Abstract
Excessive salinity in irrigation decreases crop’s growth in arid regions such as Saudi Arabia. The aim of this research was to study the combined effects of salinity and temperature on germination, plant growth, Fv/Fm, gas exchange, chlorophyll and proline content in two Sorghum bicolor cultivars, Hindi and Jaizani. The treatments contained 3 temperatures (20°C, 30°C, 40°C) and 4 NaCl concentrations (0, 50, 100, 200 mM). The results presented that as NaCl increased, germination, growth, gas exchange and Fv/Fm of the two cultivars declined, with great effects at 40°C than at the other temperatures. Proline was higher in the Hindi and Jaizani cultivars at 20°C and 30°C under the different salinity conditions. On the other hand, the chlorophyll content was increased at 20°C under increased salinity in both cultivars. The interactions between salinity and temperature significantly affected most measurements of the two cultivars; however, Hindi cultivar showed more tolerance toward stress.
1. Introduction
Salinity is one of the major problems affecting seed germination, plant growth and photosynthetic efficiency in most species in both controlled and field environments [Citation1–4]. It affects approximately 830 million hectares of farmland worldwide [Citation5]. The majority of commercially valuable crops are salt-sensitive, and salinity affects their growth and productivity [Citation6]. The most common salt in farmland is sodium chloride (NaCl). Plants which grow in salinized land accumulate high concentrations of sodium ion (Na+) that can damage plasma membrane, inhibit enzymes and produce harmful reactive oxygen species and lead to the death of plants. The accumulation of sodium ions (Na+) in plants inhibits the absorption of major nutrients such as (K+) by the root and lower the ratio of K+/Na+ [Citation7–9]. Furthermore, salinity has dual effects on plant physiological processes. It reduces the availability of water to the roots and accumulates ions to toxic levels in some tissues [Citation10]. Both factors affect the seed germination percentage and rate, the length of root and shoot and the fresh weight of root and shoot [Citation11,Citation12]. Also, salt stress has been reported to increase the proline content in plants. The accumulation of proline protects a number of mechanisms in plants [Citation13,Citation14]. Thus, improving plant tolerance to saline stress is necessary for increasing the growth and productivity of the plants.
Temperature is also one of the most significant environmental variables, and it affects a large variety of physiological processes in many plants [Citation15,Citation16]. It was found that each centigrade degree increase in the average seasonal temperature can reduce crop yields by as much as 17% [Citation17]. In addition, the temperature is known to affect all phases of plant growth, from germination and early plant growth to canopy closure, photosynthesis and chlorophyll fluorescence [Citation16–19]. Exposure plants to high temperature reduces the chlorophyll content. The high temperature decreases the biosynthesis of chlorophyll and accelerates its degradation [Citation16]. Khan et al. [Citation20] found it is important to understand the interactions between salinity and temperature to know the factors that regulate all phases of plant growth. Even though high salinity inhibits the germination of seed and plant growth, the harmful effects of salinity are mostly reduced at the optimal temperature.
Photosynthesis is considered to be one of the most physiologically sensitive processes in plants. Some environmental pressures like salinity, elevated temperature and drought, can reduce PSII photochemistry (Fv/Fm), CO2 assimilation and O2 evolution, resulting in a reduction in the response of the photosynthetic curve of light and in the light saturated-photosynthetic rate (Asat) [Citation21–23]. It was found that high rates of photosynthesis in Atriplex lentiformis (Torr.) could be achieved at low salinity levels of NaCl [Citation24]. The high concentration of NaCl impaired the saturation assimilation rate of carbon dioxide (Asat) and the maximum sum of quantum (φ) yield [Citation22,Citation23,Citation25]. Furthermore, the exposure of seven grasses to elevated temperature decreased photosynthesis of leaves [Citation26]. The elevated temperature limits the activity of photosynthesis by influencing the electron transport of thylakoid membrane and the kinetics of Rubisco and carboxylation efficiency [Citation27]. Additionally, the linear ratio of the quantum yield to the average fluorescence variable ratio (Fv/Fm) has also been found to decline at high temperatures. Thus, the combination of measuring chlorophyll fluorescence (Fv/Fm) and net parameters of gas exchange provides a good method to estimate photosynthetic output in plants under stress and gain insight into photosynthetic machinery behaviour under such stress [Citation28]. Moreover, the photorespiration rate increases with increasing temperature, which reduces the growth of the plant.
Sorghum bicolor (a C4 crop) is an important yield in arid and semi-arid areas [Citation15]. In Saudi Arabia, this crop grows in many areas with different temperatures and irrigation water salinity levels, such as Jaizan, Mecca, Asir and Al-Baha [Citation29]. Since there is little information concerning the combined effects of salinity and temperature on germination of seeds, growth and photosynthesis rates of the local Sorghum bicolor cultivars, this research was undertaken to determine the combined effects of salinity (0, 50, 100, 200 mM NaCl), and temperature (20°C, 30°C and 40°C) on germination of seeds, growth, Fv/Fm, proline content, chlorophyll content and gas exchange in two local Sorghum bicolor cultivars (Jaizani and Hindi). Information on the combined effects of salinity and temperature could be helpful in identifying the Sorghum bicolor cultivar that shows optimal growth in saline areas under different temperatures.
2. Materials and methods
2.1. Seed germination
Seeds of sorghum (Sorghum bicolor cv. Jaizani) and (Sorghum bicolor cv. Hindi) were obtained commercially from AlHilali Seed Company in AlMadinah AlMunawarh, Saudi Arabia. The experiments were conducted at the Department of Biology, Faculty of Science, Taibah University, AlMadinah AlMunawarh, Saudi Arabia. The seeds were surface sterilized by soaking for two minutes in 0.1% mercuric chloride. Then, the seeds were washed with five changes of distilled water. Ten seeds were distributed in each Petri dish (9 × 1.6 cm) containing two layers of Whatman No. 1 filter paper moistened with 10 ml of either distilled water or the appropriate NaCl solution (50, 100 and 200 mM). Four replicates for each cultivar at different NaCl concentrations were placed in an incubator at three temperatures (20°C, 30°C and 40°C) in darkness leading to a total of 96 Petri dishes. Seeds were considered germinated with the emergence of the radicles. The germination percentage was recorded daily for two weeks until no further seeds germinated.
2.2. Plant growth parameters
Seeds of sorghum cultivars were planted into plastic pots (12 × 20 cm) filled with 2 kg of coarse sand and grown in a controlled-environment chamber (JSR 314-240, JS Research Inc., 40-1 Gumsang-Dong, Gongju City, Korea) with a 14-hour photoperiod, 60% relative humidity and illuminated with fluorescent and halogen lamps giving a 400 µmol m−2 s−1. Three NaCl concentrations, 50, 100 and 200 mM, in full-strength Hoagland nutrient solution were used [Citation30]. The control was Hoagland nutrient solution with no NaCl added. Four replicates for each treatment under different NaCl concentrations were placed in the chamber at three temperatures (20°C, 30°C and 40°C) leading to a total of 96 pots for the two cultivars. Six seeds were sown in each pot, and after emergence the seedlings were thinned to three per pot. All pots were watered to field capacity with full-strength Hoagland nutrient solution every day for 7 days. On the 8th day, the NaCl treatments began.
Growth parameters, including plant height, number of leaves and fresh weight of shoot and root were determined 40 days after planting. A portable LI-3000C Leaf Area Meter was used to measure leaf area of the two cultivars (LICOR Inc, Lincoln, Nebraska, United State). The shoot and root samples were oven-dried at 80°C for 48 h to determine their dry weight.
2.3. Photosynthesis measurements
Gas exchange rates were measured on recent fully expanded leaves using a LICOR 6400XT Infra-Red Gas Analyser (LI-6400, LI-COR Inc., Lincoln, USA). Measurement followed that of Al-shoaibi [Citation31]. The fourth youngest fully expanded leaves were used for photosynthesis and dark respiration measurements. A Peltier cooling system maintained the leaf temperature at 25°C during the measurement. Gradually, leaves were illuminated with densities of photon flux from 0 to 1500 μmol m−2 s−1. The equations of Von Caemmerer and Farquar [Citation32] were used to determine the net photosynthesis per unit leaf area and intercellular CO2 concentration (ci). Asat was determined at saturating PPFD (1500 µmol m−2 s−1) and at ambient CO2 concentration of 360 µmol mol−1.
2.4. Chlorophyll content determination
The chlorophyll content was measured using a handheld chlorophyll content metre and expressed as the chlorophyll content index (Opti-Sciences, CCM-200, USA). The fourth youngest fully expanded leaves were used to measure the chlorophyll content four times for each cultivar in the different treatments, and the average was used for analysis.
2.5. Chlorophyll fluorescence measurements
The chlorophyll fluorescence of the fourth youngest fully expanded leaves of the sorghum cultivars was measured using a portable fluorimeter (PEA, Hansatech, Kings Lynn, Norfolk). The leaves were dark adapted for 20 min prior to the fluorescence measurements. The ratio of variable to maximum fluorescence (Fv/Fm) was measured four times for each cultivar in the different treatments as described by Al-shoaibi [Citation31].
2.6. Proline content
The proline content was estimated by the Bates et al. [Citation33] method. Fresh leaf samples (0.5 g) were homogenized in 3% (w/v) sulphosalicylic acid, and the homogenate was filtered through filter paper. After the addition of acid-ninhydrin and glacial acetic acid, the mixture was heated at 100°C for 1 h in a water bath. The reaction was terminated by adding 4 mL of toluene and placing the mixture in an ice bath. The absorption of the fraction with evaporated toluene was read at 520 nm. Based on the calibration curve, the concentration of proline was calculated and expressed as fresh weight μmol/g.
2.7. Statistical analysis
Statistical analyses of the data obtained from the various analysis and measurements were conducted using Two-Way and Three-Way Analysis of Variance (ANOVA), General Linear Model to test for main effects and interactions of the factors investigated in this study (Temperature; Salinity; Cultivar). In order to test significance between different levels of the factors a multiple comparison using Tukey’s test was also carried out. All analyses were performed using Minitab version 15 (Brandon Court, Unit E1-E2, Progress Way, Coventry CV3 2TE, United Kingdom). Four replicates of each treatment were used and Microsoft Excel 2016 was used to calculated standard deviations and standard errors.
3. Results
3.1. Seed germination
The combined effects of salinity and temperature on germination of seeds in the two Sorghum bicolor cultivars are summarized in Table . Seed germination significantly decreased by temperature (P < 0.001), salinity (P < 0.001) and the combined effects of salinity and temperature (P = 0.017; Table ). For the Hindi cultivar, germination approached more than 90% at 20°C and 30°C for both the control and 50 mM NaCl treatments. At salinities of 200 mM, germination rates significantly decreased compared with the control rates under all temperature regimes (P < 0.001). For the Jaizani cultivar, both temperature and salinity had pronounced effects on the rates of germination. At salinities of 100 and 200 mM, most germination rates significantly decreased compared with the control rates under all temperature regimes (P < 0.001).
Table 1. Combined effects of salinity and temperature on germination of seeds in two Sorghum bicolor cultivars. (n = 4, Mean ± S.E.).
3.2. Plant growth parameters
A variety of growth parameters have been calculated to determine how the combined effects of salinity and temperature influence the growth parameters of the two cultivars; the results are presented in Tables –. The results presented that fresh and dry weights of root were unaffected in the two cultivars by the interaction between salinity and temperature (Tables –). On the other hand, most growth parameters of the Hindi cultivar were significantly decreased (P < 0.01) in response to the treatments with high salinity and temperature compared to those of the control plants. In addition, senescence occurred in Jaizani cultivar at high salinity and temperature within one month of the emergence (Table ).
Table 2. Results of the significance of the statistical analysis of the different growth and physiological parameters of Sorghum bicolor cv. Jaizani and Sorghum bicolor cv. Hindi cultivars grown under the combined effects of salinity and temperature.
Table 3. Combined effects of salinity and temperature on the growth parameters of Sorghum bicolor cv. Hindi. (n = 4, Mean ± S.E.).
Table 4. Combined effects of salinity and temperature on the growth parameters of Sorghum bicolor cv. Jaizani. (n = 4, Mean ± S.E.).
3.3. Photosynthesis measurements
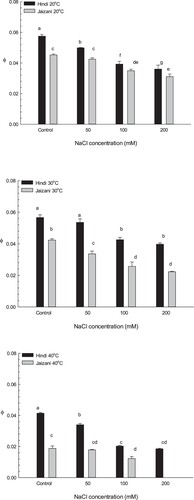
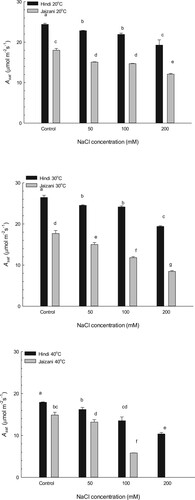
The rate of photosynthesis was determined for the two cultivars of Sorghum bicolor (Figures –). The results indicated that the interaction between temperature and salinity significantly affected the photosynthesis rate (P < 0.001; Table ). The higher temperature decreased the light-saturated photosynthetic rate significantly (Asat) and the quantum yield (φ) of both cultivars compared to those at other temperatures at the different concentrations of salinity (P < 0.001). Additionally, with increasing temperature and salinity the photorespiration levels of the two cultivars increased (Figure ). However, the Hindi cultivar showed higher photosynthetic rates at the different temperatures and salinities. This indicates that the Hindi cultivar's photosynthetic machinery is more tolerant to salinity and temperature than that of the Jaizani cultivar. The Jaizani cultivar seedlings cultivated at 200 mM NaCl and an incubation temperature of 40°C showed senescence before the measurements of photosynthesis.
Figure 1. The Photosynthetic CO2 absorption response (A) per unit area of the leaf to photon flux (Q) for two cultivars of Sorghum bicolor. CO2 absorption measurements were all made at 25°C and Ca of 360 µmol mol−1. The data is the mean of leaves; n = 4 (±SE).
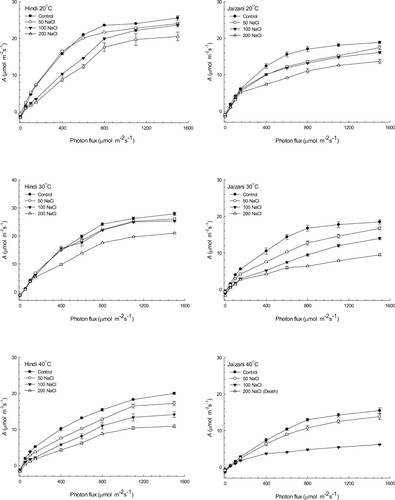
Figure 2. The photosynthetic light-saturated amount (Asat) per unit area of the leaf, estimated at 25°C and 1500 µmol m−2 s−1 photon flux for the two Sorghum bicolor cultivars. The data is the mean of leaves n = 4 (± SE). Different letters indicate significance of two-way interactions between salinity and temperature. Means that do not share a letter are significantly different.
3.4. Chlorophyll content determination
The results in Table show that the 40°C temperature significantly reduced the chlorophyll content at the concentration of 200 NaCl, to 53% for the Hindi cultivar and to 67% for the Jaizani cultivar compared to that in the control (P < 0.01). In contrast, the 20°C and 30°C treatments significantly increased the chlorophyll content of the two cultivars at 200 NaCl compared with the control group (P < 0.05). The chlorophyll content increased by 150% and 118%, respectively, for the Hindi cultivar and by 145% and 135%, respectively, for the Jaizani cultivar compared to that in the control.
Table 5. Combined effects of salinity and temperature on chlorophyll content of two Sorghum bicolor cultivars (n = 4, Mean ± S.E.).
3.5. Chlorophyll fluorescence measurements
Parameters of chlorophyll fluorescence were measured to define the effects of salinity and temperature on the electron flow of photosystem II. The results in Table show that high temperature significantly reduced the FV/Fm at 200 NaCl salinity compared to that of the control in the two cultivars of Sorghum bicolor (P < 0.05; Table ). The FV/Fm was reduced by 30% for the Hindi cultivar and by 29% for the Jaizani cultivar compared to that of the control. On the other hand, there has been a slight drop in FV/Fm at the different salinity concentrations when the two cultivars were planted at 20°C and 30°C.
Table 6. Combined effects of salinity and temperature on FV/Fm of two Sorghum bicolor cultivars. (n = 4, Mean ± S.E.).
3.6. Proline content
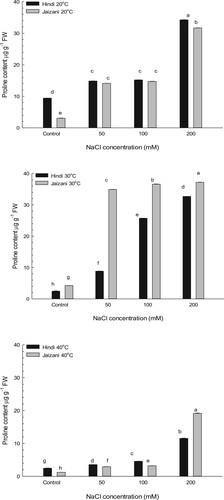
The content of proline in the two cultivars of Sorghum bicolor was determined (Figure ). The results presented that the interaction between salinity and temperature significantly affected the content of proline (P < 0.001; Table ). The high salinity increased the content of proline of the two cultivars significantly in comparison with that in the control under the different temperature regimes (P < 0.001). The content of proline increased almost 13-fold in Hindi cultivar leaves grown at 30°C after 30 days treatment of 200 mM NaCl, while it increased nearly 16-fold in the leaves of the Jaizani cultivar grown at 40°C after 30 days treatment of 200 mM NaCl, compared to that in the control plants.
4. Discussion
The present study examined the combined effects of salinity and temperature on seed germination, plant growth, Fv/Fm, proline content, chlorophyll content and gas exchange in two Sorghum bicolor cultivars, Hindi and Jaizani. Seed germination and early seedling growth are more sensitive to salinity than later stages of plant growth. Thus, successful seed germination and seedling growth are important for the establishment of a plant population [Citation34]. The results showed that the seeds of both cultivars germinated at a wide temperature and salinity range (Table ). The capability of seeds to germinate over wide salinities and temperatures ranges is essential for the formation of plants in many different regions [Citation35]. Additionally, the results indicated that the maximum germination percentage for the two cultivars (93%) was recorded for the control at 30°C, while the minimum germination percentages of 60% for Hindi and 20% for Jaizani were recorded at the higher salinity at 40°C. Similar results were found for several plants at higher temperatures and salinities [Citation20,Citation36,Citation37]. The inhibitory effects of high salinity on germination at higher temperatures may be due to water stress which causes a major alteration in the pattern of protein synthesis [Citation37]. Moreover, salinity stress causes ion imbalances due to Na and chloride toxicity and results in a delay in the seeds germination [Citation34]. However, it was found that sodium toxicity and not chloride toxicity is the main problem in maize the second stage of salinity stress [Citation34].
The results of this research presented that 40°C caused a decrease in all growth parameters of the Hindi cultivar at high salinity, while the seedlings of the Jaizani cultivar could not survive at 40°C under the high salinity treatment (Tables and ). Salinity reduces the growth of plant leaves due to a reduction in the rate of cell elongation and the number of elongating cells [Citation34,Citation38]. Also, high temperatures can have a range of morphological consequences, including sunburn on the whole shoot of the plant, leaf senescence, and growth inhibition of root and shoot, resulting in lower yield [Citation39,Citation40]. Therefore, the results showed a better resistance of the Hindi cultivar to salt stress at high temperatures than the Jaizani cultivar at the seedling stage. The tolerance of the Hindi cultivar to salinity stress at high temperature could be due to genetic diversity and variations in heredity between cultivars [Citation41]. Similar results have been reported for P. turgidum [Citation42], and nine medicinal plant species [Citation43]. Moreover, the reduction percentages of all growth parameters of the two cultivars compared to those of the control at salinities of 100 and 200 mM were greater than the reduction percentages of seed germination, suggesting that all growth parameters of the two cultivars were more sensitive than seed germination to increasing salinity. Other studies have found similar results of a higher decrease in the parameters of growth for many plants compared to the decrease in seed germination [Citation41,Citation44]. Additionally, Munns and Sharp [Citation45] found that shoots more sensitive to salt stress than roots. The results of this research found that the interaction between salinity and temperature affected shoots more than roots in the two cultivars (Table ).
Photosynthesis is very sensitive process to salinity and high temperature. Both salinity and high temperature affect photosynthesis by limiting the electron transport capacity of the thylakoid membrane and reducing the activity of Rubisco [Citation46–49]. Furthermore, stomatal limitations and degradation of chlorophyll and carotenoid contents associated with decline in the photosynthesis process in many plants under high temperature and salt stress [Citation46–49]. In this research, the photosynthetic responses of the two Sorghum bicolor cultivars to salinity and temperature were investigated. The findings in this study presented that all leaves of the Hindi cultivar grown under the control and 50 mM NaCl had similar light-saturated photosynthetic rates (Asat) and quantum yields (φ) at 20°C and 30°C (Figures –). The rates of Asat and φ were similar to those for healthy leaves previously noted in a variety of NADP-malic enzyme C4 grasses [Citation50]. This indicates that the Hindi cultivar grown under previous conditions of temperature and salinity was unstressed and did not suffer any photoinhibition. On the other hand, the rates of Asat and φ in the Jaizani cultivar grown under the control and 50 mM NaCl at 20°C and 30°C were lower than those for healthy leaves previously noted in a variety of NADP-malic enzyme C4 grasses, suggesting that the Jaizani cultivar was slightly stressed and suffered photoinhibition. Additionally, these results indicate that the photosynthetic apparatus of the Hindi cultivar is more tolerant to salinity and temperature than that of the Jaizani cultivar. Similar results were observed for two sugar beets [Citation51] and for sorghum cultivars [Citation52]. Moreover, the 40°C treatment caused significant reductions, of 42% for Asat and 55% for φ, in the Hindi cultivar at 200 salinity compared to those in the control, while the seedlings of the Jaizani cultivar senesced under the same growth conditions. This decline in the photosynthetic performance of the Hindi cultivar may have been caused by stomatal behaviour, toxicity of ions or both [Citation52–54]. Also, the significant decrease in the photosynthetic potential of the Hindi cultivar at the highest temperature and salinity may also resulted from a decrease in the chlorophyll content or inhibition in leaf development and expansion [Citation46,Citation47]. Another reason for this reduction in the photosynthetic efficiency of the Hindi cultivar may be due to reduction in the enzyme activities of bundle sheath cell such as Rubisco [Citation47,Citation48].
The current study showed that the chlorophyll content of both cultivars grown at 20°C and 30°C significantly increased at 200 NaCl compared to that in the control (Table ). Similar results were found in several plants at higher salinities [Citation55–57]. The reason for this increase might be an increase in the chloroplast number in stressed leaves or could be a result of reduction in leaf area [Citation56,Citation57]. On the other hand, significant and marked decreases in chlorophyll content were observed in both cultivars grown at 40°C and 200 mM salinity compared to that in the control. This decline in chlorophyll content might be a result of losing grana stacking or might be due to changes in the structure of thylakoid [Citation58,Citation59].
The findings from this study showed that chlorophyll fluorescence parameters were slightly decreased at the different salinity concentrations when the two cultivars were grown at 20°C and 30°C (Table ). These high FV/Fm values gave clear evidence that the two cultivars were resistant to photoinhibition under different salinity treatments when the cultivars were grown at low and moderate temperatures. Similar results have been reported previously for two different cultivars of wheat that differed in their tolerance for salinity [Citation60]. Thus, the present study suggests that the chlorophyll fluorescence parameters cannot be considered as one of the factors to regulate net CO2 assimilation rate in the two Sorghum bicolor cultivars when grown under different salinity treatments and optimal temperatures. On the other side, the high temperature significantly reduced the FV/Fm at 200 NaCl compared to that of the control in the two cultivars of Sorghum bicolor. This drop in FV/Fm might result from oxidative damage to plant photosynthesis devices, particularly under the stress of temperature [Citation61,Citation62].
Plants acclimate to osmotic stress by increasing the contents of osmotic adjustment substances and reducing the rate of transpiration [Citation63,Citation64]. Proline plays a major role in response to osmotic stress. its accumulation in leaves in relation to salinity can decrease the water potential and then help maintain the content of water in leaves [Citation64,Citation65]. The results of this research showed that high salinity under different temperature regimes caused proline content to increase significantly in the two Sorghum bicolor cultivars compared to that in the control (Figure ). A previous study reported a large accumulation of proline in sorghum grown under salt stress [Citation52]. The proline accumulation in plant leaves can reduce the water potential and then help to preserve the water content in the leaves [Citation64,Citation65].
It can be concluded that the Hindi cultivar presented more tolerance to salinity than the Jaizani cultivar for most recorded measurements at the different temperatures. The Hindi cultivar seems to be a reliable crop for saline agricultural lands.
Acknowledgements
The author is indebted to Dr Abdellah Akhkha for his help in proline content estimation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Nedjimi B. Effect of salinity and temperature on germination of Lygeum spartum. J Agric Res. 2013;2(4):340–345.
- Tian F, Hou M, Qiu Y, et al. Salinity stress effects on transpiration and plant growth under different salinity soil levels based on thermal infrared remote (TIR) technique. Geoderma. 2020;357(113961):1–10.
- Acosta-Motos JR, Maria FO, Agustina BV, et al. Plant responses to salt stress: adaptive mechanisms. Agronomy. 2017;7(1):7–18.
- Rui-dong H. Research progress on plant tolerance to soil salinity and alkalinity in sorghum. J Integr Agric. 2018;17(4):739–746.
- El Naim AM, Mohammed KE, Ibrahim EA, et al. Impact of salinity on seed germination and early seedling growth of three sorghum (Sorghum biolor L. Moench) cultivars. Sci Technol. 2012;2:16–20.
- Flowers TJ. Improving crop salt tolerance. J Exp Bot. 2004;55:307–319.
- Almeida DM, Oliveira MM, Saibo NJM. Regulation of Na+ and K+ homeostasis in plants: towards improved salt stress tolerance in crop plants. Genet Mol Biol. 2017;40:326–345.
- Assaha DVM, Ueda A, Saneoka H, et al. The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front Physiol. 2017;8(509). 10/3389/fphys.2017.0059.
- Ami K, Planchais S, Cabassa C, et al. Different proline responses of two Algerian durum wheat cultivars to in vitro salt stress. Acta Physiol Plant. 2020;42:21–42.
- Munns R, James RA, Läuchli A. Approaches to increasing the salt tolerance of wheat and other cereals. J Exp Bot. 2006;57(5):1025–1043.
- AL-Shoaibi AA, AL-Sobhi OA. Effect of NaCl salinity and incubation temperature on the germination of two cultivars of Pearl millet. Biosci Biotechnol Res Asia. 2007;4(1):1–4.
- Tang X, Mu X, Shao H, et al. Global plant-responding mechanisms to salt stress: physiological and molecular levels and implications in biotechnology. Crit Rev Biotechnol. 2015;35:425–437.
- Ben Khaled L, Morte G, Asunéion HM, et al. Effet du stress salin en milieu hydroponique sur le trèfle inoculé par le Rhizobium. Agronomie. 2003;23:553–560.
- Liu L, Huang L, Lin X, et al. Hydrogen peroxide alleviates salinity-induced damage through enhancing proline accumulation in wheat seedlings. Plant Cell Rep. 2020;39:567–575.
- Prasad PVV, Pisipati SR, Mutava RN, et al. Sensitivity of grain sorghum to high temperature stress during reproductive development. Crop Sci. 2008;48:1911–1917.
- Ashraf M, Harris PJC. Photosynthesis under stressful environments: an overview. Photosynthetica. 2013;51:163–190.
- Lobell DB, Asner GP. Climate and management contributions to recent trends in U.S. agricultural yields. Science. 2003;299(5609):1032.
- Guidi L, Piccolo E, Landi M. Chlorophyll fluorescence, photoinhibition and Abiotic stress: does it make any difference the fact to be a C3 or C4 species? Front Plant Sci. 2019;10:174.
- Yi F, Wang Z, Baskin CC, et al. Seed germination responses to seasonal temperature and drought stress are species-specific but not related to seed size in a desert steppe: implications for effect of climate change on community structure. Ecol Evol. 2019;9(4):2149–2159.
- Khan MA, Gul B, Weber DJ. Effect of salinity and temperature on the germination of Kochia scoparia. Wetl Ecol Manag. 2001;9:483–489.
- Savitch LV, Massacci A, Gray GR, et al. Acclimation to low temperature or high light mitigates sensitivity to photoinhibition: roles of the Calvin cycle and the Mehler reaction. Aust J Plant Physiol. 2000;27:253–264.
- Youssef T. Stomatal, biochemical and morphological factors limiting photosynthetic gas exchange in the mangrove associate Hibiscus tiliaceus under saline and arid environment. Aquat Bot. 2007;87:292–298.
- Stepien P, Johnson GN. Contrasting responses of photosynthesis to salt stress in the glycophyte Arabidopsis and the halophyte Thellungiella: role of the plastid terminal oxidase as an alternative electron sink. J Plant Physiol. 2009;149:1154–1165.
- Zhu J, Meinzer C. Efficiency of C4 photosynthesis in Atriplex lentiformis under salinity stress. Aust J Plant Physiol. 1999;26:79–86.
- López-Climent M, Arbona V, Pérez-Clemente RM, et al. Relationship between salt tolerance and photosynthetic machinery performance in citrus. Environ Exp Bot. 2008;62:176–184.
- Maiseyenkava YA, Pshybytko NL, Kabashnikova LF. Greening barley seedlings under high temperature. Gen Appl Plant Physiol. 2005;31(1-2):3–14.
- Posch BC, Kariyawasam BC, Bramley H, et al. Exploring high temperature responses of photosynthesis and respiration to improve heat tolerance in wheat. J Exp Bot. 2019;70:5051–5069.
- Maxwell K, Johnson GN. Chlorophyll fluorescence—a practical guide. J Exp Bot. 2000;51:659–668.
- Migahid AM. Flora of Saudi Arabia. 4th ed. Riyadh: University Libraries, King Saudi University; 1990.
- Arnon D, Hoagland DR. Crop production in artificial culture solutions and in soils with special reference to factors influencing yields and absorption of inorganic nutrients. Soil Sci. 1940;50:463–484.
- AL-Shoaibi AA. Photosynthetic response of elephant grass (Pennisetum purpureum) to NaCl salinity. J Biol Sci. 2008;8(3):610–615.
- Von Caemmerer S, Farquhar GD. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981;153:376–387.
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207.
- Farooq M, Hussain M, Wakeel A, et al. Salt stress in maize: effects, resistance mechanisms, and management. A review. Agron Sustain Dev. 2015;35:461–481.
- Qu XX, Huang ZY, Baskin JM, et al. Effect of temperature, light and salinity on seed germination and radicle growth of the geographically widespread Halophyte Shrub Halocnemum strobilaceum. Ann Bot. 2008;101:293–299.
- Aiazzi MT, Carpane PD, Rienzo JA, et al. Effects of salinity and temperature on the germination and early seedling growth of Atriplex cordobensis Gandoger et Stuckert (Chenopediaceae). Seed Sci Technol. 2002;30:329–338.
- El-Keblawy AA, Al-Rawai A. Effects of salinity, temperature and light on germination of invasive Prosopis juliflora. J Arid Environ. 2005;61:555–565.
- Munns R. Physiological processes limiting growth in saline soils: some dogmas and hypotheses. Plant Cell Environ. 1993;16:15–24.
- Wahid A, Gelani S, Ashraf M, et al. Heat tolerance in plants: an overview. Environ Exp Bot. 2007;61:199–223.
- Nahar K, Hasanuzzaman M, Ahamed KU, et al. Plant responses and tolerance to high temperature stress: role of exogenous phytoprotectantsCrop production and global environmental issuesSpringer, Cham; 2015. p. 385–435.
- Almodares A, Hadi MR, Dosti B. Effects of salt stress on germination percentage and seedling growth in sweet sorghum cultivars. J Biol Sci. 2007;7:1492–1495.
- Al-Khateeb SA. Effect of salinity and temperature on germination, growth and ion relations of Panicum turgidum Forssk. Bioresour Technol. 2006;97:292–298.
- Nadjafi F, Shabahang J, Mahdavi Damghani AM. Effects of salinity and temperature on germination and seedling growth of nine medicinal plant species. Seed Technol. 2010;32(2):96–107.
- Jafarzadeh AA, Aliasgharzad N. Salinity and salt composition effects on seed germination and root length of four sugar beet cultivars. Biología. 2007;2(5):562–564.
- Munns R, Sharp RE. Involvement of abscisic acid in controlling plant growth in soils of low water potential. Aust J Plant Physiol. 1993;20:425–437.
- Morales F, Ancín M, Fakhet D, et al. Photosynthetic metabolism under stressful growth conditions as a bases for crop breeding and yield improvement: a review. Plants. 2020;9(88). doi.org/10.3390/plants9010088.
- Sharma A, Kumar V, Shahzad B, et al. Photosynthetic response of plants under different abiotic stresses: a review. J Plant Growth Regul. 2019;10:1007.
- Yang Z, Li JL, Liu LN, et al. Photosynthetic regulation under salt stress and salt-tolerance mechanism of sweet sorghum. Front Plant Sci. 2020;10:1722.
- Qu C, Liu C, Gong X, et al. Impairment of maize seedling photosynthesis caused by a combination of potassium deficiency and salt stress. Environ Exp Bot. 2012;75:134–141.
- Ehleringer J, Pearcy RW. Variation in quantum yield for CO2 uptake among C3 and C4 plants. J Plant Physiol. 1983;73:555–559.
- Dadkhah A. Effect of salinity on growth and leaf photosynthesis of two sugar beet (Beta vulgaris L.) cultivars. J Agric Sci Technol. 2011;13:1001–1012.
- Yan K, Chen P, Shao H, et al. Responses of photosynthesis and photosystem II to higher temperature and salt stress in Sorghum. J Agron Crop Sci. 2012;198:218–225.
- Ashraf M. Some important physiological selection criteria for salt tolerance in plants. Flora. 2004;199:361–376.
- Sudhir P, Murthy SDS. Effects of salt stress on basic processes of photosynthesis. Photosynthetica. 2004;42:481–486.
- Aldesuquy HS, Gaber AM. Effect of growth regulators on Vicia faba plants irrigated by sea water. leaf area, pigment content and photosynthetic activity. Biologia Plantarum. 1993;35:519–527.
- Misra AN, Sahu SM, Misra M, et al. Sodium chloride induced changes in leaf growth, and pigment and protein contents in two rice cultivars. Biologia Plantarum. 1997;39(2):257–262.
- AL-Shoaibi AA, AL-Sobhi OA. The effect of salinity on growth of elephant grass (Pennisetum purpureum). Proceedings of 2nd Saudi Science Conference, Part 1; 2004 Mar 15–17. p. 141–147.
- Boutraa T, Akhkha A, Al-Shoaibi AA. Evaluation of growth and gas exchange rates of two local Saudi wheat cultivars grown under heat stress conditions. Pak J Bot. 2015;47(1):27–34.
- Purnama PR, Purnama ER, Manuhara YSW, et al. Effect of high temperature stress on changes in morphology, anatomy and chlorophyll content in tropical seagrass Thalassia hemprichii. AACL Bioflux. 2018;11(6):1825–1833.
- Arfan M, Athar HR, Ashraf M. Does exogenous application of salicylic acid through the rooting medium modulate growth and photosynthetic capacity in two differently adapted spring wheat cultivars under salt stress? J Plant Physiol. 2007;164:685–694.
- Yan K, Chen P, Shao H, et al. Effects of short-term high temperature on photosynthesis and photosystem II performance in sorghum. J Agron Crop Sci. 2011;197:400–408.
- Aro EM, Virgin I, Andersson B. Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim Biophys Acta. 1993;1143(2):113–134.
- Chen M, Yang Z, Liu J, et al. Adaptation mechanism of salt excluders under saline conditions and its applications. Int J Mol Sci. 2018;19:3668.
- Hayat S, Hayat Q, Alyemeni MN, et al. Role of proline under changing environments. Plant Signal Behav. 2012;7:1456–1466.
- Munns R, Tester M. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 2008;59:651–681.