?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Phytoremediation is a viable approach in order to mitigate toxic soil levels and, therefore, the associated impacts of HMs. This approach requires the assessment of contaminated sites and the selection of reportedly efficient phytoremediators, which might contribute to facilitating the practical implementation of phytoremediation in polluted soils. In a twelve-month microcosm experiment, Indian mustard (Brassica juncea) and rapeseed (Brassica napus) plants were planted over the winter season. Subsequently, maize (Zea maize) was planted after the harvest of these plants. Oil crop rotation systems might promote tested HM removal (Cu, Cd, Fe, Ni, Mn, Zn, and Pb) from the Mahd AD'Dahab mine-contaminated soil and provide higher biomass than single crops. The results indicated that both the rapeseed maize and Indian mustard maize rotation showed the highest phytoextraction efficiency compared to a single crop. The extraction of Zn, Pb, Ni, Mn, Fe, Cu, and Cd are 18, 27, 29, 24, 23, 24, and 28%, respectively, for Indian mustard-maize rotation, although 17, 27, 31, 24, 21, 23, and 26% respectively for rapeseed-maize rotation. The short rotation strategy could be helpful in landscaped and controlled access to the polluted sites. The utilization of short-rotation phytoremediation has the potential to emerge as a financially viable approach for the management of polluted areas. This method involves enhancing biomass yield through the implementation of phytomanagement techniques such as fertilization, irrigation, and coppicing, thereby capitalizing on the benefits associated with both biomass production and landscape management.
1. Introduction
The soil is a fundamental component of the terrestrial ecosystem, and it is critical to human survival and the activities of macro and microorganisms that live within it. Mining and industrial operations result in the discharge of various hazardous compounds as solid and wet waste, causing significant contamination in the surrounding environment [Citation1]. Soil contaminated with HMs from mining operations has become a worldwide environmental issue. This issue presents a substantial threat to the ecosystem due to the accumulation of HMs, their toxic properties, and their resistance to biodegradation within the food chain[Citation2]. Long-term exposure to HM contamination in mining sites causes metabolic abnormalities and reduced reproduction rates in terrestrial creatures grown in exposed regions [Citation3]. Soil contaminated by HMs is a significant concern in many countries despite variations in the level of comprehension regarding the problem, approaches to treatment, and available technological solutions [Citation4]. Renovation of such contaminated land through the implementation of economically efficient methods is necessary prior to its utilization for agricultural purposes in order to render it suitable for optimal agricultural practices [Citation5].
HM-contaminated soil can be remedied using several procedures, including immobilization, soil replacement, vitrification, and surface capping. Nevertheless, a significant number of these technologies are characterized by high costs or limited effectiveness in terms of providing sustainable solutions [Citation6]. Additionally, several other remediation techniques may negatively impact the soil's structure, fertility, and biological activity [Citation7]. The utilization of phytoremediation for the extraction of HMs from polluted soil has proven to be a successful, secure, minimally disruptive, and financially viable approach. Phytoremediation is a technique employed to mitigate the presence of detrimental contaminants by utilizing plants to enhance soil quality. Phytoremediation encompasses two distinct approaches. The initial method employed in phytostabilization involves the use of tolerant plants to stabilize HMs [Citation7]. Mobility reduction, toxicity, and bioavailability of HMs in the soil are significant aspects of phytostabilization. Using this method does not eliminate these contaminants from the site but reduces their presence. By impeding the free movement of metal pollutants, phytostabilization averts their entry into the food chain and water cycle [Citation7]. High metal concentrations can be tolerated, extracted, and accumulated by a variety of hyperaccumulator plants. Phytoextraction enables these plants to retrieve HMs from the substrate, storing them in their roots and subsequently transferring them to their shoots [Citation8,Citation9].
Phytoremediation exhibits considerable potential as a means of pollutant removal from the substrate; however, regulation of the biomass generated during the remediation process is imperative to prevent its discharge into the environment. One of the conventional and auspicious methods for integrating biomass produced by phytoremediation is the thermochemical conversion process. It has been suggested that biomass generation and commercial energy use could be integrated with phytoextraction [Citation10].
Methods have been devised to convert this biomass into products with substantial added value, including biofuels. Bioenergy production has exhibited considerable promise and has the potential to contribute to the worldwide demand for renewable energy. These biofuels can be utilized for transportation, fuel, heating, and electricity [Citation11].
The increasing global interest in bioenergy crops is due to their potential usage as an eco-friendly renewable energy source that is clean and carbon-neutral and might help meet part of the world's energy demands [Citation12]. Unfortunately, bioenergy production is insufficient to meet the expanding world population's energy demands. The polluted regions are unsafe for farmland use. Therefore, it is vitally necessary to develop energy crops in contaminated areas to produce bioenergy while ensuring social, environmental, economic, and environmental sustainability [Citation13]. Bioenergy crops must be cultivated on a large scale in polluted regions in order to remediate and meet the demand for biofuels. An alternative approach to phytoremediation in contaminated areas is the utilization of short-rotation crops [Citation13].
Maize (Zea maize), Indian mustard (Brassica juncea), and rapeseed (Brassica napus) are bioenergy crops cultivated and distributed globally. Due to their high biomass and metal accumulation capability, they considered promising possibilities for the phytoremediation of polluted sites [Citation14,Citation15]. These phytoremediators are considered efficient in remediating HM contaminated areas [Citation16–18].
Nevertheless, a thorough examination of the appropriate literature finds that little work has investigated using monoculture crops and short-rotation systems of bioenergy crops in an experiment. Consequently, this work aims to evaluate the possibility and removal efficiency of tested HMs (Zn, Pb, Ni, Mn, Fe, Cu, and Cd) from Mahd AD'Dahab mine-polluted soil. The current study is a short-rotation experiment involving a monoculture oil crop (rapeseed, Indian mustard, and maize) and two oil crop rotations (rapeseed- maize & Indian mustard- maize), (ii) investigate translocation and bioaccumulation factors, HMs uptake, and phytoextraction efficiency.
2. Materials and methods
2.1. Site description
Mahd AD'Dahab is the largest and oldest gold mine founded in Saudi Arabia. The investigated area represents the Mahd AD'Dahab geographic area, which covers over 966 hectares and is located in the Mahd AD'Dahab province (southeast of Medina). It lies between latitude (N) 23°30'18.8 and longitude (E) 40°51′23.4′′ (Figure ).
The deposit is found beneath Jabal Mahad AD'Dahab, a geological formation that reaches a height of 1,238 m, standing 200 m higher than the adjacent wadis. The gold deposit is situated beneath a volcanic sequence characterized by a stratigraphic succession commencing with andesite lithology at the base, succeeded by volcanoclastic and pyroclastic rocks as well as tuffs. The rocks in question have been subjected to intrusion by diabase microdiorite, gabbro, and porphyritic rhyolite. The mineralization in quartz veins encompasses Zn, Cu, Ag, and Au, while massive bodies and stockworks contain Cu, Pb, Zn, Au, and Ag sulfides, as well as Ni and Fe sulfide lenses. In a previous study conducted by [Citation19], it was observed that the soil in the region of Mahad AD'Dahab exhibited significantly elevated concentrations of Zn, Pb, Ni, Mn, Fe, Cu, and Cd.
2.2. Soil sampling and experimental setup
For this study, HM-contaminated soil was collected from the mining area of Mahad AD'Dahab in Saudi Arabia. The soil samples were obtained from the upper layer of the soil (0–15 cm). After that, the soil was mixed and subjected to air drying. Then, a 2-mm screen was utilized to sieve the soil samples. Following the procedure by Sparks [Citation20], numerous soil chemical and physical characteristics were estimated from soil samples (n = 4). The hydrometer technique was utilized to determine the soil texture. PH as well as electrical conductivity (EC), were assessed using soil extracts, according to Richards [Citation21]. Organic matter (OM) was estimated via the K2CrO4 oxidation process [Citation22]. For estimating the total concentration of tested HMs (Zn, Cu, Cd, Fe, Mn, Pb, and Ni), digestion of 1 g of soil samples was done by adding a tri-acid solution (20 mL) of HNO3, H2SO4, and HClO4 (5:1:1, v/v/v). Nevertheless, the soil available content of tested HMS was extracted utilizing DTPA (diethylene triamine pentaacetate), according to [Citation23]. The total and available seven tested HMs were determined via an Inductively Coupled Argon Plasma (ICAP 6500 Duo-Thermo Scientific-England). The total and DTPA-Cd, Zn, Pb, Ni Cu, Mn, and Fe and physiochemical characteristics are presented in Table .
Table 1. Soil characteristics, geo-accumulation index (Igeo), total, and DTPA- extracted Cd, Cu, Fe, Mn, Ni, Pb, and Zn (mg/Kg) contents in soil (mean ± SD, n = 3).
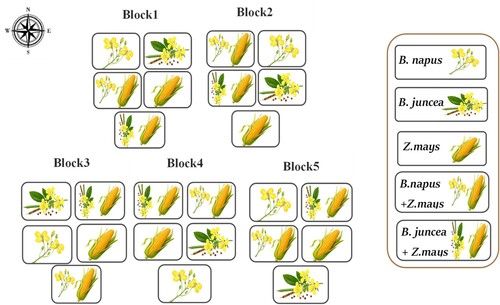
A microcosm experiment was carried out at the greenhouse (Faculty of Applied Science- Umm Al-Qura University-Saudi Arabia). Temperatures in the greenhouse were designed to be between 20 and 28°C, with a relative humidity range of 50%. Three different oil crops – maize (Zea mays), mustard (Brassica juncea), and rapeseed (Brassica napus) were utilized for a short rotation experiment to investigate the feasibility and efficiency of removing HMs from mine-polluted soil. The seeds of three oil crops (i.e. rapeseed, Indian mustard, and maize) were grown in a wooden basin (60 cm × 60 cm), a short rotation on basins filled with the mine-contaminated soil. The wooden basin was internally lined with plastic material with about 5 mm thickness. The rotation was as follows: rapeseed and Indian mustard were sown in October and harvested in May next year; maize was then planted as monoculture in each plot after the rapeseed, and Indian mustard was harvested between June and September. Routine fertilizers were added before seeding and then watered. Complete plant samples were collected within each basin and combined with soil samples (n = 4). These combined samples were randomly selected and mixed to create a composite sample for subsequent laboratory analyses. The planting layout is shown in Figure .
At the harvest stage, the examined crop plants were harvested and divided into roots and shoots for Indian mustard and rapeseed/cob maize. In order to determine the biomass (dry as well as fresh weight, g/plant), the various plant components were weighed both prior to and following oven drying at a temperature of 65 °C. Separate samples were blended in a mill and passed through a mesh filter with a pore size of 2 mm. The tested HM contents of plant samples were assessed using soil analysis procedures. Moreover, soil sample collection was done (from each basin; n = 4) and underwent preparation for HM extraction utilizing DTPA (diethylene triamine pentaacetate), according to [Citation23]. The concentrations of DTPA, extractable HMs, and their contents in different plant parts were estimated utilizing an Inductively Coupled Argon Plasma (ICAP 6500 Duo-Thermo Scientific-England).
2.3. Data analysis
2.3.1. Bioaccumulation (BCF) and translocation (TF) factors
The BCF approach is suitable for modelling HMs uptake (from soil to plant roots), though the translocation method assesses HMs transport from roots to different plant aerial organs. These measurements were calculated to assess the capability of tested crops to uptake and transport HMs from the soil to their shoots [Citation24]. It was determined as per Galal et al. [Citation25] like the following:
(1)
(1)
(2)
(2)
(3)
(3)
Where Cshoot, Croot, and Ccob and Csoil, are the HMs contents (mg/Kg) in the shoot, root, cob, and soil, respectively.
2.3.2. Phytoextraction efficiency (PE)
The tested crop phytoextraction efficiency, examining the potential of this crop for HM removal from polluted sites, was determined based on the method of Jing et al. [Citation26]:
(4)
(4) Cbc and Cac are the contents of HMs (mg/Kg) in the soil prior to and following each crop's harvest. Total uptake of metal (g/ plant) was calculated utilizing Equation (5):
(5)
(5) Where C1 (mg/Kg) is the tested HM concentration determined in all plant parts, W (g/plant) is dry weight at different plant parts.
The geo-accumulation index (Igeo) assesses the progressive fluctuation in HMs by comparing the concentration of existing metal (with the geochemical background). Based on Muller [Citation27], the Igeo may be estimated via equation Equation6(6)
(6) :
(6)
(6) Where Cn is the HM contents, Bn is HMs (n) geochemical background value. The constant 1.5 accounted for potential fluctuations in the reference value caused by lithogenic processes. Muller [Citation27] classified Igeo into seven groups: ≤ 0 (uncontaminated), 0 < Igeo <1 (moderately to uncontaminated), 1 < Igeo < 2 (moderately contaminated), 2 < Igeo < 3 (moderately to heavily polluted), 3 < Igeo < 4 (heavily polluted), 4 < Igeo < 5 (heavily to significantly contaminated) and 5 < Igeo (significantly contaminated). A reference concentration of Fe is employed to achieve normalization due to its widespread natural presence.
2.4. Statistical analysis
Two-way ANOVA was applied to test variation significance in the tested concentration of HMs tested bioenergy plant multiple organs. Before conducting an analysis of variance (ANOVA), the obtained data underwent testing to assess the homogeneity of error variances and the normality of distribution; the Shapiro test was used to test the normality. Statistical analysis was done utilizing SPSS software. In addition, multidimensional data were visualized and interpreted using a principal component analysis (PCA). It eliminates data dimensionality by original data linear combination. These new variables are designed to be both orthogonal as well as uncorrelated to one another [Citation28].
A person's correlation matrix was used to calculate the PCA according to phytoextraction efficiency of tested HMs. Cluster analysis was employed to categorize the three crops and rotation pattern into comparable groups based on their phytoextraction efficiency [Citation29].
3. Results and discussion
3.1. Physiochemical, heavy metals, and pollution levels in the soil
Descriptive statistics data on soil properties and HM concentration in mine-contaminated soil are provided in Table . In addition, the investigated soil is yellow-red in colour and has a pH of 7.9, organic matter (OM) content of 2.3 g/kg, and electrical conductivity (EC) of 388.0 µs/cm.
Total Zn, Pb, Ni, Mn, Fe, Cu, and Cd were 857.5, 373.2, 45.9, 1028.0, 1860.0, 640.7, and 22.7 mg/Kg, respectively. Since soil HMs exist in various chemical forms and exhibit multiple chemical and physical behaviours regarding potential toxicity, mobility, chemical interactions, and biological availability, analyzing total metal concentrations alone is inadequate to assess the environmental effect of polluted soil [Citation30]. The DTPA- extractable of Cd, Cu, Fe, Mn, Ni, Pb, and Zn were 10.9, 8.4, 59.5, 44.4,13.4, 28.6, and 90.9 mg/Kg, respectively. The toxicity of HMs in the soil is mainly impacted by the forms of metal available to plants rather than the total metal concentrations in the soil [Citation31]. According to Lindsay [Citation32], the total HM content, with the exception of Fe, in Mahd AD'Dahab was more significant compared to the typical soil range, as indicated in Table .
The geo-accumulation (Igeo) index was described to be more effective and valuable in assessing soil quality. According to category and Igeo values (Table ), the Mahd AD'Dahab soil samples were shown to be moderate to highly contaminated or severely polluted by Cd, Cu, Zn, and Pb. Nevertheless, the Igeo values for Ni, Fe, and Mn from the Mahd AD'Dahab site are in the <0 range, which indicates background concentrations. Earlier, various studies found significantly high contents of HMs in plant and soil plants around mining activities [Citation19]. They detected that the soils adjacent to the Mahd AD'Dahab mine in Saudi Arabia had elevated HM concentrations like Pb, Cd, Zn, Cu, and As, owing to landfilling a significant amount of mine waste and mine activity.
Our findings suggest that increased geo-accumulation indices show that Cd, Cu, Zn, and Pb in urban mining regions may have originated from anthropogenic activities. Furthermore, the high contents of tested HMs in Mahd AD'Dahab's urban environment may be of more significant concern as environmental and human life hazards. A low geo-accumulation index value for (Cu, Ni, Pb, and Zn) conveys that all of these HMs came from lithogenic sources and are primarily influenced by soil-forming variables.
3.2. Available HM contents in soil under the five patterns
For all tested oil crops (monoculture) and rotation patterns, available Zn, Pb, Ni, Mn, Cu, and Cd content in detected soil (before cultivation) were 90.9, 28.6, 13.4, 44.4, 59.4, 8.5, and 10.9 mg/Kg (Table ). Following the harvesting of plants, the seven tested HM contents of all tested crops’ soil and rotation patterns were substantially decreased (p < 0.05).
Table 2. Heavy metal contents mg/Kg (mean ± SD) of the soil supporting the growth of the three-oil crop and rotation pattern before cultivation and after harvesting.
Compared to soil content prior to cultivation, available soil Cd decreased by 9.33 in Indian mustard, 9.6 in rapeseed, 9.1 in maize, 7.9 in Indian mustard + maize, and 7.9 mg/Kg soil in rapeseed + maize. The reduction of tested HMs in different crops and rotation patterns could be arranged in decreasing order as follows: rapeseed > Indian mustard > maize > rapeseed + maize> Indian mustard + maize for seven tested HMs.
This result is consistent with the findings of [Citation33], who conducted fieldwork in a rotation experiment for one year in a gold mine area in China. Three crop rotation systems were investigated: potato-adzuki, wheat-corn, and rye-corn rotation. They found that the Hg, Cd, and Pb content decreased by 72, 82, and 22%, respectively, compared to before cultivation.
3.3. Accumulation of heavy metal in the selected crops
Tested HM contents (Zn, Pb, Ni, Mn, Fe, Cu, and Cd) in different parts for the three bioenergy crops are presented in Table . Significant remarkable variations in metal distribution were detected throughout the three studied bioenergy crops. The HM's content in the Indian mustard, rapeseed, and maize ranged from 0.9 to 4.0 for Cd, 14.9 for Cu, 28.4 to 193.2 for Fe, 12.2 to 74.3 for Mn, 1.3 to 7.4 for Ni,15.3 to 88.5 for Pb and 22.7 to 155.3 mg/Kg for Zn (Table ). The highest Cd, Mn, and Ni were detected in Indian mustard roots, while the highest Cu, Fe, and Zn content was found in maize roots. Simultaneously, the highest concentration of Pb was detected in rapeseed roots (Table ). The HM contents in plants descending were Fe > Zn > Cu > Pb > Mn > Ni > Cd.
Table 3. Cd, Cu, Fe, Mn, Ni, Pb, and Zn mg/Kg concentrations in different parts of the three oil crops (mean ± SD, n = 3).
In addition, implementing a suitable crop rotation system is regarded as a viable approach to improve soil fertility, resulting in higher agricultural product yields and the cultivation of crops tolerant to HMs [Citation14].
The crops under investigation, namely Indian mustard, rapeseed, and maize, have been documented to exhibit higher levels of metal accumulation than other crops. This characteristic makes them potentially valuable for the remediation of HMs remediation [Citation34,Citation35,Citation14], with underground parts generally accumulating significantly higher contents than shoots [Citation36].
In a five-year field study, [Citation37] compared three phytoremediation methods. These methods involved the cultivation of high-biomass amaranth, the phytoaccumulator hylotelephium, and a crop rotation of winter rapeseed and maize in soil contaminated with Cd and Pb. Their findings revealed that the optimal strategy for remediating farmland contaminated with Cd and Pb is implementing a crop rotation system involving rapeseed and maize.
The field experiment involving sesame and sunflower crop rotation, as Zhou et al. [Citation38] reported, was carried out in an industrial area within Hunan Province. Sunflowers exhibited varying Pb, Zn, Cu, and Cd concentrations ranging from 0.5 to 14.9, 32.1 to 448.7, 15.7 to 53.9, and 2.2 to 9.4 mg/kg of soil, respectively. Conversely, sesame plants showed concentrations of 0.12 to 12.2, 52.1 to 161.0, 7.3 to 32.9, and 1.5 to 4.1 mg/kg of soil for Pb, Zn, Cu, and Cd, respectively.
3.4. Remediation potential of bioenergy crops rotation system for HMs
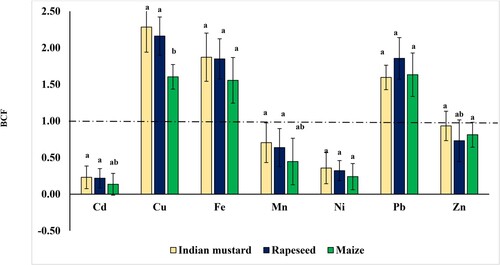
The bioconcentration factor (BCF) value significantly indicates a plant species’ ability to accumulate metal in its tissues from soil [Citation39]. According to BCF values, Baker [Citation40] classified the plants as follows: excluders when BCF value < 1, accumulators when the values between one to ten, and above ten called hyperaccumulators. The mean BCF values for the three oil crops vary, covering a range of values. These values include 0.14–0.23 for Cd, 1.60–2.28 for Cu, 1.56–1.87 for Fe, 0.45–0.70 for Mn, 0.24–0.36 for Ni, and 1.60–1.86 for Pb. The average tested HMs BCF values in decreasing order were Cu > Fe > Pb > Zn > Mn > Ni > Cd for three tested bioenergy crops (Figure ).
Figure 3. Bioconcentration factors (BCF) of heavy metals of the three oil crops: Indian mustard, rapeseed, and maize in the experimental rotation system. Means with different letters are significant at P < 0.05, the standard deviation is denoted as ± SD (n = 3).
These findings align with previous research that has demonstrated significant variability in the translocation of metals from soils to roots, and this variability depends on the specific elements and plant species [Citation41,Citation42]. Numerous studies have emphasized that the potential of metals to translocate (from soils to roots) as well as within plant tissues is influenced by numerous factors, including organic matter content, pH, temperature, reduction potential, salinity, and other metal contents [Citation43]. Additional variables, such as seasonal variations in physiology and the capacity for compartmentalization, may also impact the accumulation levels in plant species [Citation44,Citation45].
In a study conducted by [Citation33], it was found that BCF values for Hg and Pb in rye, wheat, corn, and beans < 1, while those for Cd were >1 in three crop rotation systems cultivated in mine polluted soil.
The ratio of HM content in the shoot system to that in the roots is referred to as the translocation factor (TF). It reflects a plant's ability to transport HMs from its roots to its aboveground parts [Citation46]. For three tested bioenergy crops, TF values were less than 1. The mean values of TF for three bioenergy crops in decreasing order were Cd > Ni > Pb > Mn > Zn > Cu > Fe. In the tested bioenergy crops, plants exhibited poor translocation of tested HMs to plant aboveground tissues (Figure ). The average calculated values for seven tested HMs were used to compare the translocation values in the three studied bioenergy crops. Moreover, Figure shows that maize has the highest TF value compared to other bioenergy crops tested. The same finding is consistent with other studies. Ali et al. [Citation16] observed that the assessed TF for B. junces and B. napus was less than 1 in plants cultivated in contaminated soil.
Figure 4. Translocation factors (TF) of heavy metals of the three oil crops: Indian mustard, rapeseed, and maize in the experimental rotation system. Means with different letters are significant at P < 0.05, the standard deviation is denoted as ± SD (n = 3).
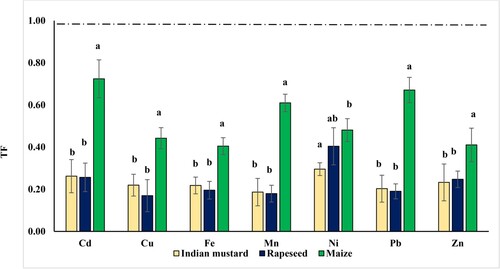
According to Goswamiv and Das [Citation47], soluble chemical elements may enter roots by crossing the plasma membrane of root cells and moving into the cytoplasm or through cell wall-free space (apoplastic route). Numerous investigations have shown that roots hinder HM translocation, protecting aerial plant organs from HM contamination and reducing oxidative stress [Citation48,Citation49].
The variations in HM contents in plant portions indicate diverse bioaccumulation cellular mechanisms that could regulate and control their translocation and partitioning in plant systems [Citation50,Citation51]. Furthermore, uptake and translocation of HMs to shoot are associated with element speciation, soil organic matter (SOM), and pH [Citation52].
3.5. Uptake of heavy metals and phytoextraction efficiency
The uptake of HMs by plants is greatly influenced by the physicochemical characteristics of soil, e.g. soil element, pH, and metal contents, and physiological plant characteristics, such as accumulation, translocation, and root metal absorption in the plant that regulate the amount of metal available to plants [Citation53,Citation54]. Consequently, metals uptake by roots and shoots of three bioenergy crops and rotation patterns was calculated and presented in Table .
Table 4. Uptake of Cd, Cu, Fe, Mn, Ni, Pb, and Zn (mg/plant) in different parts of the three oil crops and rotation pattern.
The Indian mustard harvested had a high accumulation of Cd, Cu, Fe, Mn, Ni, Pb, and Zn, which rendered the total plant uptake 15.4, 453. 6, 596.9, 251.2, 29.2, 252.1, and 490.2 mg/plant and phytoextraction efficiency 14.8, 9.9,12.1, 11.7,12.7,14.5 and 7.9%, respectively (Figure ). Correspondingly, the total plant uptake of Cd, Cu, Fe, Mn, Ni, Pb, and Zn in rapeseed is 15.6, 452.5, 646.4, 233.3, 29.4, 321.6, and 399.1 mg/plant, respectively, while the extraction was 10.4,10.8,10.0, 8.6, 12.2, 13.0 and 6.7%, respectively.
Figure 5. Phytoextraction efficiency (%) of the three oil crops and rotation pattern. ***P < 0.001. Means with different letters are significant at P < 0.05, The standard deviation is denoted as ± SD (n = 3).
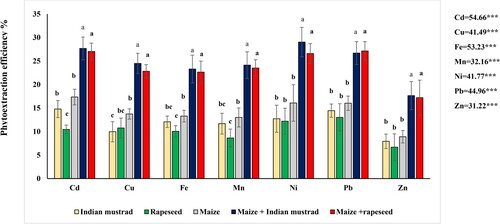
Similarly, the total uptake of maize is 38.8, 1050.6, 1863.1, 623.5, 72.3,1079.0, and 1432.4 mg/plant, respectively, for Cd, Cu, Fe, Mn, Ni, Pb, and Zn and their phytoextraction efficiency is 17.4, 13.7, 13.3, 13.0, 16.1, 16.0, 8.9%, respectively. The highest uptake of tested HMs in rotation patterns was shown in Indian mustard + maize and rapeseed + maize. The total plant uptake of Cd, Cu, Fe, Mn, Ni, Pb, and Zn are 54.2, 1504.2, 2459.9, 874.7, 101.6, 1431.2, and 1922.5 mg/plant, respectively, for Indian mustard + maize, whereas: 54.4, 1503.1, 2509.5, 856.9, 101.7, 1400.6 and 1861.5 mg/plant, respectively for rapeseed + maize. Correspondingly, the extraction of Cd, Cu, Fe, Mn, Ni, Pb, and Zn are 27.7, 24.5, 23.3, 24.1, 29.0, 26.7 and 17.7%, respectively, for Indian mustard and maize. However, 27.0, 22.8, 22.6, 23.5,30.6, 27.1 and 17.2%, respectively, for rapeseed + maize (Figure ). The uptake of the three crops and rotation pattern showed an Indian mustard + maize > rapeseed + maize > maize >Indian mustard > rapeseed.
Chen et al. [Citation55] conducted a field experiment in China to evaluate the efficiency of a three-season rotation of chicory, tobacco, and peanuts in remediating farmland contaminated with Cd. The study revealed that the bioconcentration values observed in chicory ranged from 6.6 to 11.9, in tobacco from 3.9 to 21.6, and in peanut from 1.4 to 7.0. These findings indicate that all three crops exhibited a significant tendency to accumulate Cd. Furthermore, the yearly efficiency of Cd phytoextraction was recorded at 10.3%. The proportion of the total biomass accounted for by the aboveground tissues of the three crops in the rotation experiment ranged from 83.9% to 91.2%.
The primary objective of phytoextraction in the context of metal-contaminated agricultural soils is to effectively eliminate excessive metal concentrations, thereby restoring the soil's capacity for crop cultivation and ensuring the safety of agricultural production. In the present investigation, three oil crops were cultivated and subsequently harvested within one year, with the primary objective of extracting metal contaminants from the soil of Mahd AD'Dahab.
The phytoremediation approach often faces two challenges; first, most HM cleanup products are hyper-accumulators, which local farmers often reject. Second, hyper-accumulators usually demand an environment that promotes high growth rates. These plants could remove particular HMs from polluted soil. However, they also remove other minerals, like Fe, Cu, and Zn, which are required for plant growth [Citation56–58], which can influence the growth of subsequent crops. This investigation utilized oil crops as the fundamental components for designing crop rotation schemes, with all three crops being acknowledged by farmers. Consequently, this crop rotation system may efficiently remediate HMs polluted sites while being extensively promoted as a viable economic choice.
Moreover, the same findings were recorded by Yang et al. [Citation14] on three rotation systems that provided high dry biomass yields: the rapeseed-sunflower, rapeseed-peanut, and rapeseed-sesame rotation. Additionally, in fieldwork, Zhou et al. [Citation38] investigated the remediation of Pb, Zn, Cu, and Cd under an oil crop rotation of sunflower–sesame.
In addition to HMs content in plant and biomass production, soil parameters (organic matter, soil pH, electrical conductivity) can influence bioavailability, HMS absorption, and phytoextraction rate [Citation59,Citation30]. Soil reaction (pH), considered the most significant factor affecting soil availability and metal mobility, negatively correlates with soil pH [Citation30]. Additionally, soil organic matter (SOM) is a significant sorbent for HMs; consequently, it can increase availability or decrease mobility under different soil conditions [Citation59]. The availability of HMs frequently depends on the form of the metal present in the soil, which is in equilibrium with the cation exchange sites of SOM or clays [Citation60].
Furthermore, in fieldwork, Zhou et al. [Citation38] investigated the remediation of Pb, Zn, Cu, and Cd under an oil crop rotation of sunflower–sesame. The results indicated that the extraction efficiency of sunflower-sesame rotation was 6.1% for Cd, 1.4% for Zn, 0.07% for Pb, and 1.1% for Cu.
3.6. Multivariate analysis
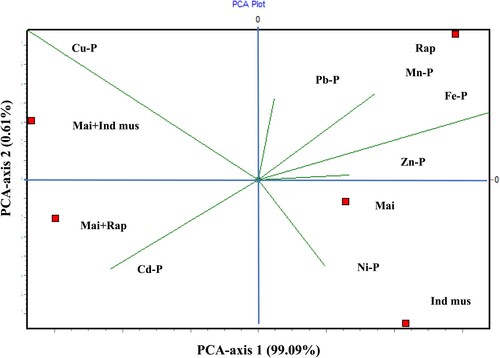
The PCA ordination indicated that the phytoextraction efficiency of Fe, Cd, and Zn contributes significantly to PC 1, while that of Pb, Mn, Cu, and Ni contribute to PC 2 (Figure ). The intercrop of maize + rapeseed was found on a high phytoextraction gradient of Cd, while maize + Indian mustard was present on a high gradient of Cu phytoextraction. Besides, Indian mustard and maize plants were significantly correlated with Ni phytoextraction, rapeseed plants were significantly contributed to Pb, Mn, Fe, and Zn phytoextraction efficiency.
Figure 6. Relationship between heavy metals and phytoextraction efficiency based on PCA, at different patterns; Mai: Maize, Ind mus: Indian mustard, Rap: Rapeseed, Mai + Ind mus: Maize and Indian mustard, Mai + Rap: Maize and Rapeseed.
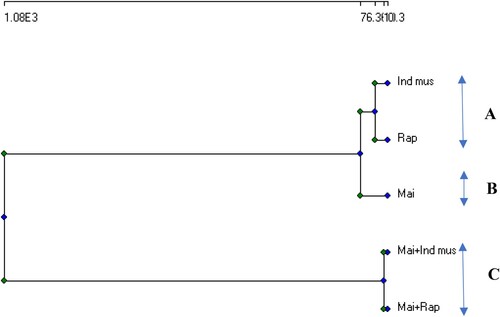
The utilization of cluster analysis algorithms is necessary in order to partition the data points into distinct clusters. Cluster analysis was utilized to identify the similarity of groups among the samples, which were categorized into three distinct clusters based on their points.
The dendrogram resulting from the application of clustering analysis, based on the phytoextraction efficiency of the study crops indicated that the intercrops of maize + Indian mustard and maize + rapeseed had similarly the highest phytoextraction potential (Figure ). The cluster analysis separated the study crops into three groups: (A) included indian mustard and rapeseed crops; (B) comprised maize plants; and (C) included maize + Indian mustard and maize + rapeseed intercrops.
4. Conclusion
The remediation of hazardous metals (HMs) in mining-contaminated soils was assessed using a short-rotation experiment. The current experiment involved five different cropping systems, including monoculture of oil crops (rapeseed, Indian mustard, and maize) and two oil crop rotations (rapeseed-maize and Indian mustard-maize), and their effectiveness was compared. A microcosm experiment assessed the phytoremediation potential of three studied oil crops. Compared to single-crop cultivation, the oil-crop rotation technique could provide high biomass and enhance the removal of Cd, Cu, Fe, Mn, Ni, Pb, and Zn from the mine-contaminated soil. Notably, both the rapeseed maize and Indian mustard maize rotations exhibited superior phytoextraction efficiency when compared to a single crop. The percentages of Cd, Cu, Fe, Mn, Ni, Pb, and Zn extracted from Indian mustard + maize are 28, 24, 23, 24, 29, 27, and 18%, respectively. Conversely, the percentages of Cd, Cu, Fe, Mn, Ni, Pb, and Zn extracted from rapeseed + maize are 26, 23, 21, 24, 31, 27, and 17%, respectively.
As demonstrated in this study, using oil rotation of crops for one year offers a viable approach for phytoremediation of sites contaminated with heavy metals (HMs). This strategy allows for effectively allocating farmland in terms of time and space, leading to substantial economic advantages. Furthermore, it ensures comprehensive, secure, and efficient remediation and utilization of agricultural soils that have been contaminated. Further investigation is necessary to evaluate the agricultural productivity of the crop and conduct a comprehensive examination of its potential adverse effects on animal models in accordance with appropriate ethical guidelines.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Baldauf RW, Lane DD, Marote GA. Ambient air quality monitoring network design for assessing human health impacts from exposures to airborne contaminants. Environ Monit Assess. 2001;66(1):63–76. doi:10.1023/A:1026428214799
- Afonso T, Demarco C, Pieniz S, et al. Bioprospection of indigenous flora grown in copper mining tailing area for phytoremediation of metals. J Environ Manag. 2020;256:109953. doi:10.1016/j.jenvman.2019.109953
- Zhang L, Wang J, Feng Y. Life cycle assessment of opencast coal mine production: a case study in Yimin mining area in China. Environ Sci Pollut Res. 2018;25:8475–8486. doi:10.1007/s11356-017-1169-6
- Khalid S, Shahid M, Niazi NK, et al. A comparison of technologies for remediation of heavy metal contaminated soils. J Geochem Explor. 2017;182:247–268. doi:10.1016/j.gexplo.2016.11.021
- Rafique MI, Usman AR, Ahmad M, et al. In situ immobilization of Cr and its availability to maize plants in tannery waste–contaminated soil: effects of biochar feedstock and pyrolysis temperature. J Soils Sediments. 2020;20(1):330–339. doi:10.1007/s11368-019-02399-z
- Xu L, Xing X, Liang J, et al. In situ phytoremediation of copper and cadmium in a co-contaminated soil and its biological and physical effects. RSC Adv. 2019;9(2):993–1003. doi:10.1039/C8RA07645F
- Siyar R, Ardejani FD, Norouzi P, et al. Phytoremediation potential of native hyperaccumulator plants growing on heavy metal-contaminated soil of Khatunabad copper smelter and refinery, Iran. Water (Basel). 2022;14(22):3597. doi:10.3390/w14223597
- Bashir S, Rehman M, Yousaf M, et al. Comparative efficiency of wheat straw and sugarcane bagasse biochar reduces the cadmium bioavailability to spinach and enhances the microbial activity in contaminated soil. Int J Phytoremediation. 2019;21(11):1098–1103. doi:10.1080/15226514.2019.1606781
- Abbas A, Azeem M, Naveed M, et al. Synergistic use of biochar and acidified manure for improving growth of maize in chromium contaminated soil. Int J Phytoremediation. 2020;22(1):52–61. doi:10.1080/15226514.2019.1644286
- Brooks RR, Chambers MF, Nicks LJ, et al. Phytomining. Trends Plant Sci. 1998;3(9):359–362. doi:10.1016/S1360-1385(98)01283-7
- Edgar V-N, Fabián F-L, Mario P-CJ, et al. Coupling plant biomass derived from phytoremediation of potential toxic-metal-polluted soils to bioenergy production and high-value by-products—A review. Appl Sci. 2021;11(7):2982. doi:10.3390/app11072982
- Sims RE, Hastings A, Schlamadinger B, et al. Energy crops: current status and future prospects. Glb Chg Bio. 2006;12(11):2054–2076. doi:10.1111/j.1365-2486.2006.01163.x
- Pandey VC, Bajpai O, Singh N. Energy crops in sustainable phytoremediation. Renewable Sustainable Energy Rev. 2016;54:58–73. doi:10.1016/j.rser.2015.09.078
- Yang Y, Zhou X, Tie B, et al. Comparison of three types of oil crop rotation systems for effective use and remediation of heavy metal contaminated agricultural soil. Chemosphere. 2017;188:148–156. doi:10.1016/j.chemosphere.2017.08.140
- Quronfulah AS, El-Morsy MHEM, Galal TM, et al. Phytoaccumulation of zinc and its associated impact on the growth performance and tolerance index of six non-food crop plants grown in Zn-contaminated soil. Environ Sci Pollut Res. 2023;30(15):43872–43885. doi:10.1007/s11356-023-25332-x
- Ali I, Khan MJ, Shah A, et al. Screening of various Brassica species for phytoremediation of heavy metals-contaminated soil of Lakki Marwat, Pakistan. Environ Sci Pollut Res. 2022;29(25):37765–37776. doi:10.1007/s11356-021-18109-7
- Marchiol L, Assolari S, Sacco P, et al. Phytoextraction of heavy metals by canola (Brassica napus) and radish (Raphanus sativus) grown on multicontaminated soil. Environ Pollut. 2004;132(1):21–27. doi:10.1016/j.envpol.2004.04.001
- Ahmad I, Malik SA, Saeed S, et al. Phytoremediating a wastewater-irrigated soil contaminated with toxic metals: comparing the efficacies of different crops. Soil Syst. 2022;6(4):77. doi:10.3390/soilsystems6040077
- Al-Farraj AS, Al-Wabel MI. Evaluation of soil pollution around mahad ad'dahab mine. J Saudi Soc Agric Sci. 2007;6(2):89–106.
- Sparks DL. Environmental soil chemistry. San Diego (CA): Harcourt Brace and Company; 1995.
- Richards IA. Diagnosis and improvement of saline and alkali soils, Handbook No 60. Washington: USDA; 1954.
- Nelson DW, Sommers LE. Total carbon, organic carbon, and organic matter. In: Page AL, editor. Methods of soil analysis; Part 2 (2nd). Madison (WI): Agron J; 1982. p. 539–579.
- Lindsay WL, Norvell WA. Development of a DTPA soil test for zinc iron manganese and copper. Soil Sci Soc Am. J. 1978;42:421–428. doi:10.2136/sssaj1978.03615995004200030009x
- Ghosh M, Singh SP. A comparative study of cadmium phytoextraction by accumulator and weed species. Environ Pollut. 2005;133:365–371. doi:10.1016/j.envpol.2004.05.015
- Galal TM, Hassan LM, Ahmed DA, et al. Heavy metals uptake by the global economic crop (Pisum sativum L.) grown in contaminated soils and its associated health risks. PLoS One. 2021;16(6):e0252229. doi:10.1371/journal.pone.0252229
- Jing Y, Yang Y, Xi-hong Z, et al. A new method for simultaneous removal of heavy metals and harmful organics from rape seed meal from metal-contaminated farmland. Sep Purif Technol. 2018. doi:10.1016/j.seppur.2018.09.056
- Muller G. Heavy-metals in sediment of the Rhine-changes since 1971. Umsch Wiss Tech. 1979;79(24):778–783.
- Nkansah K, Dawson-Andoh B, Slahor J. Rapid characterization of biomass using near infrared spectroscopy coupled with multivariate data analysis: part 1 yellow-poplar (Liriodendron tulipifera L.). Bioresour Technol. 2010;101(12):4570–4576. doi:10.1016/j.biortech.2009.12.046
- Tabachnick BG, Fidell LS, Ullman JB. Using multivariate statistics. Vol. 6. Boston (MA): pearson; 2013. p. 497–516.
- Yu L, Zhu J, Huang Q, et al. Application of a rotation system to oilseed rape and rice fields in Cd-contaminated agricultural land to ensure food safety. Ecotoxicol Environ Saf. 2014;108:287–293. doi:10.1016/j.ecoenv.2014.07.019
- Yin H, Tan N, Liu C, et al. The associations of heavy metals with crystalline iron oxides in the polluted soils around the mining areas in Guangdong Province, China. Chemosphere. 2016;161:181–189. doi:10.1016/j.chemosphere.2016.07.018
- Lindsay WL. Chemical equilibrium in soils. NewYork: Wiley Interscience; 1979.
- Jiang L, Yi X, Xu B, et al. Soil treatment and crop rotation for in situ remediation of heavy metal-contaminated agricultural soil in gold mining areas. Hum Ecol Risk Assess Int J. 2019;25(1–2):374–392. doi:10.1080/10807039.2019.1568856
- Angelova V, Ivanova R, Ivanov K. Heavy metal accumulation and distribution in oil crops. Commun Soil Sci Plant Anal. 2004;35(17–18):2551–2566. doi:10.1081/LCSS-200030368
- Shi G, Cai Q. Cadmium tolerance and accumulation in eight potential energy crops. Biotechnol Adv. 2009;27(5):555–561. doi:10.1016/j.biotechadv.2009.04.006
- Shu WS, Ye ZH, Lan CY, et al. Lead, zinc and copper accumulation and tolerance in populations of Paspalum distichum and Cynodon dactylon. Environ Pollut. 2002;120(2):445–453. doi:10.1016/S0269-7491(02)00110-0
- Guo J, Zheng G, Yang J, et al. Safe utilization of cadmium-and lead-contaminated farmland by cultivating a winter rapeseed/maize rotation compared with two phytoextraction approaches. J Environ Manag. 2022;304:114306. doi:10.1016/j.jenvman.2021.114306
- Zhou J, Chen LH, Peng L, et al. Phytoremediation of heavy metals under an oil crop rotation and treatment of biochar from contaminated biomass for safe use. Chemosphere. 2020;247:125856. doi:10.1016/j.chemosphere.2020.125856
- Melgar MJ, Alonso J, García MA. Cadmium in edible mushrooms from NW Spain: Bioconcentration factors and consumer health implications. Food Chem Toxicol. 2016;88:13–20. doi:10.1016/j.fct.2015.12.002
- Baker AJ. Accumulators and excluders-strategies in the response of plants to heavy metals. J Plant Nutr. 1981;3(1–4):643–654. doi:10.1080/01904168109362867
- Usman AR, Alkredaa RS, Al-Wabel MI. Heavy metal contamination in sediments and mangroves from the coast of Red Sea: Avicennia marina as potential metal bioaccumulator. Ecotoxicol Environ Saf. 2013;97:263–270. doi:10.1016/j.ecoenv.2013.08.009
- Bonanno G, Vymazal J, Cirelli GL. Translocation, accumulation and bioindication of trace elements in wetland plants. Sci Total Environ. 2018;631:252–261. doi:10.1016/j.scitotenv.2018.03.039
- Etesami H. Can interaction between silicon and plant growth promoting rhizobacteria benefit in alleviating abiotic and biotic stresses in crop plants? Agric Ecosyst Environ. 2018;253:98–112. doi:10.1016/j.agee.2017.11.007
- Laffont-Schwob I, Triboit F, Prudent P, et al. Trace metal extraction and biomass production by spontaneous vegetation in temporary Mediterranean stormwater highway retention ponds: Freshwater macroalgae (Chara spp.) vs. cattails (Typha spp.). Ecol Eng. 2015;81:173–181. doi:10.1016/j.ecoleng.2015.04.052
- Vymazal J. Concentration is not enough to evaluate accumulation of heavy metals and nutrients in plants. Sci Total Environ. 2016;544:495–498. doi:10.1016/j.scitotenv.2015.12.011
- Feng J, Jia W, Sulian LV, et al. Comparative transcriptome combined with morpho-physiological analyses revealed key factors for differential cadmium accumulation in two contrasting sweet sorghum genotypes. Plant Biotechnol J. 2018;16(2). doi:10.1111/pbi.12795
- Goswami S, Das S. A study on cadmium phytoremediation potential of Indian mustard, Brassica juncea. Int J Phytoremediation. 2015;17(6):583–588. doi:10.1080/15226514.2014.935289
- Panwar BS, Ahmed KS, Mittal SB. Phytoremediation of nickel-contaminated soils by Brassica species. Environ, Dev Sustainability. 2002;4:1–6. doi:10.1023/A:1016337132370
- Liu B, Wang J, Xu M, et al. Spatial distribution, source apportionment and ecological risk assessment of heavy metals in the sediments of Haizhou Bay national ocean park, China. Mar Pollut Bull. 2019;149:110651. doi:10.1016/j.marpolbul.2019.110651
- Sinha S, Gupta AK, Bhatt K. Uptake and translocation of metals in fenugreek grown on soil amended with tannery sludge: involvement of antioxidants. Ecotoxicol Environ Saf. 2007;67(2):267–277. doi:10.1016/j.ecoenv.2006.07.005
- Sharma SS, Dietz KJ. The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci. 2009;14(1):43–50. doi:10.1016/j.tplants.2008.10.007
- Kabata-Pendias A. Trace elements in soils and plants. CRC Press; 2010; doi:10.1201/b10158
- Rascio N, Navari-Izzo F. Heavy metal hyperaccumulating plants: how and why do they do it? And what makes them so interesting? Plant Sci. 2011;180(2):169–181. doi:10.1016/j.plantsci.2010.08.016
- Muhammad I, Puschenreiter M, Wenzel WW. Cadmium and Zn availability as affected by pH manipulation and its assessment by soil extraction, DGT and indicator plants. Sci Total Environ. 2012;416:490–500. doi:10.1016/j.scitotenv.2011.11.029
- Chen L, Yang W, Yang Y, et al. Three-season rotation of chicory–tobacco–peanut with high biomass and bioconcentration factors effectively remediates cadmium-contaminated farmland. Environ Sci Pollut Res. 2022;29:64822–64831. doi:10.1007/s11356-022-20400-0
- Deng DM, Shu WS, Zhang J, et al. Zinc and cadmium accumulation and tolerance in populations of Sedum alfredii. Environ Pollut. 2007;147(2):381–386. doi:10.1016/j.envpol.2006.05.024
- Xv L, Ge J, Tian S, et al. A Cd/Zn Co- hyperaccumulator and Pb accumulator, Sedum alfredii, is of high Cu tolerance. Environ Pollut. 2020;263:114401. doi:10.1016/j.envpol.2020.114401
- Zu Y, Li Y, Christian S, et al. Accumulation of Pb, Cd, Cu, and Zn in plants and hyperaccumulator choice in Lanpinglead–zinc mine area, China. Environ Int. 2004;30(4):567–576. doi:10.1016/j.envint.2003.10.012
- Zeng F, Ali S, Zhang H, et al. The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ Pollut. 2011;159(1):84–91. doi:10.1016/j.envpol.2010.09.019
- MacCarthy P. The principles of humic substances. Soil Sci. 2001;166(11):738–751. doi:10.1097/00010694-200111000-00003