ABSTRACT
In the present study, we synthesized carbon nanoparticles with a different method using chitosan, a natural polymer. As a result, carbon nanomaterials were diffused directly through the intestinal membrane, and carbon nanoparticles were obtained. Surface and chemical characterizations of the obtained carbon nanoparticles were determined by different techniques such as FTIR, DLS, SEM, EDX, and UV-visible. Moreover, the cytotoxic effects of the carbon nanoparticles on Saos-2, MCF-7 cancer cells, and HUVEC non-cancerous cells were evaluated with the MTT test. Although the size of these carbon nanoparticles is not as small as carbon nanodots, these nanoparticles were very effective on cancer cells. Interestingly, our results showed that drug-free carbon nanoparticles were remarkably cytotoxic on MCF-7 and Saos-2 cancer cells. In addition, 5FU loaded carbon nanoparticles (IC50: 12 µg/mL) were 3.5 times more effective than 5FU alone (IC50: 43 µg/mL) on MCF-7 cell lines.
GRAPHICAL ABSTRACT
1. Introduction
In recent years, the emergence of nanoscience has opened new horizons for researchers. With the developments in nanotechnology, particularly in biology and medicine, new and complex nanomaterials are used, and incredibly multifunctional nanocarrier systems for the diagnosis and treatment of common cancer types and some diseases are being used studied. In particular, carbon nanomaterials are used in many kinds of research and continue to attract increasing attention. Carbon nanostructures have unique properties such as high surface area, environmentally friendly materials, and non-toxic and biocompatible materials. At the same time, the fact that these materials can be synthesized in simple and low-cost ways has made them more interesting [Citation1,Citation2]. Carbon nanoparticles, especially carbon nanodots, are widely used in different fields such as fluorescence imaging (FL), two-photon FL, Raman imaging, magnetic resonance imaging (MRI), tomography (CT), photoacoustic imaging (PAI), computed positron emission tomography/single photon emission computed tomography (PET/SPECT), and multimodal imaging[Citation3,Citation4]. Carbon nanotubes, on the other hand, are preferred for energy storage due to their large surface area conductivity. At the same time, carbon nanotubes are also involved in the repair of DNA. However, carbon nanoparticles have recently broken new ground in technology with the production of aromatic structures with conjugated double bonds at the Nanoscale. In addition to imaging, carbon nanoparticles are also the focus of attention of researchers in drug release studies and the design and construction of electrochemical sensors [Citation2–4].
To date, researchers have tried many methods to produce carbon nanoparticles. Initially, the logic was similar to metal nanoparticle production, where a reducing reagent (such as NaBH4 or LiAlH4) is used to prevent the formation of an ion pair layer around the metal because of the positive valence. In contrast, it is coated with a reagent (such as trisodium citrate) using the core–shell relationship against the risk of oxidation in the aqueous medium simultaneously [Citation5]. Carbon nanoparticles were coated with a polymer such as Oxa (IV) COOH or Poly (N-isopropyl acrylamide) [Citation6,Citation7]. However, in recent years, different methods have been tried to synthesize carbon nanoparticles, and new synthesis methods continue to be investigated [Citation2,Citation8–10].
Researchers focused on carbon nanodots, excellent nanocarriers with large functional surface groups below 10 nm when their properties were discovered. The easiest production method known so far is to treat lemon juice and onion juice in the microwave to produce carbon nanodots [Citation11,Citation12]. However, this method has been determined to have low repeatability when tried by other researchers. Later, another technique became more popular, where carbon nanoparticles are extracted by reverse diffusion under pressure from a microwaved sample after the carbonization process, such as a dialysis machine. As can be seen, one-pot synthesis of nano-structured carbon materials has been the focus of researchers [Citation13]. However, the dialysis device is expensive, and the method requires a process, which led to the exploration of different methods.
In this study, we put the chitosan, for which there are no previous examples in the literature, in gel form and caramelize it in the microwave. Unlike the literature, we obtained the carbon nanoparticles by naturally diffusing the caramelized carbonaceous material through an animal intestine membrane. The carbon nanoparticle obtained by this method has the potential to be used directly as a drug in the treatment of cancer.
2. Material methods
The surface properties of the powders were examined using a Gemini 500 computer-controlled digital Scanning Electron Microscopy (SEM). Quantitative elemental analysis was performed with an EDX spectrometer attached to SEM. FTIR device (BRUKER ALPHA, having a resolution of 4 cm x 1; equipped with a DTGS detector, performing ten scans for each spectrum) was used to record vibration peaks. Nanoparticle measurements reported in this paper were made at a temperature of 25°C on a Zetasizer Nano Z.S. (Malvern Instruments Ltd, Malvern, U.K.) fitted with a high-concentration zeta potential cell (ZEN1010).
2.1. Cell culture and cytotoxicity test (MTT assay)
In this study, human breast cancer (MCF-7), human osteosarcoma (Saos-2), and human umbilical vein endothelial cells (HUVEC) cell lines obtained from the ATCC (American Type Culture Collection) were used. The cells were maintained in Dulbecco's Modified Eagle Medium (DMEM; Sigma) supplemented with 10% heated-inactivated fetal bovine serum (FBS; Sigma) and 100 units/mL penicillin and 100 µg/mL of streptomycin (Sigma) in a humidified incubator 5% CO2 at 37°C atmospheres. The cell lines were passaged after reaching 80% monolayer confluency.
The cytotoxic effects of free-carbon nanoparticles, 5-FU loaded carbon nanoparticles, and free 5FU were determined using the MTT method on the cells for 72 h [Citation14]. The MTT reduction characteristic of cells is a test that measures cell vitality, staining intensity acquired at the end of MTT analysis demonstrates the correlation with the number of living cells. For this purpose, the cells were cultured in 96-welled plates with 1 × 105 cells/ mL. The cells grown in DMEM were exposed to increasing concentrations of each free carbon nanoparticle, 5FU-loaded carbon nanoparticles, and free 5FU. After 72 h of incubation, MTT solution (10 µl) was added at 37°C for four h, and the medium was taken away from the environment cells were lyzed with 100 µl DMSO in which formazan crystals formed by MTT dissolved. Absorbance was read in an enzyme-linked immunosorbent assay (ELISA) plate reader.
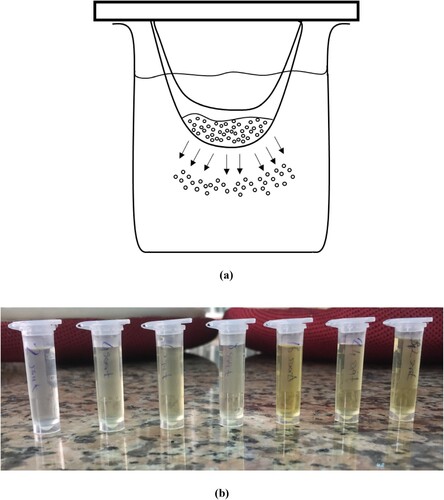
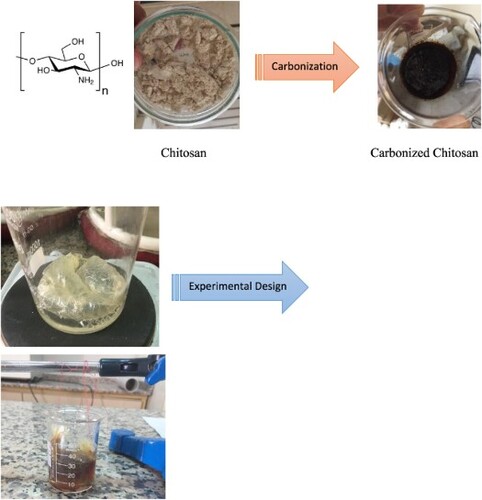
1 g chitosan (purchased analytical grade) was gelled with 1 mL of glacial acetic acid and dissolved in 10-20 mL of distilled water. The resulting sol gel was caramelized in a microwave oven, at 800 W, for at least 12 min. The caramelized product added up to 200-250 mL of pure water. Approximately 15 mL of this solution taken from the surface with a dropper was put into a 3-layer animal intestine membrane, which was previously kept in organic liquids such as acetone, ethanol, and chloroform for one day, purified by washing with distilled water several times. Both sides of the intestine were tightly tied and fixed to a metal (the tied edge of the intestinal membrane did not touch the solution in the beaker). The intestine was placed in a baker. The pure water is added to the baker at 15-20 mL. At certain time intervals (4-6-12 h), water was added again instead of the water in the beaker. Carbon nanoparticles diffused into the pure water through the intestine were taken using a dropper for a certain period. Pure water was added to the beaker to replace the liquid, and the times were scanned (in Figure ). The detail of the progress in producing Carbon nanoparticles is seen in Figure . The first image is the carbonization process, the second image is the experimental design, and the third image is the simulation of the occurring carbon nanoparticles in Figure .
3. Results and discussion
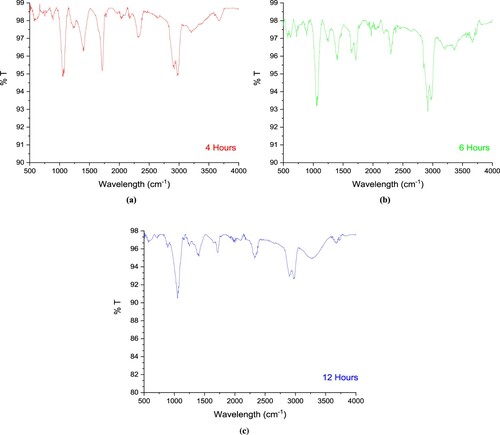
Figure (a) shows the FTIR spectrum of carbon nanoparticles. Figure shows FTIR spectra and %T values plotted against wavenumbers. % T mean is the transmission. At 3200-3500 cm−1, weak vibration signals from the –N.H. group covered the vibration signals from the –O.H. group. The following vibrations were observed: -CH aliphatic symmetrical stretching vibrations at 2976 and 2914 cm−1, –C = O symmetrical vibration signals at 2324 cm−1, C = O stretching at 1712cm−1, –CH bending at 1400 cm−1, –C–H stretching at 1224cm−1, –CH stretching band at 1054 cm−1, -CH bending out of the plane vibrations at 885 cm−1 [Citation15]. In addition, the vibration signals of the amine and hydroxyl groups belonging to chitosan could be detected very weakly. The infrared spectrum gives us information about the functional groups in the structure. According to this infrared spectrum, aliphatic and hydroxide functional groups are the predominant functional groups of carbon nanoparticles. Hence, the functional carbon nanoparticle is synthesized according to the FTIR spectrum.
Figure 2. (a) FTIR spectrum of carbon nanoparticles (4 h). (b) FTIR spectrum of carbon nanoparticles (6 h). (c) FTIR spectrum of carbon nanoparticles (12 h).
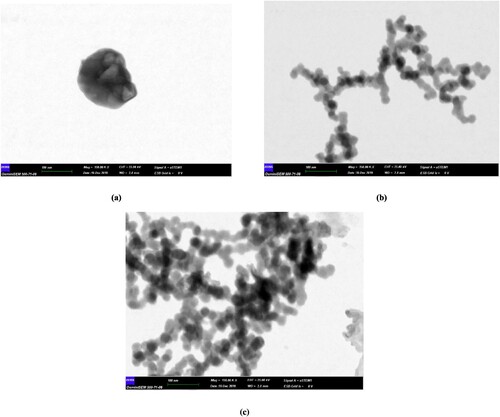
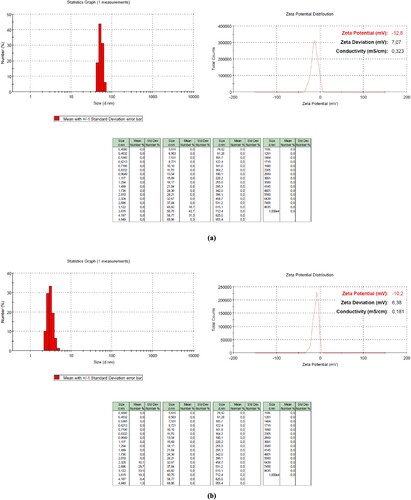
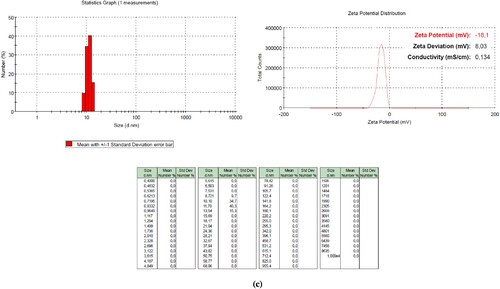
SEM images of carbon nanoparticles are given in Figure (b)(c)(d), whereas DLS analyses are given in Figure . The SEM images of the carbon nanoparticles are not edited or screened. Carbon nanoparticles below 100 nm were diffused in 4 h, carbon nanodots below 5 nm were obtained in 6 h, and carbon nanoparticles around 10 nm were obtained in 12 h. A careful examination reveals that in 6-hour diffusion, the particle sizes were below 10 nm. The samples are prepared for SEM analysis by coating with Au/Pt after being dropped and dried on the conductive layer; therefore, agglomerations can be observed. However, nanoparticles are of a discernible size. The diffusion time scans were then tested for 24–36 and 48 h, but after these times, carbon nanoparticles with a size of 100-200 nm were detected (in Figure ).
Figure 4. DLS analysis of carbon nanoparticles.
Figure shows the UV-VIS spectrum of the carbon nanodot obtained by 6-hour diffusion. In Figure , UV-VIS spectrum, Absorbance values were plotted against wavelengths. Maximum peaks are indices in the UV-VIS spectrum in Figure . This ridge, which generally corresponds to π-π * absorption transitions in the literature range of 240-270 nm, gave us a small transition peak corresponding to n – π * absorption transition around 332 nm. The difference in our U.V. spectrum from the others is chitosan's starting material. After caramelization, the vibration signals of chitosan's functional groups, such as -N.H., -O.H., also seem pretty weak in the FTIR spectrum. This is because –C.H. and –C = O functional groups are predominant in the structure rather than –N.H. and-OH groups.
Figure 5. UV-VIS. The Spectrum of Carbon nanoparticles. *The drug-free carbon nanoparticles were diluted with a medium in ratios of 1/2, 1/4, and 1/8.
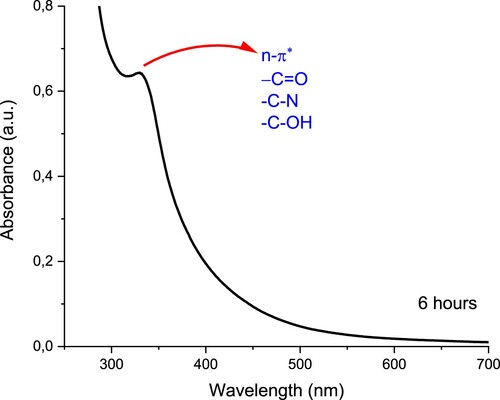
Nevertheless, such a peak at this point indicates that nano-sized carbon material was produced (<5 nm). Usually, to observe adsorption at 240-270 nm, –C = C groups must be present in the structure. Multiple studies in the literature are compatible with this type of U.V. spectrum [Citation16,Citation17]. This U.V. spectrum supports the FITR spectrum because the hydroxide functional groups are the main ones responsible for π-π * absorption electron transitions. Electron pairs (on the oxygen) in the lower energy level π transfer to the higher energy level π*.
In our study, MCF-7 and Saos-2 cancer cells were used. In addition, cytotoxicity results were compared with non-cancerous HUVEC cell lines as a control. The cells were analyzed by MTT test with drug-free carbon nanoparticles, 5-FU loaded carbon nanoparticles, and free 5-FU at various concentrations for 72 h. In Figure , interestingly, drug-free carbon nanoparticles were remarkably cytotoxic on MCF-7 and Saos-2 cancer cells. However, these drug-free nanoparticles are only effective at a higher rate on non-cancerous HUVEC cell lines than on MCF-7 and Saos-2 cancer cells. As the drug-free nanoparticles ratio increases, cytotoxicity also increases. Drug-free nanoparticles were the most cytotoxic on Saos-2 cell lines. The IC50 values of drug-free carbon nanoparticles differ on different cells. Accordingly, the IC50 values of drug-free carbon nanoparticles were reached when the nanoparticles were diluted at approximately 1/4 on Saos-2 cells, approximately 1/2 on MCF-7 cells, and undiluted (in 1 ratio) on HUVEC cells (Table ). However, it was an important finding that drug-free carbon nanoparticles showed low toxicity in non-cancerous HUVEC cells.
Figure 6. Cytotoxicity (%) of drug-free carbon nanoparticles at various ratios on HUVEC, MCF-7, and Saos-2 cells for 72 h.
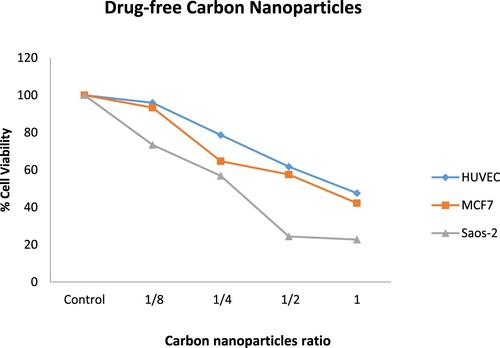
Table 1. Cytotoxicity of free-carbon nanoparticles, 5-FU loaded carbon nanoparticles and free 5-FU obtained by MTT on MCF-7, Saos-2 and HUVEC cell lines.
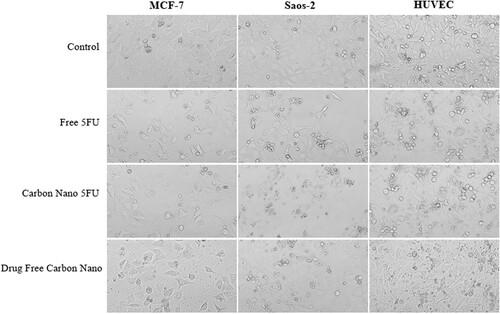
Figure shows MCF-7, Saos-2, and HUVEC cells proliferated in colonies. In general, control groups are surface-bound and interconnected in all cell lines. At the same time, it was observed that the control group cells were denser. After treatment with the compounds, the connections between the cells appear to be significantly reduced, and cell integrity is impaired in some of them. The number of cells has decreased significantly compared to control group cells.
5FU loaded nanoparticles (IC50: 12 µg / mL) were 3.5 times more effective than 5FU alone (IC50: 43 µg / mL) on MCF-7 cell lines. Thus, it is possible to achieve significant cytotoxicity with a lower concentration of 5FU on MCF-7 cancer cells. This finding is significant in reducing the anticancer drug's side effects. When IC50 values of 5FU-loaded nanoparticles on non-cancerous HUVEC and cancerous MCF-7 cells were compared, and IC50 value on HUVEC cells was approximately 2-times higher than MCF-7 cells with 21 µg/ mL. Hence, the concentration used for MCF-7 cells was slightly toxic for HUVEC cells (Table ).
As an exciting finding, drug-free nanoparticles appear to be more effective on Saos-2 cells than 5FU-loaded nanoparticles (in Figure ). It is reported that Saos-2 cells show strong adhesion with carbon nanoparticles [Citation18]. In addition, it is an essential finding that carbon nanoparticles are effective without drug loaded. When the effects of drug-free nanoparticles on non-cancerous HUVEC and cancerous Saos-2 cells are compared, the ratio of drug-free nanoparticles used on HUVEC cells is at least two times more effective on Saos-2 cell lines (Table ). The nanoparticles we prepared are promising compounds that can be very effective on cancer cells in light of these findings.
Figure 7. Cytotoxicity (%) of HUVEC, MCF-7, and Saos-2 cells were incubated with 5-FU-loaded carbon nanoparticles and free 5-FU at various concentrations for 72 h.
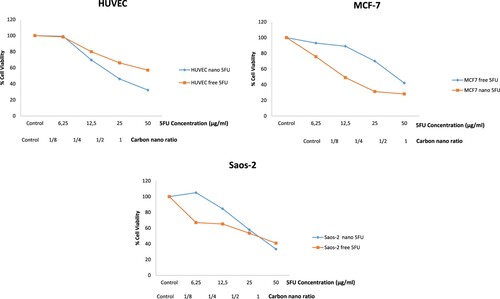
Figure 8. Demonstration of MCF-7, Saos-2, and HUVEC cell lines treated with free 5FU, carbon nano 5FU, and drug-free carbon nano in IC50 concentrations. Images were taken with a light microscope. Magnification: X100.
According to the researchers, chitosan leads to environmental protonation due to amine groups. Thus, the water-soluble bio adhesive structure also allows hydroxyl groups to easily attach to mucosal and basement membrane surfaces with negative functional groups. This polar structure of the chitosan facilitates its transport to epithelial surfaces [Citation19,Citation20]. With its antimetastatic activity, chitosan is suggested to trigger the permeation-enhancing mechanism in both in vitro and in vivo environments [Citation21]. Moreover, it is reported that chitosan nanoparticles are promising for liver damage by affecting the P53, iNOS, VEGF, PCNA, and CD68 pathways [Citation22]. In another study, Zhang et al. claimed that the nano cancer drug “mifepristone” they produced with chitosan increased anticancer activity, which explains the outcome of our test performed with 5-FU cancer drug and chitosan. Besides all these mechanisms, the triggering of cellular Apoptotic Mechanism by low molecular weight chitosan supports our results. Low molecular weight chitosan has more charge density. According to research, low molecular weight chitosan approaches cells with coulomb interactions and attacks the cell wall [Citation23,Citation24], which prevents substance exchange of the cancer cell and its connection with other cancerous cells. Therefore, both the Cellular Apoptotic Mechanism and the antimetastatic mechanism explain how nano chitosan that we produced affected the cancerous cell at this high cytotoxicity.
4. Conclusion
In this study, we used the intestinal membrane directly for the first time to produce carbon nanoparticles. Furthermore, we obtained a new method for mass production of nanoparticles. Ultimately, we hope this new method will contribute to the broader use of carbon nanoparticles in health and medicine.
Interestingly, drug-free carbon nanoparticles were remarkably cytotoxic in this study on MCF-7 and Saos-2 cancer cells, whereas they were low cytotoxic to normal HUVEC cells. Moreover, 5FU-loaded carbon nanoparticles were 3.5 times more effective than 5FU alone on MCF-7 cell lines. However, it was lower toxic than normal HUVEC cells. Thus, it was possible to achieve significant cytotoxicity with a lower concentration of 5FU on MCF-7 cancer cells. This finding is significant in reducing the anticancer drug's side effects. The nanoparticles we prepared are promising compounds for cancer cells in light of these findings.
We have developed a method suitable for mass production with high concentration and low investment cost with this study. Thus, this method is both innovative according to the literature and has differed from other carbon nanoparticle production methods in terms of outcomes. Its effects on cancer cells are also a breakthrough in this area.
5. Ethics approval and consent to participate
This study does not need any Ethics report.
6. Consent for publication
The Authors give consent for publication.
7. Availability of data and materials
All data and materials of the paper are available to the public.
8. Authors’ contributions
İ. Afşin Kariper: Design, investigation, supervisor
Ceylan Hepokur: Experimental
Ferdane Danışman Kalındemirtaş: Experimental, writing
Serpa Erdem Kuruca: Supervisor, writing, editing
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Cuenca AG, Jiang H, Hochwald SN, et al. Emerging implications of nanotechnology on cancer diagnostics and therapeutics. Cancer. 2006;107:459–466. https://doi.org/10.1002/CNCR.22035.
- Asadian E, Ghalkhani M, Shahrokhian S. Electrochemical sensing based on carbon nanoparticles: a review. Sens Actuators B Chem. 2019;293:183–209. https://doi.org/10.1016/J.SNB.2019.04.075.
- Yan QL, Godin M, Zhao FQ, et al. Highly energetic compositions based on functionalized carbon nanomaterials. Nanoscale. 2016;8:4799–4851. https://doi.org/10.1039/C5NR07855E.
- Lin J, Chen X, Huang P. Graphene-based nanomaterials for bioimaging. Adv Drug Deliv Rev. 2016;105:242–254. https://doi.org/10.1016/J.ADDR.2016.05.013.
- Wang Y, Hu A. Carbon quantum dots: synthesis, properties, and applications. J Mater Chem C Mater. 2014;2:6921–6939. https://doi.org/10.1039/C4TC00988F.
- Sharma A, Das J. Small molecules derived carbon dots: synthesis and applications in sensing, catalysis, imaging, and biomedicine. J Nanobiotechnol. 2019;17 :1–24. https://doi.org/10.1186/S12951-019-0525-8.
- Monte-Filho SS, Andrade SIE, Lima MB, et al. Synthesis of highly fluorescent carbon dots from lemon and onion juices for determination of riboflavin in multivitamin/mineral supplements. J Pharm Anal. 2019;9:209–216. https://doi.org/10.1016/J.JPHA.2019.02.003.
- Xie L, Yan M, Liu T, et al. Kinetics-controlled super-assembly of asymmetric porous and hollow carbon nanoparticles as light-sensitive smart nanovehicles. J Am Chem Soc. 2022;144:1634–1646. https://doi.org/10.1021/JACS.1C10391/SUPPL_FILE/JA1C10391_SI_010.MP4.
- Salama DM, Abd El-Aziz ME, El-Naggar ME, et al. Synthesis of an eco-friendly nanocomposite fertilizer for common bean based on carbon nanoparticles from agricultural waste biochar. Pedosphere. 2021;31:923–933. https://doi.org/10.1016/S1002-0160(21)60024-3.
- Fang Y, Guo S, Li D, et al. Easy synthesis and imaging applications of cross-linked green fluorescent hollow carbon nanoparticles. ACS Nano. 2012;6:400–409. https://doi.org/10.1021/NN2046373/SUPPL_FILE/NN2046373_SI_001.PDF.
- Namdari P, Negahdari B, Eatemadi A. Synthesis, properties and biomedical applications of carbon-based quantum dots: an updated review. Biomed Pharmacother. 2017;87:209–222. https://doi.org/10.1016/J.BIOPHA.2016.12.108.
- Schneider EM, Bärtsch A, Stark WJ, et al. Safe one-pot synthesis of fluorescent carbon quantum dots from lemon juice for a hands-on experience of nanotechnology. J Chem Educ. 2019;96:540–545. https://doi.org/10.1021/ACS.JCHEMED.8B00114/ASSET/IMAGES/MEDIUM/ED-2018-00114E_0001.GIF.
- Liu Y, Xiao N, Gong N, et al. One-step microwave-assisted polyol synthesis of green luminescent carbon dots as optical nanoprobes. Carbon N Y. 2014;68:258–264. https://doi.org/10.1016/J.CARBON.2013.10.086.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. https://doi.org/10.1016/0022-1759(83)90303-4.
- Negrea P, Caunii A, Sarac I, et al. The study of infrared spectrum of chitin and chitosan extract as potential sources of biomass. Dig J Nanomater Biostruct. n.d.;10:1129–1138.
- Emam AN, Loutfy SA, Mostafa AA, et al. Cytotoxicity, biocompatibility and cellular response of carbon dots–plasmonic-based nano-hybrids for bioimaging. RSC Adv. 2017;7:23502–23514. https://doi.org/10.1039/C7RA01423F.
- Lei B, Hu C, Hu G, et al. Synthesis of modified carbon dots with the performance of ultraviolet absorption used in sunscreen. Opt Express. 2019;27(5):7629–7641. https://doi.org/10.1364/OE.27.007629.
- Akasaka T, Yokoyama A, Matsuoka M, et al. Adhesion of human osteoblast-like cells (Saos-2) to carbon nanotube sheets. Biomed Mater Eng. 2009;19:147–153. https://doi.org/10.3233/BME-2009-0574.
- Emam-Djomeh Z, Hajikhani M. Chitosan/poly (ethylene glycol)/ZnO bionanocomposite for wound healing application. In: Öchsner A, da Silva LFM, Altenbach H, editors. Biodegradable and environmental applications of bionanocomposites. Cham: Springer; 2023. p. 31–65. https://doi.org/10.1007/978-3-031-13343-5_2.
- Thanou M, Verhoef JC, Junginger HE. Chitosan and its derivatives as intestinal absorption enhancers. Adv Drug Deliv Rev. 2001;50:S91–S101. https://doi.org/10.1016/S0169-409X(01)00180-6.
- Nam KS, Shon YH. Suppression of metastasis of human breast cancer cells by chitosan oligosaccharides. J Microbiol Biotechnol. 2009;19:629–633. https://doi.org/10.4014/JMB.0811.603.
- Shaheen S, Arafah MM, Alshanwani AR, et al. Chitosan nanoparticles as a promising candidate for liver injury induced by 2-nitropropane: implications of P53, iNOS, VEGF, PCNA, and CD68 pathways. Sci Prog. 2021;104: 1–19. https://doi.org/10.1177/00368504211011839/ASSET/IMAGES/LARGE/10.1177_00368504211011839-FIG2.JPEG.
- Takimoto H, Hasegawa M, Yagi K, et al. Proapoptotic effect of a dietary supplement: water-soluble chitosan activates caspase-8 and modulating death receptor expression. Drug Metab Pharmacokinet. 2004;19:76–82. https://doi.org/10.2133/DMPK.19.76.
- Zhang J, Xia W, Liu P, et al. Chitosan modification and pharmaceutical/biomedical applications. Mar Drugs. 2010;8 :1962–1987. https://doi.org/10.3390/md8071962.