Abstract
The current work reports the synthesis of novel hydrazones and bis-hydrazones containing 1,2,3-triazole moiety. Base-assisted aldol condensation of 4-acetyl-1,2,3-triazole 1a (R = NO2) and anisaldehyde (2) gave the corresponding chalcone 3 in 88% yield. 4-(1-(2-(2,4-Dinitrophenyl)hydrazineylidene)-3-(4-methoxyphenyl)allyl)-5-methyl-1-(4-nitrophenyl)-1H-1,2,3-triazole (5) was synthesized in 85% yield from the reaction of 3 and 2,4-dinitrophenylhydrazine (4) in boiling ethanol in the presence of a catalytic amount of HCl. Condensation of 1b (R = OMe) and N-phenylhydrazinecarbothioamide(6) in an acidic medium led to unexpected bis-hydrazones 8 rather than carbothioamide 7. An acid catalyzed one-pot three-component reaction of carbohydrazide 9, isatoic anhydride (10), and 1b,c (c: R = F, d: R = H) gave the corresponding hydrazones 12a–12c in high yields, instead of the expected dihydroquinazolin-4(1H)-ones 11. The resulting hydrazones were fully characterized using different techniques including 1H NMR, 13C NMR, X-ray and elemental analysis. Antibacterial screening revealed that some of the synthesized heterocycles showed moderate activity against Staphylococcus aureus ATCC-47077 and Listeria monocytogenes ATCC-35152.
1. Introduction
Scientists and researchers spend a lot of time and effort developing new antibacterial drugs that benefit human health [Citation1]. Recently, a wide range of organic compounds have been created specifically for this purpose, with various molecular hybridization procedures used to add structural properties, or reconfigure structural features, in both old and new biologically active compounds [Citation2]. Numerous nitrogen-containing heterocyclic compounds have revealed interesting biological activity and have received interest from medicinal chemists because of the numerous functional uses potentially on offer [Citation3]. Due to their broad range of biological applications, hydrazone linkages and 1,2,3-triazoles have been identified as two of the most fascinating scaffolds in medicinal chemistry [Citation4].
Hydrazones, sometimes known as “Schiff bases”, are remarkable chemical compounds, which have a broad range of application in medicine. Owing to their highly tunable features and exceptional properties, hydrazones have become essential in the fields of organic synthesis and bioorganic chemistry and have been reported to exhibit fascinating pharmacological activity, such as antibacterial, antifungal, antiviral, anti-Alzheimer, anti-depressant, insecticidal, and enzyme inhibition [Citation5,Citation6]. Besides their unique properties, the tunable nature of hydrazones has facilitated the development of valuable novel molecules with potential applications as preservers, drugs, polymers, glues, and many other applications [Citation3–6]. Structurally, hydrazones are generally synthesized from the acidic condensation of carbonyl compounds and hydrazine derivatives [Citation7–11].
Furthermore, the 1,2,3-triazole core is a significant building block with several biomedical applications, including anti-cancer [Citation12] anti-fungal [Citation13,Citation14], anti-bacterial [Citation15,Citation16], analgesic [Citation17], anti-inflammatory [Citation18], anti-tubercular [Citation19,Citation20], anti-tyrosinase [Citation21] and anti-convulsant activity [Citation22].
Numerous FDA-approved medications have 1,2,3-triazole cores, such as the anticonvulsant Rufinamide, the anti-HIV medicine TSAO, the antibiotic Cefatrizine, the antibacterial Tazobactam, the anticancer agent CAI, and antiviral ribavirin analogues [Citation23–26]. Recently, great attention has been devoted to the development of structural hybrid molecules from bioactive chemical substances, which is a novel and promising method for enhancing drug discovery and the development of prototypes [Citation27,Citation28].
This motivated us to develop a new hydrazone based-1,2,3-triazole core, as well as investigate their biological applications as a continuation of our interest in the synthesis of novel bioactive nitrogen containing heterocycles [Citation29–31].
2. Reagents and employed methods
2.1. General
Induction heating melting point equipment was used to estimate melting points. The 1H (Five Hundred MHz) and 13C NMR (One Hundred Twenty-five MHz) spectra were measured using a JEOL NMR 500 MHz spectrometer. Compounds 1 [Citation32] and 9 [Citation33] were prepared based on procedures in the literature. A Nonius Kappa CCD diffractometer (Bruker, Madison, WI, USA) was utilized to assemble the X-ray crystal diffraction parameters. The X-ray crystallographic data for compounds under inspection 3, 5, 8, 12a and 12b were deposited at the Cambridge Crystallographic Data Center with 2178311-2178315CCDC reference numbers. The bacteria used were collected from the American Type Culture Collection (ATCC; Rockville, MD, USA).
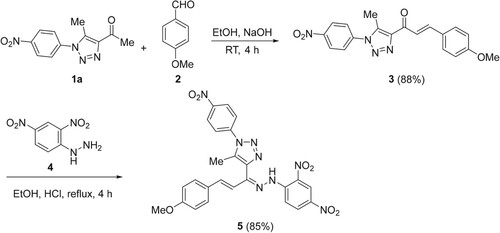
2.2. Synthesis and characterization of (E)-3-(4-methoxyphenyl)-1-(5-methyl-1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)prop-2-en-1-one (3)
To a stirred solution of 1a (0.74 g, 3 mmol) in an ethanolic solution of NaOH (0.40 g, 10 mmol in 10 mL H2O and 30 mL EtOH), compound 2 (0.41 g, 3 mmol) was added, and stirring was continued for 4 h. The resultant reaction mixture was then poured into crushed ice water (50 mL) with stirring for 30 min. The solid obtained was filtered, washed with H2O (10 mL) and EtOH (10 mL), dried, and recrystallized from EtOH to give compound 3 in 88% as a colorless solid, mp 195-196 °C. 1H NMR (DMSO): 2.63 (3H, s, CH3), 3.88 (3H, s, OCH3), 6.97 (1H, d, J = 8.6 Hz, H-Ar), 7.73 (1H, d, J = 8.6 Hz, H-Ar), 7.77 (1H, d, J = 8.6 Hz, CH=), 7.80 (1H, d, J = 8.6 Hz, CH=), 7.96 (2H, d, J = 8.6 Hz, H-Ar), 8.43 (2H, d, J = 8.6 Hz, H-Ar) ppm. Anal. Calcd. for C19H16N4O4 (364.1172): C, 62.63; H, 4.43; N, 15.38. Found: C, 62.88; H, 4.52; N, 15.59%.
2.3. Synthesis and characterization of 4-(1-(2-(2,4-dinitrophenyl)hydrazineylidene)-3-(4-methoxyphenyl)allyl)-5-methyl-1-(4-nitrophenyl)-1H-1,2,3-triazole (5)
Compound 4 (0.4 g, 2 mmol) was added to an ethanolic solution (20 mL) of compound 3 (0.73g, 2 mmol) containing concentrated HCl (0.3 mL). The reaction mixture was then refluxed for 4h. The mixture was cooled and the solid obtained was collected via filtration, washed with EtOH (10 mL), dried, and recrystallized from EtOH to give compound 5 in 85% as a red solid, mp 238-240 °C. 1H NMR (DMSO): 2.39 (3H, s, CH3), 3.75 (3H, s, OCH3), 6.91-7.11 (4H, m, Ar-H), 7.57 (2H, d, J = 7.6 Hz, CH=CH), 8.00 (1H, d, J = 8.6 Hz, H-Ar), 8.04 (2H, d, J = 8.6 Hz, H-Ar), 8.33 (1H, d, J = 8.6 Hz, H-Ar), 8.48 (2H, d, J = 8.5 Hz, H-Ar), 8.74 (1H, s, H-Ar), 12.37 (1H, s, NH D2O, exch.) ppm. 13C NMR (DMSO): 11.0 (CH3); 55.8 (OCH3); 114.8, 117.0, 123.3, 123.6, 125.7, 126.8, 129.0, 129.5, 130.3, 130.4, 135.9, 137.0, 137.7, 138.1, 140.8, 144.3, 145.3, 148.4, 160.8 (C-Ar, C=N) ppm. Anal. Calcd. for C25H20N8O7 (544.1455): C, 55.15; H, 3.70; N, 20.58. Found: C, 55.23; H, 3.84; N, 20.72%
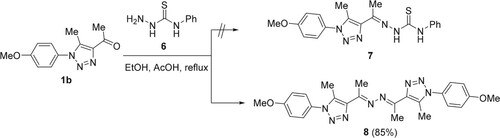
2.4. Synthesis and characterization of (1E,2E)-1,2-bis(1-(1-(4-methoxyphenyl)-5-methyl-1H-1,2,3-triazol-4-yl)ethylidene)hydrazine (8)
A mixture of 1b (0.46 g, 2 mmol) and 6 (0.33 g, 2 mmol) in dry EtOH (20 ml) containing concentrated AcOH (0.2 mL) was heated under reflux for 3 hours. The resultant solid was filtered, and then dried and crystallized from dimethylformamide (DMF) to yield compound 8 in 85% as a white solid, mp 238-240 °C. 1H NMR (CDCl3): 2.62 (6H, s, 2 x CH3), 2.64 (6H, s, 2 x CH3), 3.88 (6H, s, 2 x OCH3), 7.06 (4H, d, J = 8.6 Hz, H-Ar), 7.40 (4H, d, J = 8.6 Hz, H-Ar) ppm. 13C NMR (CDCl3): 11.4 (CH3); 15.4 (CH3); 55.7 (OCH3); 114.8, 126.9, 129.0, 133.9, 143.4, 157.6, 160.6 (C-Ar, C=N) ppm. Anal. Calcd. for C24H26N8O2 (458.2179): C, 62.87; H, 5.72; N, 24.44; Found: C, 62.83; H, 5.79; N, 24.88%.
2.5. General procedure for the synthesis of compounds 12a-12c
A mixture of hydrazide 9a or 9b (2 mmol) and/or 10 (0.33 g, 2 mmol) in EtOH (10 mL) was refluxed for 1 h; then compound 1c and/or 1d (2 mmol) and H3PO3 (0.03 g, 20 mol%) were added to the mixture and the reflux was continued for 6 h. After cooling, the colorless solid produced was collected via filtration, and dried and recrystallized from DMF, yielding the corresponding 12a-12c.
2.5.1 Characterization of N'-(1-(1-(4-Fluorophenyl)-5-methyl-1H-1,2,3-triazol-4-yl)ethylidene)-1-(4-methoxyphenyl)-5-methyl-1H-1,2,3-triazole-4-carbohydrazide (12a)
Yield: 85%, mp 203-204 °C. 1H NMR (DMSO): 2.47 (3H, s, CH3), 2.54 (6H, s, 2 x CH3), 3.83 (3H, s, OCH3), 7.13 (2H, d, J = 8.2 Hz, H-Ar), 7.48-7.53 (4H, m, H-Ar), 7.71 (2H, d, J = 8.6 Hz, H-Ar), 10.69 (1H, s, NH, D2O exch.) ppm. Anal. Calcd. for C22H21N8O2F (448.1772): C, 58.92; H, 4.72; N, 24.99; Found: C, 58.99; H, 4.81; N, 25.09%.
2.5.2 Characterization of 1-(4-Fluorophenyl)-N'-(1-(1-(4-fluorophenyl)-5-methyl-1H-1,2,3-triazol-4-yl)ethylidene)-5-methyl-1H-1,2,3-triazole-4-carbohydrazide (12b)
Yield: 85%, mp 248-249 °C. 1H NMR (DMSO): 2.54 (3H, s, CH3), 2.55 (3H, s, CH3), 2.58 (3H, s, CH3), 7.48 (d, 2H, J = 7.7 Hz, H-Ar), 7.45-7.53 (2H, m, H-Ar), 7.69-7.73 (4H, m, H-Ar), 10.78 (1H, s, NH, D2O exch.) ppm. 13C NMR (DMSO): 9.8 (CH3); 11.0 (CH3); 14.4 (CH3); 117.2 (C-Ar); 117.3 (2C, d, 2Jc,F = 22.5 Hz, C-5 and C-3); 117.4 (C-Ar); 128.4 (2C, d, 3Jc,F = 8.7 Hz, C-6 and C-2); 129.1, 132.2, 132.6 (C-Ar); 137.8 (1C, d, 4Jc,F = 3.4 Hz, C-1); 138.5, 142.6, 150.8, 157.5, 162.0 (C-Ar, C=O); 164.1 (1C, d, 1Jc,F = 247.5 Hz, C-4) ppm. Anal. (Calculated) for C21H18N8OF2 (436.1572): C, 57.79; H, 4.16; N, 25.68; (Found): C, 57.86; H, 4.22; N, 25.79%.
2.5.3 1-(4-Fluorophenyl)-5-methyl-N'-(1-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethylidene)-1H-1,2,3-triazole-4-carbohydrazide (12c)
Yield: 85%, mp 217-218 °C. 1H NMR (DMSO): 2.54 (3H, s, CH3), 2.55 (3H, s, CH3), 2.59 (3H, s, CH3), 7.49 (2H, t, J = 7.8 Hz, H-Ar), 7.60-7.64 (5H, m, H-Ar), 7.69-7.71 (2H, m, H-Ar), 10.78 (1H, s, NH, D2O exch.) ppm. 13C NMR (DMSO): 9.8 (CH3), 11.1 (CH3), 14.4 (CH3), 117.3 (2C, d, 2Jc,F = 22.5 Hz, C-5 and C-3); 125.9 (C-Ar); 128.5 (2C, d, 3Jc,F = 8.7 Hz, C-6 and C-2); 129.1, 130.3, 132.6 , 136.0 (C-Ar); 138.0 (1C, d, 4Jc,F = 3.4 Hz, C-1); 142.2, 150.4, 157.5, 162.0 (C-Ar, C=O); 164.1 (1C, d, 1Jc,F = 250.0 Hz, C-4) ppm. Anal. (Calculated) for C21H19FN8O (418.1666): C, 60.28; H, 4.58; N, 26.78; (Found): C, 60.34; H, 4.66; N, 26.83%.
2.6. Antibacterial Activity
The agar well diffusion procedure was used as the medium to investigate the antibacterial activity of the newly synthesized heterocycles [34, 35]. Vancomycin and ampicillin served as benchmark drugs for comparability. Bacteria (seventy µL) were smeared on nutrient agar-coated plates. On the implanted agar plates, a borehole (6 mm in diameter) was drilled out, and each sample (200 mg in 1 mL of DMSO) was then added. The agar-inoculated plates were coated with the reference antibiotic disks (30 and10 µg/disk of vancomycin and ampicillin, respectively). The plates were kept at 4°C for 2 h before incubation to permit the diffusion to occur. With the exception of yeast strains, which were incubated at 28°C for 24 hours, the plates were kept at 37°C. The inhibitory zone's diameter (in mm) was estimated, and the averages were computed after five replications of the tests.
3. Results and discussion
3.1. Chemistry
Aldol condensation of 1-(5-methyl-1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)ethan-1-one (1a, R = NO2) and anisaldehyde (2) were stirred in an aqueous ethanol solution containing sodium hydroxide, at room temperature for 4h, which gave (E)-3-(4-methoxyphenyl)-1-(5-methyl-1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)prop-2-en-1-one (3) in 88% yield (Scheme 1). The targeted hydrazone-based 1,2,3-triazole 5 was synthesized in 85% yield from the condensation reaction of chalcone 3 and 2,4-dinitrophenylhydrazine (4) for 4 h in boiling EtOH in the presence of concentrated hydrochloric acid (HCl) as a catalyst (Scheme 1).
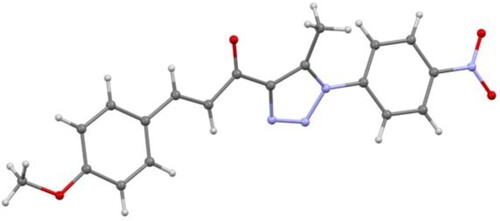
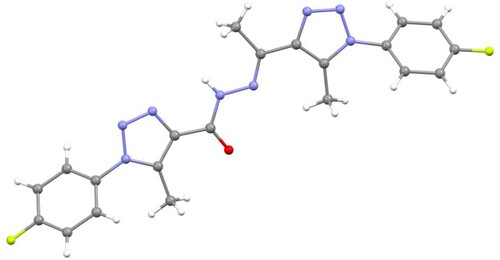
The 1H NMR spectrum of 3 showed that the two chalcone (=CH) protons appeared as two doublets (J = 8.6 Hz) in the aromatic region. Additionally, it displayed two singlets that appeared at 3.88 and 2.63 ppm, with three protons each, corresponding to the methoxy (OCH3) and methyl (CH3) protons, respectively. On the other hand, the 1H NMR spectrum of 5 showed an exchangeable diagnostic singlet at 12.37 ppm, attributed to the amino proton (NH), which confirmed the success of the condensation reaction. Its 13C NMR spectrum displayed two signals at 11.0 and 55.8 ppm attributable to the methyl and methoxy carbons, respectively. Further evidence for the formation of compounds 3 and 5 was established using the X-ray crystallography of a single crystal (Figures and , respectively).
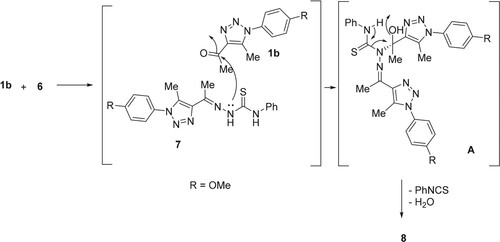
Surprisingly, the condensation of 1b (R = OMe) with N-phenylhydrazinecarbothioamide (6), in boiling dry EtOH in the presence of a sufficient quantity of glacial acetic acid (AcOH) for 3 h, afforded the unexpected bis-hydrazones 8 in 85% yield rather than the expected thiosemicarbazone adduct 7 (Scheme 2).
The formation of compound 8 involved the reaction of 1b with 6 to produce intermediate 7. The reaction of 7 and 1b produced intermediate A, which lost phenyl isothiocyanate and water to give 8 (Scheme 3).
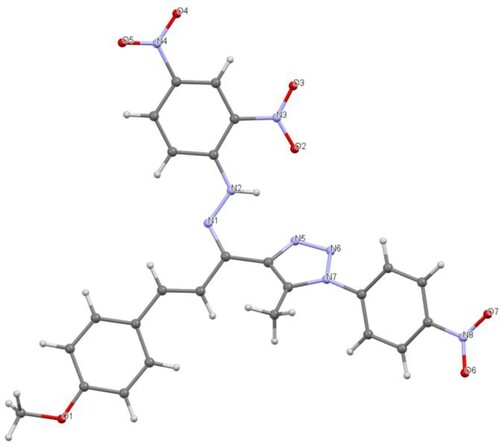
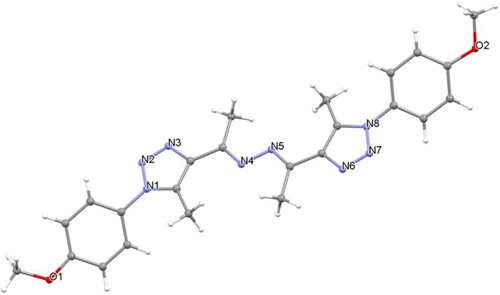
The absence of diagnostic thiosemicarbazide NH protons in the resulting product's 1H NMR spectrum rules out the formation of thiosemicarbazone 7. The 1H NMR spectrum of the resulting compound 8 displayed three singlets, with six protons each, which resonated at 2.62 (2 CH3), 2.64 (2 CH3), and 3.89 ppm (2 OCH3). Its 13C NMR spectrum revealed the presence of 10 signals. For example, the two methyl carbons appeared at 11.4 and 15.9 ppm, while the methoxy carbons appeared at 55.7 ppm. The aromatic protons and carbons appeared in their appropriate regions. These results confirmed the formation of compound 8 and not 7. Additionally, the structure of 8 was clearly supported using X-ray crystallography of a single crystal (Figure ).
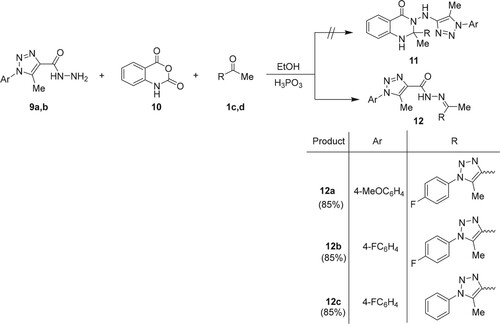
Spiro-quinazolinones have been synthesized and described in the literature [Citation36] using one-pot reactions of isatoic anhydride, cyclic ketones, and hydrazides using phosphorous acid (H3PO3) as a catalyst. The reactions of isatoic anhydride (10), 1,2,3-triazole-4-carbohydrazides 9a,b (X = OMe, F), and 4-acetyltriazoles 1c,d (R = F, H) in the presence of H3PO3 were investigated under the same conditions. Surprisingly, the corresponding hydrazones 12a–12c, rather than the expected dihydroquinazolin-4(1H)-ones 11, were formed (Scheme 4).
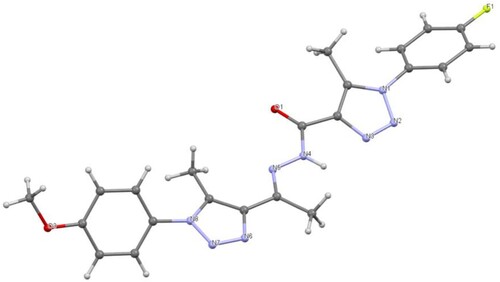
NMR spectroscopy and x-ray crystallography (Figures and ) were used to establish the structures of 12a–12c. The 1H NMR spectra of 12a–12c, for example, revealed the presence of a distinct exchangeable singlet at 10.69–10.78 ppm, corresponding to the hydrazone NH proton.
3.2. Antibacterial activity
According to the results of the antibacterial screening (Table ), compounds 3, 5, and 8 were found to be active against Staphylococcus aureus ATCC-47077 (S. aureus) and Listeria monocytogenes ATCC-35152 (L. monocytogenes). The compounds tested showed moderate activity compared with ampicillin and vancomycin (Table ). The minimal inhibitory concentrations of 3, 5, and 8 were in the range of 100–120 μg/mL.
Table 1. Antibacterial activity (mm) of heterocycles 3, 5, and 8.
4. Conclusions
Novel hydrazones and bis-hydrazones containing a 1,2,3-triazole ring system were synthesized from commercially available chemicals using simple synthetic procedures. Their structures were confirmed and their antibacterial activities evaluated.
Acknowledgements
I would like to thank Dr Benson M. Kariuki, Cardiff University, United Kingdom for the X-Ray crystal structure determination.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Yuan H, Ma Q, Ye L, et al. The traditional medicine and modern medicine from natural products. Molecules. 2016;21:559.
- Pawełczyk A, Sowa-Kasprzak K, Olender D, et al. Molecular consortia—various structural and synthetic concepts for more effective therapeutics synthesis. Int J Mol Sci. 2018;19:1104.
- Kerru N, Gummidi L, Maddila S, et al. A review on recent advances in nitrogen-containing molecules and their biological applications. Molecules. 2020;25:1909.
- Bozorov K, Zhao J, Aisa HA. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: a recent overview. Bioorg Med Chem. 2019;27:3511–3531.
- Brum JOC, França TCC, LaPlante SR, et al. Synthesis and biological activity of hydrazones and derivatives: a review. Mini Rev Med Chem. 2020;20:342–368.
- Popiołek Ł. The bioactivity of benzenesulfonyl hydrazones: a short review. Biomed Pharmacother. 2021;141:111851.
- Larsen D, Kietrys AM, Clark SA, et al. Exceptionally rapid oxime and hydrazone formation promoted by catalytic amine buffers with low toxicity. Chem Sci. 2018;9:5252–5259.
- Hajipour AR, Mohammadpoor-Baltork I, Bigdeli M. A convenient and mild procedure for the synthesis of hydrazones and semicarbazones from aldehydes or ketones under solvent-free conditions. J Chem Res. 1999;1999:570–571.
- Parkinson DR. Comprehensive sampling and sample preparation: analytical techniques for scientists. Oxford (UK): Elsevier, 2012; p. 559–595.
- Safari J, Gandomi-Ravandi S. Structure, synthesis and application of azines: a historical perspective. RSC Adv. 2014;4:46224–46249.
- Khidre RE, Mohamed HA, Kariuki BM, et al. Facile, mild and efficient synthesis of azines using phosphonic dihydrazide. Phosphorus Sulfur Silicon Relat Elem. 2020;195:29–36.
- Banerji B, Chandrasekhar K, Sreenath K, et al. Synthesis of triazole-substituted quinazoline hybrids for anticancer activity and a lead compound as the EGFR blocker and ROS inducer agent. ACS Omega. 2018;3:16134–16142.
- Peng XM, Cai GX, Zhou CH. Recent developments in azole compounds as antibacterial and antifungal agents. Curr Top Med Chem. 2013;13:1963–2010.
- Peyton LR, Gallagher S, Hashemzadeh M. Triazole antifungals: a review. Drugs Today. 2015;51:705–718.
- Aouad MR, Mayaba MM, Naqvi A, et al. Design, synthesis, in silico and in vitro antimicrobial screenings of novel 1,2,4-triazoles carrying 1,2,3-triazole scaffold with lipophilic side chain tether. Chem Cent J. 2017;11:117–117.
- Abdel-Wahab BF, Abdel-Latif E, Mohamed HA, et al. Design and synthesis of new 4-pyrazolin-3-yl-1,2,3-triazoles and 1,2,3-triazol-4-yl-pyrazolin-1-ylthiazoles as potential antimicrobial agents. Eur J Med Chem. 2012;52:263–268.
- Dheer D, Singh V, Shankar R. Medicinal attributes of 1,2,3-triazoles: current developments. Bioorg Chem. 2017;71:30–54.
- Bonandi E, Christodoulou MS, Fumagalli G, et al. The 1,2,3-triazole ring as a bioisostere in medicinal chemistry. Drug Discov Today. 2017;22:1572–1581.
- Keri RS, Patil SA, Budagumpi S, et al. Triazole: a promising antitubercular agent. Chem Biol Drug Des. 2015;86:410–423.
- Kumar SS, Helen PK. Synthesis and biological applications of triazole derivatives – a review. Mini-Rev Org Chem. 2013;10:40–65.
- Celik F, Unver Y, Barut B, et al. Synthesis, characterization and biological activities of new symmetric bis-1,2,3-triazoles with click chemistry. Med Chem. 2018;14:230–241.
- Varala R, Bollikolla HB, Kurmarayuni CM. Synthesis of pharmacological relevant 1,2,3-Triazole and its analogues–a review. Curr Org Synth. 2021;18:101–124.
- Smit FJ, Seldon R, Aucamp J, et al. Synthesis and antimycobacterial activity of disubstituted benzyltriazoles. Med Chem Res. 2019;28:2279–2293.
- Ali A, Gogoi A, Chaliha D, et al. Synthesis and biological evaluation of novel,2,3-triazole derivatives as anti-tubercular agents. Bioorganic Med Chem Lett. 2017;27:3698–3703.
- Zhang S, Xu Z, Gao C, et al. Triazole derivatives and their anti-tubercular activity. Eur J Med Chem. 2017;138:501–513.
- Zhang K, Wang P, Xuan L-N, et al. Synthesis and antitumor activities of novel hybrid molecules containing 1,3,4-oxadiazole and 1,3,4-thiadiazole bearing Schiff base moiety. Bioorg Med Chem Lett. 2014;24:5154–5156.
- Bérubé G. An overview of molecular hybrids in drug discovery. Expert Opin Drug Discov. 2016;11:281–305.
- Viegas-Junior C, Danuello A, da Silva Bolzani V, et al. Molecular hybridization: a useful tool in the design of new drug prototypes. Curr Med Chem. 2007;14:1829–1852.
- Baashen MA. Synthesis of novel fluorinated 1H-pyrazole-1-carbothioamide and 5(1H-pyrazol-1-yl) thiazoles containing 1,5-diphenyl-1H-1,2,3-triazole moiety. Indian J Heterocycl Chem. 2021;31:583–588.
- Baashen MA. Synthesis of N,N'-diacylhydrazines and their use in various synthetic transformations. Curr Org Chem. 2021;25:1394–1403.
- Baashen MA, Abdel-Wahab BF, Hegazy AS, et al. The crystal structure of 4-(4-bromophenyl)−2-(3-(4-bromophenyl)−5-(4-fluorophenyl)−4,5-dihydro-1H-pyrazol-1-yl)thiazole, C24H16Br2FN3S. Z Kristallogr – New Cryst Struct. 2021;236:425–427.
- Kamalraj VR, Senthil S, Kannan P. One-pot synthesis and the fluorescent behavior of 4-acetyl-5-methyl-1,2,3-triazole regioisomers. J Mol Struct. 2008;892:210–215.
- Chu C-H, Hui X-P, Zhang Y, et al. Synthesis and antifungal activities of ω-[4-aryl-5-(1-phenyl-5-methyl-1,2,3-triazol-4-yl)−1,2,4-triazol-3-thio]-ω-(1H-1,2,4-triazol-1-yl)acetophenones. J Chinese Chem Soc. 2001;48:121–125.
- Alvand ZM, Rajabi HR, Mirzaei A, et al. Ultrasonic and microwave assisted extraction as rapid and efficient techniques for plant mediated synthesis of quantum dots: green synthesis, characterization of zinc telluride and comparison study of some biological activities. New J Chem. 2019;43:15126–15138.
- Hannan PCT. Guidelines and recommendations for antimicrobial minimum inhibitory concentration (MIC) testing against veterinary mycoplasma species. Vet Res. 2000;31:373–395.
- Tajbakhsh M, Ramezanpour S, Balalaie S, et al. Novel one-pot three-component reaction for the synthesis of functionalized spiroquinazolinones. J Heterocycl Chem. 2015;52:1559–1564.