ions [Citation6,Citation7]. The partial ions substitution at Fe3+ site leads to change the net magnetic moment per unit and tailor magnetic properties of BaFe12O19. The enhancement of the net magnetization for this magnetic material can be occurred when the substituted ions occupy spin down sites [Citation8]. Many researchers [Citation9–12] have been investigated the impact of using the suitable transition ions substation ratio at Fe site on coercivity, anisotropy and saturation magnetization for barium hexaferrite. A significant change in both magnetic and structural properties was obtained as a result of the influence of transition ions on the grains growth. On the other hand, the structural and lattice parameters, crystallite and grain sizes, as well as the dislocation density of hexaferrite samples were strongly affected by the composition and preparation conditions. Several techniques can be used for synthesizing hexaferrites such as micro-emulsion [Citation13], sol–gel [Citation14,Citation15], combustion [Citation16], co-precipitation [Citation17], hydro-thermal [Citation18], etc. The most efficient technique for preparing hexaferrites is co-precipitation technique due to its low cost, energy-efficient, easy method and short reaction time [Citation19].
The aim of the present study is not only to obtain the optimum calcination temperature of the preparation of barium hexaferrite nanoparticles by the chemical co-precipitation method but also to study the effect of Hg2+ ions substitution on the structural and magnetic properties of it. In order to improve its properties to be a suitable candidate in for different industrial application.
2. Experimental techniques
2.1. Samples preparation
Barium hexaferrite nanoparticles, BaFe12O19 were synthesized by an efficient co-precipitation technique at different annealing temperature (700, 800, 900 and 1000°C) to obtain the optimum annealing temperature necessary for achieving pure phase of barium hexaferrite. The starting materials for this synthesis are barium chloride, (BaCl2.2H2O), ferric chloride (FeCl3) and sodium hydroxide (NaOH). Stoichiometric amounts ratios of these chlorides were dissolved in distilled water, then mixed through magnetic stirrer at 60°C. An alkaline solution (NaOH) was put into the salt solution till pH was reached to 12.0. The resulting solution was heated at 90°C with continuous stirring for two hours. The precipitate was collected by magnet and washed with distilled water many times until the pH of the filtrate reached to 7.0 in order to remove unwanted impurities. Then, the precipitate was dried at 80°C. Finally, the dried powders were put into a ceramic crucible and annealed at different temperatures: 700°C (B-700), 800°C (B-800), 900°C (B-900) and 1000°C (B-1000) for four hours and followed by a slow cooled to room temperature. Nanoparticle samples were finally ground to fine nano powder.
In order to investigate the effect of partial substitution of Hg2+ ions on BaFe12O19 nanoparticles, a series of BaFe12-xHgxO19 nanoparticle, x = 0.00, 0.05. 0.10, 0.20 and 0.30, were synthesized through the indicated co-precipitation technique under the best verified conditions (annealed at 1000°C).
2.2. Measurements
Phase identification of barium hexaferrite is carried out by X-ray powder diffractometer (Bruker D8 advance) with Cu-Kα radiation (λ = 1.54056 Å) in 2θ range 10–80°.
The lattice parameters and
are determined using the following formula [Citation20]:
(1)
(1) where
is the crystal face distance and (
) are the Miller indices.
The unit cell volume for barium hexaferrite can be estimated using the lattice parameters and
from the following relation:
(2)
(2) The theoretical (X-ray) density (
) and bulk density (
) of the prepared powders are determined by applying Eqs.3,4, respectively.
(3)
(3)
(4)
(4) where Z = 2 indicating 2 formula units,
is material molecular weight, m is the pellet mass, r is the pellet radius and h is the pellet thickness.
The percentage porosity () of the samples is estimated in terms of both the theoretical and bulk densities of the samples as follows [Citation21]:
(5)
(5) The average nanoparticle size is determined using Jeol transmission electron microscope JEM- 2100, carried out at 200 kV through dispersing the powders in diluted HCl on a copper grid. The elemental compositions of barium hexaferrites are studied by scanning electron microscopy (SEM/ EDS; JSM-7200F). Infrared absorption spectra of the samples are recorded in the range of 400–4000 cm−1 using FTIR 8400S Shimadzu spectrophotometer. The magnetic properties of the prepared samples are studied by vibrating sample magnetometer VSM, Lakeshore 7410, at room temperature. EPR spectra are measured by EPR spectrometer, Bruker Elexsys 500, at room temperature.
3. Results and discussion
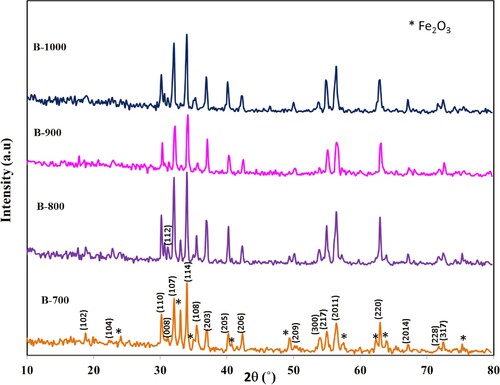
In order to study the effect of annealing temperature on phase formation, the percentage, the type of impurities formed and crystallite size were determined. XRD analysis was applied on the samples. Figure shows XRD pattern of the barium hexaferrite, BaFe12O19, samples annealed at different temperatures 700°C (B-700), 800°C (B-800), 900°C (B-900) and 1000°C (B-1000). The estimated values of crystallite size and lattice parameters at different annealing temperature are listed in Table . Table clarifies that with increasing temperature, the percentage of intermediate phase Fe2O3 decreases, while the percentage of barium hexaferrite increases until reached to single phase at annealing temperature 1000°C. The crystallite size increases with increasing annealing temperature as a result of the tendency of particles, where they are collected together and form large particles. The estimated value of lattice parameters for samples B-700, B-800 and B-900 are inconsistent with the reported values as a result of the presence of impurities beside the basic phase. On the other hand, the lattice parameters for sample B-1000 are consistent with published values [Citation22,Citation23].
Table 1. Barium hexaferrite and intermediate impurity phase percentages, lattice constants and average crystallite size for BaFe12O19 nanoparticles prepared at different annealing temperature.
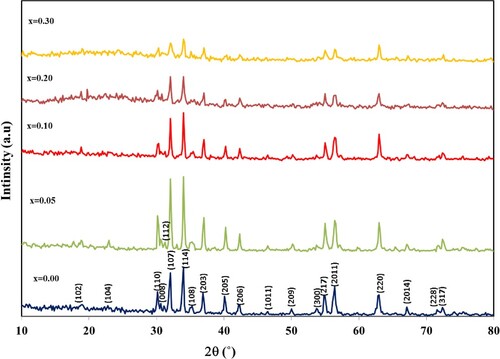
Figure shows XRD patterns of hexaferrite BaFe12-xHgxO19 nanoparticles annealed at 1000°C for 4 h, x = 0.00, 0.05, 0.10, 0.20 and 0.30. All diffraction peaks in the pattern were belonging to those of M-type barium hexaferrite BaFe12O19 with space group P63/mmc (194) from JCPDS card number 01-084-0757. From the diffraction patterns, they show the crystallite as single phase of the prepared samples, with no impurity phases. The calculated lattice parameters for undoped sample, = 5.893 Å and
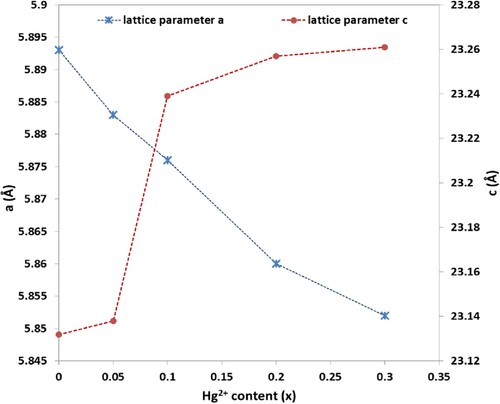
= 23.132 Å, are consistent with JCPDS card number 01-084-0757 [Citation24]. Figure shows the lattice parameters variation versus x. It is seen that the lattice parameter
increases with increasing x, while the lattice parameter
slightly decreases. The increase in
can be attributed to the partial substitution of the smaller ionic radius Fe3+ ions (0.64 Å) by larger ionic radius Hg2+ ions (1.02 Å). More variation is observed for lattice parameter
because of c-axis is easy axis which the spins can freely rotate around this axis, comparing with a-axis. The ratio
has been calculated for all the prepared samples and found to be in the range 3.925–3.983, confirming with Vegard’s law as the ratio
should be less than 3.98 for M-type hexaferrite [Citation24]. Moreover, the unit cell volume decreases from 695.688 Å3 for x = 0.00–688.106 Å3 for x = 0.30 (Table ). This decrease is due to the strongly dependence of unit cell volume on
(
α
) more than
The calculated values of X-ray density (
), bulk density (
) and percentage of porosity (
) are listed in Table versus Hg+2 ions content. It is displayed the increase of (
) and (
) with increasing x, which is attributed to the larger atomic weight and density of Hg 200.59 g mol−1 and 13.56 g cm−3, respectively, comparing with Fe of density 7.87 g cm−3 and atomic weight 55.84 g mol−1. It is observed that
is higher than
due to the presence of pores which affected by sintering temperature [Citation25] and the syntheses conditions [Citation26]. The results showed that porosity follows the inverse behaviour of
, it is found to increase as the Hg2+ ion content increases. The increase in porosity may be attributed to the diminution of oxygen vacancies or the formation of a solid solution [Citation27].
Table 2. XRD parameters of BaFe12-xHgxO19 nanoparticles annealed at 1000°C for 4 h.
The average crystallite size (L) for the prepared samples is estimated by taking the average value of the most intense diffraction peaks using the Debye Scherrer formula [Citation28] and their values are tabulated in Table . The average crystallite size is calculated from Scherer equation and is found to increase from 36.077 nm at x = 0.00 to 37.544 nm at x=0.10 and then it decreases to minimum value 26.971 nm at x = 0.30. The decreasing trend of crystallite size may be due to the high concentration of Hg2+ ions content which reduces the grain growth because of isolation on or close to the grain boundary which impedes its movement.
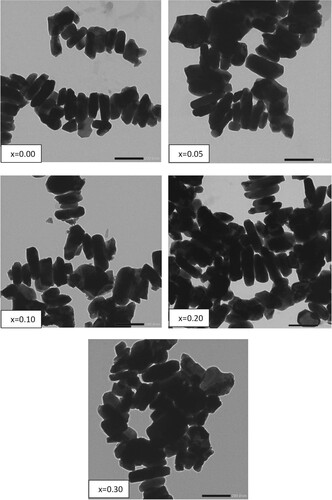
The morphologies of hexaferrite BaFe12-xHgxO19 nanoparticles are shown in Figure . It is clear that the samples are composed of approximately lamellar grains. There are agglomerations between the particles which may be due to the high magnetization and the magnetic interaction between the particles [Citation29] or related to particles chemical reaction at the time of calcination procedure. The particle size (w) of BaFe12-xHgxO19 nanoparticles obtained at 1000°C, is mainly between 41.86 and 53.29 nm. Table shows that the values of w are slightly higher than those obtained from XRD (L). This is because of X-rays can detect the individual crystallite parts into the nanoparticle, as TEM deals with the grain which might consist of a number of crystallites. As present in Table , it is clear that w has the same behaviour of L with Hg2+ ions content.
The elemental analysis of BaFe12-xHgxO19 nanoparticles is investigated by Energy dispersive X-ray analysis (EDS). The results indicate the presence of Ba, Fe, Hg, and O peaks as seen in Figure , affirming the formation of BaFe12-xHgxO19 solid solution and indicates Hg2+ ions incorporation in the BaFe12O19 host lattice. Experimental and theoretical atomic percentages (%) of BaFe12-xHgxO19 nanoparticles, x = 0.00, 0.10 and 0.30, are inserted in the EDS Figs. It is clear the rapprochement between the calculated and experimental values of the atomic percentages for all elements. FTIR absorption spectra of M-type hexaferrite BaFe12-xHgxO19 nanoparticles recorded in range of 400–4000 cm−1 are seen in Figure (a). Two main absorption bands υ1 and υ2 in the ranges 560–590 cm−1 and 430–450 cm−1 are observed which are characteristic to M-type barium hexaferrite. The band υ1 is attributed to the stretching vibrations of the tetrahedral A-site and υ2 to the octahedral B-site vibrations [Citation12]. It is observed that the values of υ1 are higher than υ2, indicating that the vibration normal mode for A-sites is larger than that of B-sites. This is attributed to shorter bond length of the A-site clusters than that of the B-site clusters [Citation30]. Also, the band υA shows up in the spectra at around 880 cm−1 and is attributed to increment in the concentration of divalent ions among the A-sites [Citation30]. The appearance of this band confirms the represents the divalent metal- oxygen vibrations and may assign to the existence of Hg2+– O2− or/and Fe2+– O2− among A-sites. Carbonyl group (C = O) with asymmetric stretching is detected at 1633 cm−1. The detection of band around 3438 cm−1 is assigned to the O–H bond of water with stretching vibration which represents the appearance of hydrogen-bonded chains [Citation31].
Figure 5. EDX spectra and elemental mapping images of BaFe12-xHgxO19 nanoparticles, x = 0.00, 0.10 and 0.30.
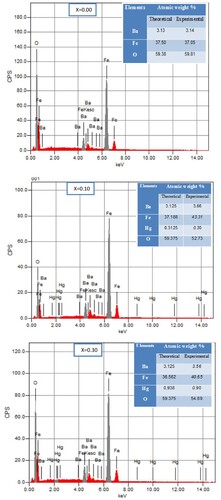
Figure 6. (a) FTIR spectra of BaFe12-xHgxO19 nanoparticles and (b) The variation of Debye temperature against Hg2+ ions content.
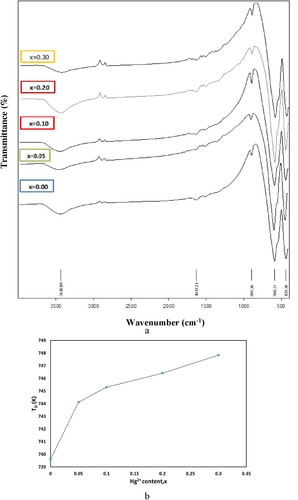
The thermal properties of M-type hexaferrite can be discussed in terms of mean value of wave number υav of the characteristic peaks υ1 and υ2 as follows [Citation32], and their values are listed in Table versus Hg+2 ions content.
(6)
(6) where
is Debye temperature in Kelvin and
is the velocity of light.
The variation of versus Hg2+ ions content is shown in Figure (b). It is demonstrated that
values show increment behaviour against x. The variation of
is attributed to the variation of the mean value of the characteristic wave number υav of IR bands [Citation33]. For n-type materials, according to specific heat theory, the conduction electrons can absorb part of the heat resulting in a decrease in Debye temperature. And therefore, the increasing trend of
confirms the reduction of conduction electrons (i.e.n-type) and increment of the holes contribution (i.e.p-type) by increasing Hg2+ ions [Citation34].
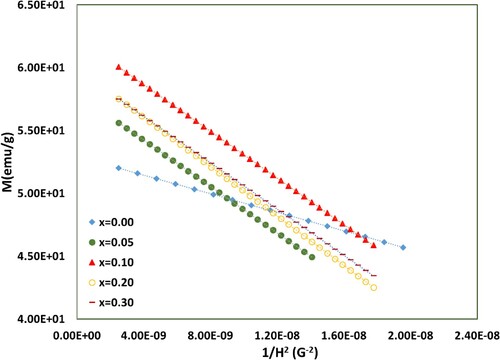
The magnetic hysteresis loops, at room temperature for BaFe12-xHgxO19 nanoparticles are shown in Figure . The magnetic measurements are carried out under an applied field of ±20 kG. It is displayed wide hysteretic behaviour curves (large coercivity Hc) which distinguish the hard magnetic materials [Citation35,Citation36]. The saturation magnetization values () are estimated from the approximation of Stoner-Wohlfarth (S-W) using the following equation [Citation37]:
(7)
(7) According to Equationeq. (7
(7)
(7) ), the plot of
versus 1/
(Figure ) leads to a straight line with slope =
. The intersection of this straight line with y-axis gives the values of saturation magnetization
. The estimated values of
and α are displayed in Table versus Hg+2 ions content. It is observed that
and remnant magnetization
rise with increasing x and attained the maximum value at x = 0.10, beyond which they decrease. The increase in
is mainly attributed to the strengthening of super-exchange interactions among nanoparticles due to the partial substitution of Fe3+ ions by the Hg2+ ions. The ions radii Hg2+ ions (1.02 Å) is larger than that Fe3+ ions (0.64 Å). This leads to decrease the different magnetic ions distances and subsequently enhance the strength of the super-exchange interactions [Citation38]. On the other hand, the Fe3+ ions in barium hexaferrite have five sites are up-spins (↑), whereas two sites are down-spins (↓) [Citation24]. The increase in
is attributed to the occupation of Hg2+ ions in the down-spin states. The net spin in upward direction consequently increases, leading to enhance
up to x = 0.10. While the decreasing of
and
at high Hg2+ ions content may be resulting from the enhancement of nonmagnetic Hg2+ ions concentration and existing of spin canting due to magnetic collinearity loss.
Figure 7. Room temperature magnetic hysteresis loops of BaFe12-xHgxO19 nanoparticles, x = 0.00, 0.05, 0.10, 0.20 and 0.30.

Table 3. The distinguishing wave number υ1, υ2 corresponding to different vibrations and Debye temperature of BaFe12-xHgxO19 nanoparticles.
Table 4. Magnetic parameters of BaFe12-xHgxO19 nanoparticles.
The magnetic moment () per formula unit in Bohr magneton (µB) can be calculated through the following equation [Citation39]:
(8)
(8) where
is the molecular weight of each composition. The obtained values of magnetic moment are presented in Table versus x. It is observed that
has the same trend with x as
. The increase in the crystallite size L and the decrease in spin canting angle lead to enhance
,
and the strength of A–B super-exchange magnetic interactions [Citation39].
The magneto crystalline anisotropy constant () and the intrinsic coercivity (
) can be deduced from the following relations [Citation40,Citation41]:
(9)
(9)
(10)
(10)
The estimated values of and
are also presented in Table versus x. It is obvious that
slightly decreases as Hg2+ ions content increases till x = 0.10, and then increases with further increase in x, and become close to that of x = 0.00. The reduction in
may be attributed to the non-magnetic Hg2+ ions which make the inter-sublattice interactions weak, leading to decrease the anisotropy energy and
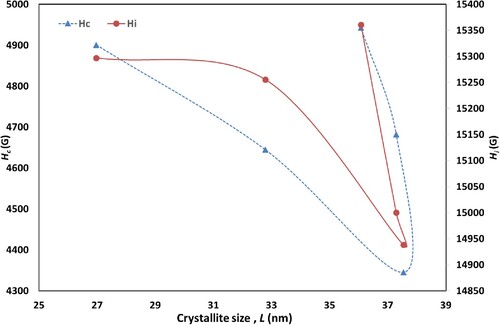
[Citation30]. Coercivity
also decreases from 4943 G at x = 0.00 to 4345 G at x = 0.10 and then rises to 4900 at x = 0.30. The variation of
is associated with the extrinsic effect which related to porosity and the distribution of grain size, as well as the intrinsic effect which directly related to the anisotropy field [Citation42]. From the results listed in Table , it is observed that
decreases with increasing the grain size and with decreasing the porosity which improves the inter grain connectivity across the crystal lattice. The other reason of the decrease in
may be ascribed to the decrease in magneto crystalline anisotropy. Frequently, we can use the variations in
values to explain the
variations [Citation43]. The high values of
indicate the hard magnetic behaviour of the samples. Figure shows that
of the samples is proportional to
and depends on crystallite size. Usually, the values range of coercivity determines the application in which nanomaterials are used, for example the substances that have
<1200 G is applicable in the field of perpendicular magnetic recording media [Citation44]. Moreover, as-prepared hexaferrites nanoparticles of
in the range (4943–4345 G) with
>
, are known as permanent magnets which have a potential candidate for numerous applications.
The study of EPR of ferrites is very important for investigation high frequency magnetic properties as the resonance originates from the spins-electromagnetic waves interaction [Citation45]. Figure shows the first-derivative electron paramagnetic resonance absorption spectra at room temperature for BaFe12-xHgxO19 samples with 0.00 ≤ x ≤ 0.30. EPR spectra contain an isotropic with relative narrow EPR line, characterizing Ba ferrite samples [Citation46]. The isotropic behaviour of EPR line, for all substituted samples, reflects its symmetry about the central position. This indicates the absence of skin effect [Citation47]. In ferrites, Fe3+ ions are the main responsible for EPR spectra as EPR signal for Fe2+ ions can be only observed at very low temperature (∼ 4 K) because of their very short spin–lattice relaxation time [Citation48]. On the other hand, the two main sources which are responsible for the EPR signal linewidth for any ferrite material are the interaction between magnetic dipoles among particles and the super-exchange interactions between magnetic ions through the ions of oxygen [Citation49].
Figure 10. First-derivative absorption spectra for BaFe12-xHgxO19 nanoparticles versus the magnetic field.
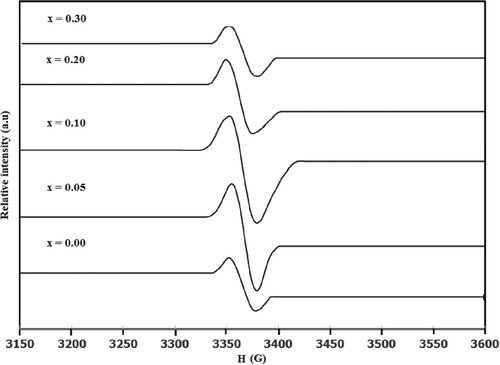
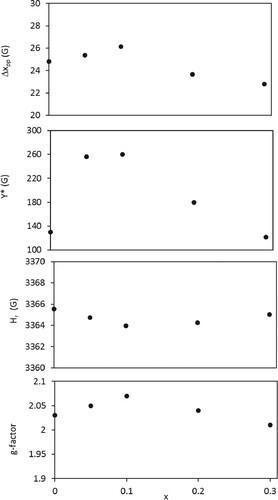
The strong EPR intensity display slightly shift for the point of maximum derivative with increasing Hg substitution. This makes a slight change in g-factor. The g-factor was calculated from knowing the g-factor of CuSO4.5H2O [Citation50]. The g-factor variation with x for BaFe12-xHgxO19 samples is shown in Figure . The g-factor is near ≈ 2, corresponding to barium ferrite sample [Citation46]. There is a slight increase in g-factor with increasing Hg2+ ions content up to x = 0.10, followed by a decrease with further increase in x. The slight reduction of g-factor for x ≥ 0.20 reflect the decrease in the magnetic interaction. Reverse behaviour for the resonance field Hr versus x is plotted in Figure . The EPR line intensity of peak-to-peak amplitude Y* increases with increasing Hg substitution up to 0.10, beyond which it decreases as represented in Figure . This enhancement indicates the enhancement of unpaired electrons in BaFe12-xHgxO19 samples. Moreover, the variation of peak-to-peak line width Δxpp with Hg2+ ions content is also shown in Figure . It is clear that Δxpp increases with increasing Hg2+ ions content up to x = 0.10, beyond which it decreases. The increase in Δxpp reflects the strengthening of the dipolar interaction and indicates the existence of critical grain size with 0.00 ≤ x ≤ 0.10 as the crystalline size increases up to x = 0.10. On the other hand, the decrease in Δxpp for x ≥ 0.20 can be interpreted as the reduction of magnetic interaction effect, according to the reduction of magnetic ions (Fe3+ ions), confirming with the model of solid solutions with magnetic dilution; [Citation51]. The number of spins N participating in the resonance are estimated from comparing the area under the absorption curve with that for CuSO4.5H2O [Citation50]. N enhances as Hg2+ ions content increases up to x = 0.10, beyond which it decreases as listed in Table . This enhancement of N, as well as g-factor and
reflects the increase of the crystalline degree for BaFe12-xHgxO19 samples as x increases up to 0.10, which improve the dipole interaction that in turn give large
, g-factor and N [Citation52]. The paramagnetic susceptibility χ at room temperature is calculated through the following relation [Citation53]:
(11)
(11) where
is Bohr magneton and
is total angular momentum. The values of paramagnetic susceptibility versus Hg2+ ions content are listed in Table . It is clear that χ increases with increasing x up to 0.10, confirming with the behaviour of N with x. The order of paramagnetic susceptibility is comparable with that obtained for BaFe12O19 sample [Citation10]. The spin relaxation process is characterized by a time constant that is a function of magnetic field. It depends on the rate at which the energy of microwave can be emitted or absorbed to the surrounding spins. The spin–spin relaxation time constant T can be calculated through the following relation [Citation54]:
(12)
(12) where
is Bohr magneton,
is the linewidth at half height of absorption peak. The values of spin–spin relaxation time constant versus Hg2+ ions content for BaFe12-xHgxO19 samples are also listed in Table . One can notice that T decreases with increasing Hg2+ ions content up to x = 0.10, followed by a decrease for further increase in x. The behaviour could be explained according to the change in electron motion and the strength of super-exchange interaction between magnetic ions through oxygen.
Table 5. Variation of N, χ and τ versus x for BaFe12-xHgxO19 nanoparticles.
4. Conclusion
Barium hexaferrite nanoparticles were prepared at different temperature through a co-precipitation method. XRD diffraction patterns revealed that the annealing temperature played an important role in the crystallization of barium hexaferrite. A series of BaFe12-xHgxO19 nanoparticle, 0.00 ≤x≤ 0.30 were successfully prepared at the optimum annealing temperature 1000°C. XRD and FTIR analysis confirmed the single phase formation and the absence of impurities. Lattice parameter c increased with the increase in x, whereas a slightly decreased. M-H curves displayed wide hysteretic behaviours with higher coercivity which is characteristic of the typical hard magnetic materials, leading to recommend these materials to be a potential candidate for numerous applications such as recording media applications. M-H curves indicated the enhancement in saturation magnetization values () and remnant magnetization
up to x = 0.10 followed by a decrease up to x = 0.30. On the other hand the intrinsic coercivity (
) and coercivity
diminished as Hg2+ content increased till x=0.10, beyond which they enhanced. The decrease in
might be ascribed to the increase in the grain size and the decrease in magneto crystalline anisotropy. The increase of Hg2+ content up to 0.10 for BaFe12-xHgxO19 nanoparticle improved the dipole interaction that in turn made an enhancement for the values of g-factor, peak-to-peak line width, number of spin and paramagnetic susceptibility. The enhancement of these EPR parameters confirmed the improvement of the crystalline degree as Hg2+ content increased up to 0.10, reflecting the existence of critical grain size with 0.00 ≤ x ≤ 0.10.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Mosleh Z, Kameli P, Ranjbar M, et al. Effect of annealing temperature on structural and magnetic properties of BaFe12O19 hexaferrite nanoparticles. Ceram Int 2014;40:7279–7284. doi: 10.1016/j.ceramint.2013.12.068
- Pullar RC. Hexagonal ferrites: A review of the synthesis, properties and applications of hexaferrite ceramics. Prog Mater Sci. 2012;57(7):1191–1334. doi: 10.1016/j.pmatsci.2012.04.001
- Harris VG, Geiler A, Chen Y, et al. Recent advances in processing and applications of microwave ferrites. J Magn Magn Mater 2009;321:2035–2047. doi: 10.1016/j.jmmm.2009.01.004
- Chen D, Liu Y, Li Y, et al. Microstructure and magnetic properties of Al-doped barium ferrite with sodium citrate as chelate agent. J Magn Magn Mater 2013;337-338:65–69. doi: 10.1016/j.jmmm.2013.02.036
- Almessiere MA, Slimani Y, El Sayed HS, et al. Investigation of Microstructural and magnetic properties of BaVxFe12−xO19 Nanohexaferrites. J Supercond Nov Magn. 2019;32:1437–1445. doi: 10.1007/s10948-018-4856-8
- Maria Lumina Sonia M, Anand S, Maria Vinosel V, et al Effect of lattice strain on structure, morphology and magneto-dielectric properties of spinel NiGd x Fe 2−x O 4 ferrite nano-crystallites synthesized by sol-gel route. J Magn Magn Mater 2018;466:238–251. doi: 10.1016/j.jmmm.2018.07.017
- Elayakumar K, Dinesh A, Manikandan A, et al. Structural, morphological, enhanced magnetic properties and antibacterial bio-medical activity of rare earth element (REE) cerium (Ce3+) doped CoFe2O4 nanoparticles. J Magn Magn Mater. 2019;476:157–165. doi: 10.1016/j.jmmm.2018.09.089
- Rai GM, Iqbal MA, Kubra KT. Effect of Ho3+ substitutions on the structural and magnetic properties of BaFe12O19 hexaferrites. J Alloy Compd. 2010;495:229–233. doi: 10.1016/j.jallcom.2010.01.133
- Chen W, Wu W, Mao M, et al. Improvement of the magnetization of barium hexaferrites Induced by substitution of Nd3+ ions for Fe3+ ions. J Supercond Nov Magn. 2017;30:707–714. doi: 10.1007/s10948-016-3886-3
- Slimani Y, Baykal A, Manikandan A. Effect of Cr 3+ substitution on AC susceptibility of Ba hexaferrite nanoparticles. J Magn Magn Mater 2018;458:204–212. doi: 10.1016/j.jmmm.2018.03.025
- Topkaya R. Effect of Zn substitution on temperature dependent magnetic properties of BaFe12O19 hexaferrites. J Alloy Compd. 2017;725:1230–1237. doi: 10.1016/j.jallcom.2017.07.248
- Amer MA, Meaz TM, Attalah SS, et al. Structural and magnetic studies of Ti4+substituted M-type BaFe12O19 hexa-nanoferrites. Mater Sci Semicond Process 2015;40:374–382. doi: 10.1016/j.mssp.2015.07.007
- Ali I, Islam MU, Ashiq MN, et al. Investigation of the magnetic properties of nanometric SrSmCoNi ferrite/PST matri. Ceram Int. 2015;41:8748–8754. doi: 10.1016/j.ceramint.2015.03.097
- Kaur T, Kaur B, Bhat BH, et al. Effect of calcination temperature on microstructure, dielectric, magnetic and optical properties of Ba0.7La0.3Fe11.7Co0.3O19 hexaferrites. Physica B. 2015;456:206–212. doi: 10.1016/j.physb.2014.09.003
- Khan HM, Islam MU, Xu Y, et al. Structural and magnetic properties of TbZn-substituted calcium barium M-type nano-structured hexa-ferrites. J Alloys Compd 2014;589:258–262. doi: 10.1016/j.jallcom.2013.11.107
- Sharma R, Agarwala RC, Agarwala V. A study on the heat-treatments of nanocrystalline nickel substituted BaW hexaferrite produced by low combustion synthesis method. J Magn Magn Mater. 2007;312:117. doi: 10.1016/j.jmmm.2006.09.021
- Yu H. Bafe12o19 powder with high magnetization prepared by acetone-aided coprecipitation. J Magn Magn Mater. 2013;341:79–85. doi: 10.1016/j.jmmm.2013.04.030
- Tang X, Hong RY, Feng WG, et al. Ethylene glycol assisted hydrothermal synthesis of strontium hexaferrite nanoparticles as precursor of magnetic fluid. J Alloys Compd 2013;562:211–218. doi: 10.1016/j.jallcom.2013.02.049
- Ghoneim AI, Amer MA, Meaz TM, et al. Dielectric properties of Ti4+ substituted BaFe12O19 nanoparticles. Physica B. 2017;507:1–12. doi: 10.1016/j.physb.2016.11.032
- Catellanos PAM, Jarque JCS, Rivera JA. Magnetic and microstructural properties of the BaFe(12−(4/3)x)SnxO19 ceramic system. Physica B. 2005;362:95–102. doi: 10.1016/j.physb.2005.01.480
- Safaan SA, Abo El Ata AM, El Messeery MS. Study of some structural and magnetic properties of Mn-substituted SrCu hexagonal ferrites. J Magn Magn Mater 2006;302:362–367. doi: 10.1016/j.jmmm.2005.09.041
- Kumar S, Manglam MK, Supriya S, et al. Lattice strain mediated dielectric and magnetic properties in La doped barium hexaferrite. J Magn Magn Mater. 2019;473:312–319. doi: 10.1016/j.jmmm.2018.10.085
- Kaur P, Chawla SK, Narang SB, et al. Effect of Cu-Co-Zr Doping on the properties of Strontium hexaferrites synthesized by Sol-Gel Auto-combustion method. J Supercond Nov Magn. 2017;30:635–645. doi: 10.1007/s10948-016-3835-1
- Almessiere MA, Slimani Y, Tashkandi NA, et al. The effect of Nb substitution on magnetic properties of BaFe12O19 nanohexaferrites. Ceram Int 2019;45:1691–1697. doi: 10.1016/j.ceramint.2018.10.048
- Islam MU, Ahmad I, Abbas T, et al. pp. 19–23, 155–158. Proceedings of 6th International Symposium on Advanced Materials, Islamabad (1999).
- Deraz NM, Alarif A. Int J Electrochem Sci 2012;7:4585.
- Verma A, Thakur OP, Prakash C, et al. Temperature dependence of electrical properties of nickel–zinc ferrites processed by the citrate precursor technique. Mater Sci Eng B. 2005;116:1–6. doi: 10.1016/j.mseb.2004.08.011
- Cullity BD. Powder photographs, Debye Scherrer Method, 150–151, Elem. of X-ray Diffr., Addision Wesley Publishing Companym Inc., Reading, Massachusetts, M. Cohen, Printed in U.S.A; 1956.
- Kumar ER, Jayaprakash R, Kumar S. The role of annealing temperature and bio template (egg white) on the structural, morphological and magnetic properties of manganese substituted MFe2O4 (M=Zn, Cu, Ni, Co) nanoparticles. J Magn Magn Mater 2014;351:70–75. doi: 10.1016/j.jmmm.2013.09.055
- Amer MA, Meaz TM, Mostafa AG, et al. Time effect of annealing on phase transformations of Cu–Al–Cr nano-ferrites prepared by a co-precipitation method. Mater Sci Semicond Process 2015;32:68–75. doi: 10.1016/j.mssp.2014.12.034
- Singh J, Singh C, Kaur D, et al. Elucidation of phase evolution, microstructural, mössbauer and magnetic properties of Co2+Al3+ doped M-type Ba Sr hexaferrites synthesized by a ceramic method. J Alloys Compd 2017;695:1112–1121. doi: 10.1016/j.jallcom.2016.10.237
- Patange SM, Shirsath SE, Lohar KS, et al. Infrared spectral and elastic moduli study of NiFe2−xCrxO4 nanocrystalline ferrites. J Magn Magn Mater 2013;325:107–111. doi: 10.1016/j.jmmm.2012.08.022
- Patange SM, Shirsath SE, Jadhav SP, et al. Elastic properties of nanocrystalline aluminum substituted nickel ferrites prepared by co-precipitation method. J Mol Struct 2013;1038:40–44. doi: 10.1016/j.molstruc.2012.12.053
- Mazen SA, Mansour SF, Dhahri E, et al. The infrared absorption and dielectric properties of Li–Ga ferrite. J Alloy Compd. 2009;470:294–300. doi: 10.1016/j.jallcom.2008.02.035
- El-Sayed SM, Meaz TM, Amer MA, et al. Magnetic behavior and dielectric properties of aluminum substituted M-type barium hexaferrite. Physica B. 2013;426:137–143. doi: 10.1016/j.physb.2013.06.026
- Teh GB, Nagalingam S, Jefferson DA. Preparation and studies of Co(II) and Co(III)-substituted barium ferrite prepared by sol–gel method. Mater Chem Phys 2007;101:158–162. doi: 10.1016/j.matchemphys.2006.03.008
- Amir M, Gungunes H, Slimani Y, et al. Mössbauer Studies and magnetic properties of Cubic CuFe2O4 nanoparticles. J Supercond Nov Magn. 2019;32:557–564. doi: 10.1007/s10948-018-4733-5
- Almessiere MA, Slimani Y, Baykal A. Structural and magnetic properties of Ce-doped strontium hexaferrite. Ceram Int 2018;44:9000–9008. doi: 10.1016/j.ceramint.2018.02.101
- Bobade DH, Rathod SM, Mane ML. Sol–gel auto-combustion synthesis, structural and enhanced magnetic properties of Ni2+ substituted nanocrystalline Mg–Zn spinel ferrite. Physica B. 2012;407:3700–3704. doi: 10.1016/j.physb.2012.05.017
- Slimani Y, Baykal A, Amir M, et al. Substitution effect of Cr3+ on hyperfine interactions, magnetic and optical properties of Sr-hexaferrites. Ceram Int 2018;44:15995–16004. doi: 10.1016/j.ceramint.2018.06.033
- Almessiere MA, Slimani Y, Baykal A. Structural, morphological and magnetic properties of hard/soft SrFe12-xVxO19/(Ni0.5Mn0.5Fe2O4)y nanocomposites: effect of vanadium substitution. J Alloy Compd. 2018;767:966–975. doi: 10.1016/j.jallcom.2018.07.212
- Ali I, Islam MU, Awan MS, et al. Effect of Tb3+ substitution on the structural and magnetic properties of M-type hexaferrites synthesized by sol–gel auto-combustion technique. J Alloys Compd 2013;550:564–572. doi: 10.1016/j.jallcom.2012.10.121
- Slimani Y, Güngüneş H, Nawaz M, et al. Magneto-optical and microstructural properties of spinel cubic copper ferrites with Li-Al co-substitution. Ceram Int 2018;44:14242–14250. doi: 10.1016/j.ceramint.2018.05.028
- Ali I, Islam MU, Awan MS, et al. Effects of Ga–Cr substitution on structural and magnetic properties of hexaferrite (BaFe12O19) synthesized by sol–gel auto-combustion route. J Alloys Compd 2013;547:118–125. doi: 10.1016/j.jallcom.2012.08.122
- Gazeau F, Bacri JC, Gendron F, et al. Magnetic resonance of ferrite nanoparticles. J Magn Magn Mater 1998;186:175–187. doi: 10.1016/S0304-8853(98)00080-8
- Koksharov YA, Pankratov DA, Gubin SP, et al. Electron paramagnetic resonance of ferrite nanoparticles. J Appl Phys 2001;89:2293–2298. doi: 10.1063/1.1332417
- Bejjit L, Haddad M. EPR study, in the normal and superconducting states, of GdBa2Cu3O7 single crystal before and after grinding. Physica C. 2002;371:339–343. doi: 10.1016/S0921-4534(01)01105-4
- Sastry MD, Nagar YC, Bhushan B, et al. An unusual radiation dose dependent EPR line at geff = 2.54 in feldspars: possible evidence of Fe3+O2−↔ Fe2+O− and exchange coupled Fe3+–Fe2+–nO−. J Phys Condens Matter. 2008;20:025224. doi: 10.1088/0953-8984/20/02/025224
- Singh JP, Dixit G, Srivastava RC, et al. Magnetic resonance in superparamagnetic zinc ferrite. Bull Mater Sci 2013;36:751–754. doi: 10.1007/s12034-013-0528-2
- Weil JA, Bolton JR. Electron paramagnetic resonance – Elementary theory and Practical applications. 2nd ed. Hoboken (New Jersey): John Wiley & Sons, Inc.; 2007.
- Mendoza A, Prado J, Almanza O. Structural and magnetic Features of Mn1-xZnxFe2O4 nanoparticles. Acta Phys Pol A. 2012;121:950–953. doi: 10.12693/APhysPolA.121.950
- Li X, Lu G, Li S. Synthesis and characterization of fine particle ZnFe2O4 powders by a low temperature method. J Alloys Compd. 1996;235:150–155. doi: 10.1016/0925-8388(95)02022-5
- Aschcroft NW, Mermin ND. Solid State Physics. New York: Harcourt College Publishers; 2001.
- Shahane GS, Kumar A, Arora M, et al. Synthesis and characterization of Ni–Zn ferrite nanoparticles. J Magn Magn Mater 2010;322:1015–1019. doi: 10.1016/j.jmmm.2009.12.006