?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The present work aimed to investigate the effect of plasma-activated water (PAW) on seed germination and seedling growth of mung bean sprouts. Distilled water was exposed to non-thermal plasma for 15, 30, 60, and 90 s to prepare PAW, which was defined as PAW15, PAW30, PAW60, and PAW90, respectively. The germination rate, growth characteristics, total phenolic and flavonoid contents were all maximized when mung bean seeds were treated by PAW15, then followed by a decline over the prolonged plasma activation time (30–90 s). PAW15 caused no remarkably changes in the antioxidant capacity of sprouts as compared to DW (p > 0.05). However, the antioxidant activity of sprouts prepared with PAW30, PAW60, and PAW90 were decreased significantly (p < 0.05) compared with that of sprouts soaked in distilled water. In summary, PAW may be used to improve the production of sprouts, but the process parameters should be optimized for each application.
GRAPHICAL ABSTRACT
1. Introduction
Mung bean sprouts are widely consumed as one of the most popular vegetables for their greater health benefits and fewer calories. In decade years, numbers of physical, chemical, and combination intervention strategies have been implemented to stimulate seeds germination and production of bean sprouts [Citation1,Citation2], such as gamma irradiation, ultrasound, X-irradiation, electromagnetic field, cold plasma, electrolyzed oxidizing water, and pesticides [Citation3,Citation4]. However, these technologies are associated with multiple serious adverse effects, such as high cost, time consuming, leaving chemical residues to environment, and raising ecological issues. Therefore, it is still necessary to develop cost-efficient and environment-friendly technologies to improve the production of sprouts.
Over the past decade, plasma-activated water (PAW) has shown promising applications at various stages of the food supply chain, such as inactivation of microorganisms [Citation5,Citation6], preservation of fruits [Citation7], vegetables [Citation8], and seafood products [Citation9] with less adverse effects on the quality characteristics of food products. Recently, the application of PAW has been extended to agriculture, especially for regulating seeds germination and plant growth. It has been reported that the germination rate of PAW-treated rye seeds was increased when compared to the untreated samples [Citation10]. Similar findings were also observed in PAW treated-soybean seeds [Citation1]. However, a decrease in germination rate of PAW-watered lentil seeds was observed by Zhang et al. [Citation5].
Nowadays, limited research has been conducted on the effects of PAW on germination and growth characteristics of mung bean seeds, especially the phytochemicals and bioactivities of sprouts. Therefore, the purpose of this study was to examine the effects of PAW on seed germination, seedling growth, phytochemical components, and antioxidant capacity of mung beans sprouts. In addition, the main physical and chemical properties of PAW were also evaluated to explain the underlying mechanisms of PAW.
2. Materials and methods
2.1. Materials and chemicals
Mung bean seeds (Zhonglv 5) were provided by the Institute of Crop Sciences, Chinese Academy of Agricultural Sciences (Beijing, China). Folin-Ciocalteu’s phenol reagent, ferrous ammonium sulfate, xylenol orange, 2,2-dipheny l-1-picrylhydrazyl (DPPH•), potassium sulphate, 2,4,6-tris(2-pyridyl)-1,3,5-triazine (TPTZ), Trolox, gallic acid, 2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), and rutin were purchased from Aladdin Industrial Co. (Shanghai, China).
2.2. Preparetion of PAW
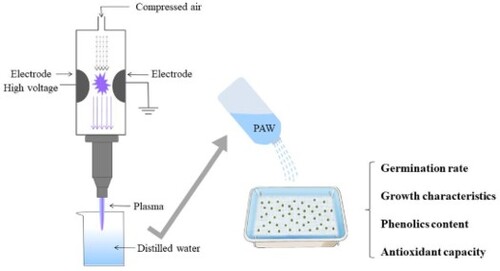
The atmospheric pressure plasma jet (APPJ) device was used to prepare PAW [Citation11]. The plasma generator was energized by a high-frequency high-voltage power supply (5 kV, 40 kHz). The distance between the atmospheric-pressure plasma jet nozzle and the water surface was 5 mm and the plasma discharge power was set at 750 W. Approximately 200 mL of distilled water was exposed to plasma for 15, 30, 60, and 90 s to prepare PAW, which was referred to as PAW15, PAW30, PAW60, and PAW90, respectively.
2.3. Measurements of properties of PAW
The pH and electric conductivity of PAW were measured with a pHS-3C digital pH metre and a DDS-307A electrical conductivity metre (INESA Scientific Instrument Co., Ltd., Shanghai, China), respectively. ORP was determined by using a 501 rechargeable ORP composite electrode (INESA Scientific Instrument Co., Ltd, Shanghai, China) connected to a dual channel pH metre. The levels of H2O2 and NO2− in PAW were measured according to the method described in the previous study [Citation6]. The contents of NO3− in PAW were measured according to the method reported by Shen et al. [Citation12].
2.4. Production of mung bean sprouts
Mung bean seeds (approximately 20 g) of similar size and shape were soaked in 100 mL of distilled water, PAW15, PAW30, PAW60, and PAW90 for 6 h at ambient temperature, respectively [Citation13]. Then, the hydrated seeds were placed in seedling trays filled with wet filter paper (20.5 cm × 13.5 cm) and all seedlings were incubated for 5 days, keeping away from illumination at 25 °C and 85% relative humidity. The seeds were watered daily with distilled water, PAW15, PAW30, PAW60, and PAW90 (100 mL × 3 times) until the harvesting [Citation14].
2.5. Determination of water uptake and germinability of mung bean seeds
Water uptake of seeds was determined by measuring the weight of mung bean seeds (approximately 20 g) before and after the soaking in distilled water or PAW. At fixed intervals of 0, 2, 4, 6, 8, and 10 h, the seeds were taken out, dried with absorbent paper, and weighted. The water absorption rate (Wa) of mung bean seeds in each group was calculated according to the following formula:
(1)
(1) Where W0 is the initial weight of seeds; W1 is the final weight of seeds after absorbing water at fixed intervals of time [Citation1].
Germination percentage of mung bean seeds were measured after being placed in seedling trays with distilled water or PAW for 12, 15, 18, 21, and 24 h, respectively. The emergence of radicle elongated to 2 mm from the seed coat was taken as the criteria for germination [Citation15]. Germination rates of mung bean seeds were calculated as following:
(2)
(2) Where N0 is the total number of seeds in each group and N1 is the number of germinated seeds.
2.6. Growth characteristics of mung bean seeds
The stem and root length of mung bean sprouts were measured with a graded ruler after sowing for 5 days [Citation16]. The final results were expressed as the average length and weight of mung bean sprouts in each group. Meanwhile, the weight of sprouts was also determined.
2.7. Preparation of extract
The extract of mung bean sprouts were prepared by the reported method [Citation17]. In brief, freeze-dried mung bean sprouts were prepared and grinded into powder. The acetone/water/acetic acid mixture (70: 29.5: 0.5, v/v/v) was selected as the extraction solvent. The ground sprouts samples (1.0 g) were extracted with 10.0 mL of extraction solvent at room temperature for 3 h with continuous shaking at 150 rpm. After centrifugation at 6800 × g for 15 min at 25 °C, the supernatant was stored and the residues were re-extracted. The obtained supernatants were combined and made up to 9.0 mL with the fresh extracting solvent. All extracts were stored in a refrigerator at 4 °C until analysis.
2.8. Measurement of total phenolic and flavonoid contents
Total phenolic contents in extracts of mung bean sprouts were measured using the Folin–Ciocalteu assay [Citation11] and the results were expressed as gallic acid equivalent (GAE) in milligrams per gram of dry weight (DW) of mung bean sprouts (mg GAE/g DW). The total flavonoid content was estimated by aluminium chloride (AlCl3) colorimetric method and the results were shown as mg rutin equivalent (RE) per gram dry weight (mg RE/g DW) [Citation18].
2.9. Analysis of antioxidant properties of mung bean sprouts extracts
The antioxidant capacity of mung bean sprouts extracts was measured by ABTS•+ and DPPH• assays [Citation11]. The results were expressed as milligrams trolox equivalent per gram dry weight (mg Trolox/g DW). The ferric reducing antioxidant power (FRAP) of mung bean sprouts extracts was estimated according to the method previously reported by Benzie and Strain [Citation19] and the results were expressed as mg FeSO4/g DW.
2.10. Statistical analysis
The data were expressed as mean ± standard deviation (SD). All statistical analyses were performed with SPSS for Windows (version 21.0, IBM, Chicago, IL, USA). Statistical significance between multiple groups was evaluated by one-way analysis of variance (ANOVA) with the least significant difference (LSD) test at p < 0.05. Correlations among the total phenolic content, total flavonoid content, and antioxidant activity were evaluated by Pearson’s correlation analysis.
3. Results and discussion
3.1. Physicochemical characteristics of PAW
During plasma treatment, reactive oxygen and nitrogen species (RONS) are generated in PAW, such as •OH, O2•−, 1O2, O3, H2O2, NO2−, and NO3− [Citation20]. In agreement with previous studies, a clear formation of NO2−, NO3−, and H2O2 was observed in PAW (Figure A, 1B, and 1C). After being activated by plasma for 90 s, the concentration of NO2−, NO3−, and H2O2 in PAW increased almost linearly to 62.05, 118.39, and 0.90 mg/L (p < 0.05), respectively. These long-lived reactive species are supposed to play significant roles in the regulation of seed dormancy and germination [Citation21,Citation22].
Figure 1. Physicochemical characteristics of PAW. The values of (A) NO2-, (B) NO3-, (C) H2O2, (D) pH, (E) ORP, and (F) electric conductivity of PAW were measured as described in the Materials and methods section. Different letters indicate significant differences between groups (p < 0.05).
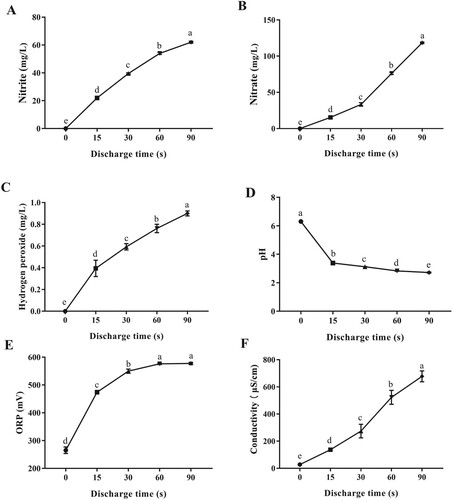
Moreover, as noticed in Figure D, the pH value of PAW significantly decreased with the increasing discharge time. After being activated by plasma for 15 s, the pH value drastically reduced to 3.38 compared with 6.30 of distilled water. Water acidification during plasma discharge have been reported in previous studies [Citation23], which may be related to the formation of nitrous acid, nitric acid, and peroxynitrous acid inside liquid water during plasma activation. ORP refers to the ability of solution to gain or lose electrons. According to Figure E, the ORP of PAW samples remarkably increased over plasma discharge time from 15 s to 90 s. A similar increase in ORP of PAW was also reported by Ma et al. [Citation24]. Additionally, as a measure of the ability of water to pass an electrical current, electric conductivity has been widely used to estimate the level of active ions in PAW [Citation20]. From Figure F, the electric conductivity of PAW samples significantly increased as a function of plasma activation time (p < 0.05) and soared to 677.67 µS/cm at 90 s, indicating the formation of active ions in PAW.
3.2. Influences of PAW on water uptake of mung bean seeds
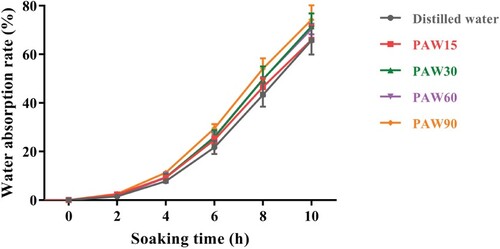
Water uptake is an essential step for the initiation of seed germination, leading to swelling and softening seed coat or testa [Citation25]. As displayed in Figure , PAW significantly increased the water absorption rate of mung bean seeds with the prolonged discharge time. After being soaked for 10 h, the water absorption rate of PAW90-soaked seeds reached to 74.26 ± 5.94%, which was significantly (p < 0.05) higher than that of distilled water-treated seeds (65.70 ± 5.79%). In another study, a sharply changed structure was observed on the surface of PAW-treated seeds by scanning electron micrograph [Citation26]. The author speculated that the high concentration of RONS produced in PAW might be responsible for the chapping of seed coat, and further could facilitate seed to absorb more water and nutrients.
3.3. Influences of PAW on germination rate of mung bean seeds
The effects of PAW treatment on seed germination are presented in Figure . When the seeds were treated by PAW15 for 30 h, the germination rate increased to 93.01 ± 2.59%, which was higher than 84.41 ± 8.62% of distilled water-treated seeds (p < 0.05). However, PAW prepared with longer discharge time (30–90 s) significantly inhibited germination of mung bean seeds. At the same incubation time of 30 h, the germination rate of mung bean seeds watered by PAW30, PAW60, and PAW90 decreased by 0.29%, 3.47%, and 6.07%, respectively, when compared with that of PAW15-treated samples. In other studies, inconsistent results were found for the effects of PAW treatment on germination rates of agricultural seeds, such as the increased effect [Citation10], the inhibition [Citation5], and also no significant change [Citation1].
Figure 3. Average germination rate of mung bean seeds watered by distilled water or PAW during the indicated incubation time.
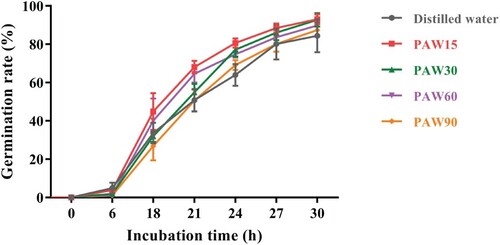
As shown in Figure , the reactive species in PAW, such as H2O2, NO2−, and NO3− was clearly observed and lasting increased over the plasma discharge time. Generally, reactive species are recognized as playing crucial roles in seed physiology, depending on the dynamic equilibrium between their generation and metabolism [Citation21, Citation22]. At low concentration, reactive species may act as positive signal molecules to alleviate seed dormancy and stimulate seed germination by involving in abscisic acid/gibberellic acid signalling pathways and so on [Citation27]. However, the excessive accumulation of reactive species can lead to toxic effects to seeds [Citation27]. Nowadays, due to the different conditions of each experiment such as discharge mode, feed gas, and solution types, the chemical compositions of PAW are quite different [Citation28]. So, the various types and concentration of reactive species are possibly the main reasons for the inconsistent results in seeds responding to PAW treatment, while the exact mechanisms are not completely understood. Further work is still needed to clearly illuminate the role of each reactive species in PAW on the germination of seeds and the underlying molecular mechanisms.
3.4. Effect of PAW treatment on seedling growth
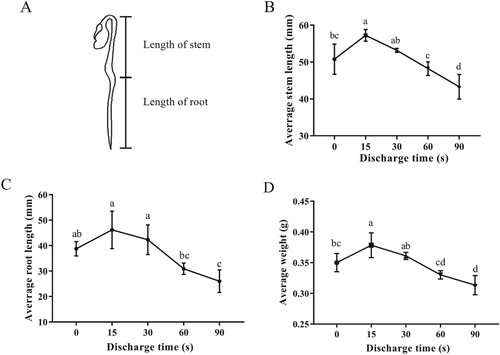
The growth characteristics of mung bean sprouts grown after 5 days, such as stem length, root length, and weight, are recorded in Figure . It was observed that the sprouts prepared with PAW15 showed significantly increase in stem length and average weight by 8.09% and 12.75%, respectively, compared to distilled water-treated samples (p < 0.05). However, PAW prepared with longer discharge time (>15 s) had significant negative effect on seedling growth. The stem length, root length and average weight of PAW90-watered sprouts were significantly decreased by 14.73%, 32.83%, and 10.46% (p < 0.05), respectively, as compared with those of distilled water-treated sprouts. Similar results have been reported by Porto et al. [Citation1]. In this study, PAW prepared with plasma for 1 min achieved more efficient in average stem length than that of 5 min. The authors revealed that the negative effect might be attributed to the more acidity of the latter than former, affecting the beneficial microorganism, which could degrade organic matter and transfer nutrients for plant [Citation1]. In another research, Sivachandiran & Khacef explored the influences of cold plasma and PAW on germination and growth of three types of seeds: tomato, radish, and sweet pepper [Citation23]. They observed that the average stem length of tomato seeds treated by PAW was shorter than that of control group, while contrary to the results in radish. In summary, there was great difference in the effects of PAW on various seeds. Hence, the plasma discharge time and PAW treatment time should be optimized for each type of seeds.
Figure 4. Effects of PAW treatment on the growth characteristics of mung bean sprouts. (A) Schematic diagram of mung bean sprout, (B) stem length, (C) root length, (D) weight of mung bean sprouts after sowing for 5 days. Different letters indicate statistically significant differences between groups (p < 0.05).
3.5. Changes in total phenolic and flavonoid contents
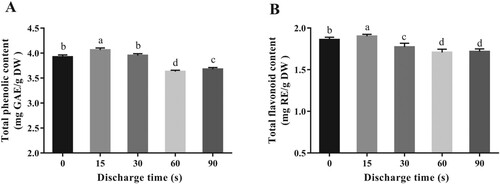
Phenolic and flavonoid compounds are the major bioactive compounds in mung bean sprouts, which offer many promising health benefits [Citation29]. As shown in Figure , the changes in the total phenolic and flavonoid contents were very similar with the tendency in seed germination and growth. After sowing for 5 days, the total phenolic and flavonoid contents of PAW15-watered samples remarkably increased by 3.53% and 2.22% (p < 0.05), respectively, compared with that of control group. However, when the plasma exposure time prolonged to 90 s (PAW90), the contents of total phenolic and flavonoid were significantly reduced to 3.68 mg GAE/g DW and 1.72 mg RE/g DW, respectively, which were lower than that of the sprouts produced with distilled water (p < 0.05). Mildaziene et al. found a substantial increase in phenolic acids (cichoric acid, caftaric acids, and chlorogenic acid), vitamin C, and radical scavenging activity of purple coneflower seeds after a short-time treatment of cold plasma, which might be beneficial to improve the defensive response against unfavourable conditions [Citation30]. Besides, Michalak also pointed out that the regulation of phenolic metabolism serves as a response for oxidative damage induced by heavy metal stress [Citation31]. Therefore, summarizing these findings, plant tissue might accumulate phenolic compounds as a defensive response against oxidative stress induced by PAW.
3.6. Changes in antioxidant activity of mung bean sprouts
The influences of PAW on the antioxidant capacity of mung bean sprouts extract was also investigated in the present work. According to Table , the PAW15 treatment didn’t lead to remarkably changes in the in vitro antioxidant potential of mung bean sprouts as compared to distilled water (p > 0.05). In addition, the three different analysis methods resulted in a consistent foundation that the antioxidant capacity of mung bean sprouts extract decreased with plasma exposure durations range of 15–90 s (p > 0.05). For instance, as measured by ABTS assay, the antioxidant activity reduced by 16.44% in PAW90-watered sprouts in contrast with the control group of 8.69 mg Trolox/g DW. Beside known phenolic and flavonoid compounds, other components in sprouts, such as polypeptides and polysaccharides, also show potential antioxidant activity [Citation32], which may produce the inconsistent trends between the changes in the antioxidant activity and total phenolic and flavonoid contents of mung bean sprouts.
Table 1. Effect of PAW treatment at different exposure durations on the antioxidant activity of mung bean sprouts.
As shown in Table , there was a strong correlation between the antioxidant activity of mung bean sprouts extract with total phenolic and total flavonoid content (0.716≤R2≤0.873, p < 0.01), indicating that phenolic and flavonoid compounds contributed greatly to the antioxidant potential of mung bean sprouts.
Table 2. Pearson’s correlation analysis between total phenolic and flavonoid content & antioxidant activity.
4. Conclusion
These results indicated that PAW15 could significantly stimulate mung bean seeds germination and growth. However, the negative effects of PAW on germination of mung bean seeds were observed when the plasma discharge time increased from to 30 s to 90 s. Similar changing trends were also observed in the growth parameters as well as total phenolic and flavonoid content, which might be related to the active components in PAW, such as NO2−, NO3−, and H2O2. In summary, PAW may be considered as a promising technology to improve the seeds germination and seedling growth. The effects of PAW on germination and growth of seeds are influenced by many factors, such as seeds types, the gas used for plasma discharge, plasma activation time, and PAW treatment time. Thus, the process parameters should be optimized for each application of PAW. In addition, the underling molecular mechanisms of PAW-regulated seed germination and growth should be clarified in future work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Lo Porto C, Ziuzina D, Los A, et al. Plasma activated water and airborne ultrasound treatments for enhanced germination and growth of soybean. Innov Food Sci Emerg Technol. 2018;49:13–19.
- Shakir SK, Kanwal M, Murad W, et al. Effect of some commonly used pesticides on seed germination, biomass production and photosynthetic pigments in tomato (Lycopersicon esculentum). Ecotoxicology. 2016;25(2):329–341.
- Rifna EJ, Ramanan KR, Mahendran R. Emerging technology applications for improving seed germination. Trends Food Sci Tech. 2019;86:95–108.
- Singh R, Prasad P, Mohan R, et al. Radiofrequency cold plasma treatment enhances seed germination and seedling growth in variety CIM-Saumya of sweet basil (Ocimum basilicum L.). J Appl Res Med Aromat Plants. 2019;12:78–81.
- Zhang S, Rousseau A, Dufour T. Promoting lentil germination and stem growth by plasma activated tap water, demineralized water and liquid fertilizer. RSC Adv. 2017;7:31244–31251.
- Xiang QS, Kang CD, Niu LY, et al. Antibacterial activity and a membrane damage mechanism of plasma-activated water against Pseudomonas deceptionensis CM2. LWT-Food Sci Technol. 2018;96:395–401.
- Guo J, Huang K, Wang X, et al. Inactivation of yeast on grapes by plasma-activated water and its effects on quality attributes. J Food Prot. 2017;80(2):225–230.
- Xu YY, Tian Y, Ma RN, et al. Effect of plasma activated water on the postharvest quality of button mushrooms, Agaricus bisporus. Food Chem. 2016;2016(197):436–444.
- Liao XY, Su Y, Liu DH, et al. Application of atmospheric cold plasma-activated water (PAW) ice for preservation of shrimps (Metapenaeus ensis). Food Control. 2018;94:307–314.
- Naumova IK, Maksimov AI, Khlyustova AV. Stimulation of the germinability of seeds and germ growth under treatment with plasma-activated water. Surf Eng Appl Electrochem. 2011;47(3):263–265.
- Xiang QS, Liu XF, Liu SN, et al. Effect of plasma-activated water on microbial quality and physicochemical characteristics of mung bean sprouts. Innov Food Sci Emerg Technol. 2019;52:49–56.
- Shen J, Tian Y, Li YL, et al. Bactericidal effects against S. aureus and physicochemical properties of plasma activated water stored at different temperatures. Sci Rep. 2016;6:28505.
- Tiansawang K, Luangpituksa P, Varanyanond W, et al. GABA (γ-aminobutyric acid) production, antioxidant activity in some germinated dietary seeds and the effect of cooking on their GABA content. Food Sci Technol. 2016;36(2):313–321.
- Liu R, Zhang DC, He XL, et al. The relationship between antioxidant enzymes activity and mungbean sprouts growth during the germination of mungbean seeds treated by electrolyzed water. Plant Growth Regul. 2014;74:83–91.
- Kaur R, Kaur J, Bains TS. Screening of mung bean genotypes for drought tolerance using different water potential levels. J Adv Agric Technol. 2017;4(2):159–164.
- Liu R, Hao JX, Liu HJ, et al. Application of electrolyzed functional water on producing mung bean sprouts. Food Control. 2011;22(8):1311–1315.
- Xu BJ, Chang SKC. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J Food Sci. 2007;72(2):159–166.
- Tian M, Xu XY, Liu YL, et al. Effect of Se treatment on glucosinolate metabolism and health-promoting compounds in the broccoli sprouts of three cultivars. Food Chem. 2016;190:374–380.
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem. 1996;239(1):70–76.
- Thirumdas R, Kothakota A, Annapure U, et al. Plasma activated water (PAW): chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci Tech. 2018;77:21–31.
- Atia A, Debez A, Barhoumi Z, et al. ABA, GA3, and nitrate may control seed germination of Crithmum maritimum (Apiaceae) under saline conditions. C R Biol. 2009;332(8):704–710.
- Barba-Espín G, Hernández JA, Diaz-Vivancos P. Role of H2O2 in pea seed germination. Plant Signal Behav. 2012;7(2):193–195.
- Sivachandiran L, Khacef A. Enhanced seed germination and plant growth by atmospheric pressure cold air plasma: combined effect of seed and water treatment. RSC Adv. 2017;7(4):1822–1832.
- Ma RN, Yu S, Tian Y, et al. Effect of non-thermal plasma-activated water on fruit decay and quality in postharvest Chinese bayberries. Food Bioprocess Tech. 2016;9(11):1825–1834.
- Obroucheva NV, Sinkevich IA, Lityagina SV, et al. Water relations in germinating seeds. Russ J Plant Physiol. 2017;64(4):625–633.
- Zhou RW, Li JW, Zhou RS, et al. Atmospheric-pressure plasma treated water for seed germination and seedling growth of mung bean and its sterilization effect on mung bean sprouts. Innov Food Sci Emerg Technol. 2018;53:36–44.
- Kumar PJ, Siddegowda RP, Banerjee R, et al. Seed birth to death: dual functions of reactive oxygen species in seed physiology. Ann Bot. 2015;116(4):663–668.
- Sarangapani C, Patange A, Bourke P, et al. Recent advances in the application of cold plasma technology in foods. Annu Rev Food Sci Technol. 2018;9(1):609–629.
- Ganesan K, Xu BJ. A critical review on phytochemical profile and health promoting effects of mung bean (Vigna radiata). Food Sci Hum Wellness. 2018;7(1):11–33.
- Mildaziene V, Pauzaite G, Naucienė Z, et al. Pre-sowing seed treatment with cold plasma and electromagnetic field increases secondary metabolite content in purple coneflower (Echinacea purpurea) leaves. Plasma Process Polym. 2018;15(2):e1700059.
- Michalak A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol J Environ Stud. 2006;15(4):523–530.
- Tang DY, Dong YM, Ren HK, et al. A review of phytochemistry, metabolite changes, and medicinal uses of the common food mung bean and its sprouts (Vigna radiata). Chem Cent J. 2014;8(1):4.