Abstract
The present research highlights the critical importance of understanding S. mansoni infection in global public health. Using a network-based pharmacological approach, this study explores parasite biology, disease mechanisms, and potential treatments. Praziquantel and its derivatives are identified as key drugs for treating schistosomiasis. ADMET and molecular docking predict the preferred binding orientation of drug candidates, like Mol4, with target proteins. Analyzing network pharmacology, disease classification, enrichment studies, and pathways reveals the biological processes influenced by these candidates. The research emphasizes the need for comprehensive healthcare in endemic regions and identifies critical pathways and target proteins, such as zinc-binding proteins and endopeptidases, as promising drug targets. The integration of molecular docking and network pharmacology provides a strong platform for advancing drug development and devising effective treatment strategies against this debilitating parasitic infection, ultimately contributing to the enhancement of global public health.
1. Introduction
Schistosomiasis, a parasitic disease caused by Schistosoma parasites, remains a significant global health challenge, particularly in regions with inadequate sanitation and limited access to clean water [Citation1]. The disease affects millions of individuals in over 70 countries, predominantly in tropical and subtropical regions. Schistosomiasis is responsible for substantial morbidity and mortality, in terms of socioeconomic and public health impact. The World Health Organization (WHO) has classified it as a neglected tropical disease, emphasizing the urgent need for effective control and treatment strategies [Citation2]. Schistosoma parasites have a complex life cycle involving freshwater snails as intermediate hosts and humans as definitive hosts [Citation3]. The life cycle of Schistosoma involves several stages. It begins when eggs, passed in the faeces or urine of infected individuals, contaminate freshwater environments [Citation4]. In the water, these eggs hatch, releasing miracidia, which are microscopic larvae. The miracidia then seek out and penetrate specific snail hosts, where they develop into sporocysts and later into cercariae within the tissues of the snail [Citation5]. Cercariae are the free-swimming, fork-tailed larvae that emerge from infected snails into the water [Citation6]. They actively seek and penetrate the skin of the definitive human host during activities like swimming or wading in infested water. Once inside the human host, the cercariae shed their tails, becoming schistosomulae, and travel through the bloodstream to reach their final destination in the blood vessels surrounding the intestines or bladder [Citation7].
The cercariae stage is crucial in the transmission of schistosomiasis from snail hosts to humans [Citation8]. Preventing contact with cercariae-infested water sources is a key strategy in controlling schistosomiasis. Efforts to interrupt the life cycle at this stage, through improved sanitation [Citation9], control of snail populations and public health education, are important for reducing the incidence of schistosomiasis [Citation10]. Infection occurs when individuals come into contact with water contaminated by Schistosoma cercariae [Citation11], typically during physical activities (such as swimming, bathing or washing clothes) in infested water bodies. The cercariae penetrate the skin and enter the bloodstream, eventually maturing into adult worms within the blood vessels surrounding the intestines or bladder [Citation12]. The eggs produced by these worms can cause chronic inflammation, tissue damage and various complications, including liver and spleen enlargement [Citation13]. Praziquantel, a broad-spectrum antiparasitic drug, has been the mainstay of schistosomiasis treatment for decades due to its efficacy, safety and relatively low cost [Citation14]. However, concerns about drug resistance and the necessity for effective control programs that incorporate preventative measures, alongside advancements in drug discovery and development, underscore the need to explore new therapeutic strategies [Citation15].
This study endeavours to provide a comprehensive understanding of S. mansoni infection and its associated disease mechanisms, employing a network-based pharmacological approach. Through molecular docking and network pharmacology analyses, potential drug targets and pathways critical for parasite survival and infection progression will be elucidated. Additionally, the study aims to shed light on the potential inhibitory roles of certain drug candidates in crucial pathways and binding pockets, highlighting their promise as novel drug targets. The ultimate goal is to contribute to the advancement of drug discovery and the development of innovative treatment strategies to combat schistosomiasis, thereby alleviating the burden of this devastating disease on global public health.
2. Materials and methods
2.1. Similarity screening for Praziquantel
Swiss Similarity is an online tool with a user-friendly interface for ligand-based virtual screening of chemical libraries, to find compounds that are similar to a query molecule. Swiss Similarity is available at http://www.swisssimilarity.ch. The tool retrieves compounds from several libraries such as approved drugs, known bioactive molecules, synthetically accessible compounds and commercially available compounds. Swiss Similarity tool is useful for searching similar compounds that are marketed drugs or clinical drug candidates and compounds with determined experimental structures that are docked with protein targets [Citation16]. Additionally, Swiss Similarity can be used to confirm the chemical novelty of de novo-designed molecules. For the present study, the Swiss Similarity tool was used for the prediction of a similar structure of Praziquantel using the module “combined” 2D/3D screening method. This method is applied based on both FP2 and ES5D similarity metrics [Citation17]. The scoring is carried out using the logistic regression method which combines FP2 and ES5D similarity scores. The combined score generated provides a quantitative measure of the likelihood that the two compounds have a shared protein target. Higher scores would indicate a higher probability of the compounds having a common protein target, while lower scores suggest a lower probability.
2.2. Prediction of pharmacokinetic properties
The smiles structures of the standard drug (Praziquantel) and five compounds obtained from a similarity search were downloaded from the PubChem database (Table ). The pharmacokinetic properties of the ligands were evaluated by ADMETlab 2.0 web server (https://admetmesh.scbdd.com) [Citation18]. The SMILES of standard drug and their derivatives were predicted for different properties (physicochemical, toxicity and pharmacokinetics). The molecular properties such as molecular weight and LogP were determined [Citation19]. In the absorption category, skin permeability, HIA penetration, human colon adenocarcinoma cell lines (Caco2) were determined. The role of chemical compounds in the distribution was scored for CNS and BBB permeability [Citation20]. The metabolism of the compounds was determined through CYP450 inhibitor and substrate activity. Total clearance of the chemical compounds was predicted for the excretion of the compounds from the human system [Citation21,Citation22]. The toxic effects of the compounds were determined through the AMES test, hepatotoxicity, skin sensitization and minnow toxicity. Overall, the scoring values of these compounds were compared – with the standard drug and the best ligand was determined from the study.
Table 1. The compounds used for the study and their CID.
2.3. Binding site prediction and molecular docking
The target protein serpin (6SSV.pdb) was downloaded from the PDB database and the protein was prepared before docking. The whole protein was subjected for binding site prediction using Prank webserver (https://prankweb.cz/). The drug control Praziquantel and five test ligands were retrieved from the PubChem database. Docking studies were performed using AutoDock Tools (ADT) [Citation20]. The protein and ligand structures used for the study were subjected to minimization and converted to pdbqt files [Citation21]. The structures were validated using PLIP (Protein–Ligand Interaction Profiler) to identify non-covalent interactions between protein and their ligand complex. The atom-level information on binding characteristics was observed to provide insightful interactions with complex [Citation23]. Further, AutoGrid was used to create the grid box for the target proteins in the respective site of binding. The parameters such as the addition of hydrogen, Kollman charges, and solvation were applied using ADT. Two binding pockets were predicted and both sites were prepared with the respective grid size, grid centre and spacing for the target proteins. For the binding pocket 1, the grid centre was determined as x = 51.5 y = −28.6 and z = 20.3. For the binding pocket 2, the grid centre was determined as x = 28.9; y = −37.05; z = 20.94. AutoDock Vina was employed for docking ligands into the binding pocket of the proteins [Citation24]. The ligands with the lowest binding affinity were extracted and aligned with the receptor for further analysis. Electrostatic surface potentials (ESP) analysis for the ligand–protein complex was performed using APBS electrostatics integrated with PyMOL version 3.0. Chimera X was for predicting the relative solvent accessibility (RSA). The structure-editing tool, attributes calculator was used to determine the solvent-excluded surface area of each type of amino acid.
2.4. Protein–protein interaction (PPI) network
Serpins produced by Schistosoma are known to inhibit host proteases, including proteases involved in the host immune response. Hence, to understand Serpin and interacting protein partners, the protein name was imported into STRING database (https://string-db.org/) [Citation25] to construct a PPI network. Studying PPIs provides the knowledge of cellular and molecular landscape of how proteins function individually and collectively. Additionally, it paves the way for advancements in drug development, disease understanding, and systems biology. Serpins and their interacting partners investigated against the Schistosoma mansoni database. The network score was set to default such as the medium score of 0.4 and maximum interactions in the 1st shell as “not more than 10”. The hub nodes were screened for the active interaction sources such as experiments, co-expression, databases, gene fusion, neighbourhood and co-occurrence. The network edges were set based on the evidence.
2.5. Construction of the compoundtarget network (C-T-N)
The network of component–target interactions was established between Praziquantel their derivatives (Mol1 and Mol4) and the target proteins. Swiss Target Prediction online server was used (http://www.swisstargetprediction.ch) for prediction. A cytoscape is a special map for studying the different proteins or genes in the human system that interact and perform their functions. Additionally, the tool is used for the multi-target approach that aims to develop drugs that can modulate several targets related to the disease, disrupting the disease network more effectively and used to create drugs that can target specific areas more efficiently. From the network obtained the expression of proteins, phenotypes and their molecular profiles was linked. In the present study, Cytoscape 3.8.2 (http://www.cytoscape.org/) was used for establishing C-T-N using the data obtained from the target protein prediction [Citation26]. The nodes in the graph represent biological entities (e.g. proteins, genes), and the edges represent the connections between them (e.g. interactions, relationships). Networks prepared are integrated with different colour codes to simplify the visualization and comprehend the interaction pattern. Thus a network-based approach prepared using Cytoscape aids in preparing for the experimental validations, allowing researchers to select the most promising targets for further investigation based on the network analysis.
2.6. Gene ontology (GO) analysis
Functional annotation of biological processes, cell composition, molecular function and pathways of the proteins are studied using the DAVID (database for annotation, visualization and integrated discovery) enrichment tool. This annotation database allows ordering of the gene based on different categories such as GO analysis (DAVID 6.8) [Citation27]. The scoring method (EASE) was applied for sorting the genes and validating the enrichment data of the C-T-N. The hub genes of both networks were annotated using the GO terms based on gene distribution the hierarchy is explained.
2.7. KEGG enrichment analysis
The functional attributes of the proteins distributed in the C-T-N were enriched in the pathways [Citation28]. The KEGG enrichment analysis was studied for the network and default parameters such as gene count >5 and p < 0.05 were used for performing the experiments. This allows the prediction of the target genes from the distinct pathways and to determine their functional attributes.
3. Results
3.1. Prediction of pharmacokinetic properties
The standard drug (Praziquantel) and five compounds obtained from a similarity search were subjected to ADMET analysis to study different pharmacokinetic and toxicity properties (Table ). The LogP (partition coefficient) is a measure of the lipophilicity or hydrophobicity of a chemical compound. It quantifies the distribution of a compound between an organic solvent (typically octanol) and water. The water solubility of the compound was >−4 (log mol/L), except Mol5. Higher the LogP value, there is an increase in the tendency for the compound to be soluble in organic solvents relative to water. Except Mol5 all the compounds have logP > 2.5 representing the tendency to be soluble in organic solvents.
Table 2. ADMET prediction for the compounds used for the study.
3.1.1. Absorption
Prediction of the absorption index in the human intestine showed that standard drugs and Mol 1, Mol2, Mol2 and Mol4 have excellent absorption index values (>92%) therefore recorded as suitable for oral bioavailability. The Caco-2 predictive model with values >0.9 considered with high Caco-2 permeability. Caco-2 permeability of the standard drug and derivatives (Mol1–4) were observed with values >1.0. From the skin permeability, the compounds with log Kp > −2.5 are considered to have low skin permeability. Compared with the standard drug, the compounds Mol 2, Mol 3 and Mol 4 have log Kp values −2.7 expected to show better skin permeability. The compounds with CNS permeability values >−2.0 are considered to have higher permeability.
3.1.2. Distribution
From the prediction results, only Mol 5 has been predicted to have CNS permeability. Remarkably, all the compounds have blood–brain barrier (BBB) permeability.
3.1.3. Metabolism
From the study, it was observed that Praziquantel and five compounds do not act as CYP2D6 substrates but act as inhibitors. However, all the compounds act as a substrate for CYP3A4. Interestingly, Mol 4 is the only compound predicted as a CYP1A2 inhibitor. In addition, Mol2, Mol3 and Mol3 are the inhibitors of CYP3A4. A drug candidate to selectively inhibit cytochrome P450 enzymes (CYP2C9 and CYP3A4) while not inhibiting CYP2D6 is a precise and targeted approach in drug development.
3.1.4. Excretion
The total clearance predicted for the present study for the six compounds shows all the compounds have CLTotlog of >1.0 except Mol 1 which has the value of 0.826.
3.1.5. Toxicity
All the compounds from AMES toxicity prediction showed that the compound did not show mutagenic potential or induce mutations. All the compounds are predicted non-toxic to the skin and can be used for development and formulation to reduce or eliminate sensitizing components. Minnow toxicity predicted that lethal concentration values (LC50) represent the concentration of a molecule necessary to cause the death of 50% of the flathead minnows. The values of minnow toxicity LC50 < −0.3 is predicted to be high acute toxicity. From the prediction, it was observed all the compounds have excellent minnow toxicity. Mol1, Mol2 and Mol5 are predicted to be hepatotoxic, whereas the standard drugs Mol3 and Mol4 do not disrupt with the normal function of the liver. Thus ADMET-based prediction allows for the modulation of specific metabolic pathways, potentially improving drug efficacy and minimizing unwanted interactions.
3.2. Binding site prediction
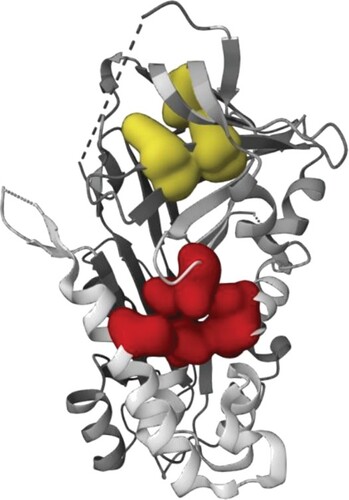
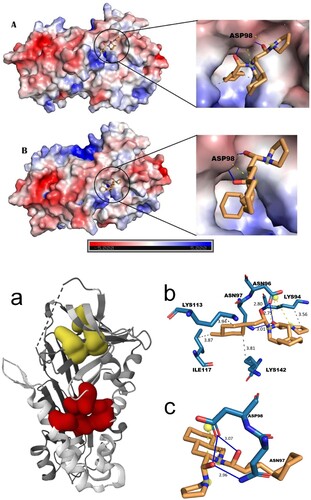
Understanding and utilizing binding sites in drug discovery facilitate the rational design and optimization of drug candidates, improving drug selectivity, efficacy and safety. Therefore it is a critical approach that helps monitor the entire drug development process, from target identification to clinical trials. In the present study, with protein target serpin two binding pockets were identified (Figure a). Pocket 1 was predicted with several residues such as Thr23, Phe22, Leu 44, Leu47, Lys 94, Leu96, Asn 97, Asp 98, Gly101, Lys 113, Val115, Ile 117, Lys 142, Met392. In pocket 2, the residues such as Leu293, Val 244, Val 257, Phe 255 and Lys 246 were discriminated as the active residues.
Figure 1. (a) Serpin from S. mansoni identified with two binding pockets. Yellow colour represents binding pocket 1 and red colour represents binding pocket 2. (b) 3D pictorial representation Mol1 showing interaction with the binding pocket 1 active site residue. (c) The molecular interaction of Mol4 into the binding pocket1 and their active residues. (d) Electrostatic potential surfaces (ESP) of protein and ligand complex (A) Mol1 and (B) Mol2.
3.3. Molecular docking
From the study, it was observed the ligands docked into the binding pocket 1 have, the best binding affinity compared to pocket 2. Additionally, Mol 1 and Mol4 have high binding affinity values of −10.3 (kcal/mol) and −10.1 (kcal/mol) respectively. The ligands have better affinity compared to the standard drug that is currently used for therapeutic purposes. The investigation of the interacting residues showed that all the compounds interact with Asp98 (Table ). However, Mol1 showed their interaction with binding pocket residues such as Lys94, Asn97, Asp98, Gly101, Lys113, Val115, Ile117 and Lys142. Similarly, Mol4 is involved in interaction with major binding pocket residues (Figure b,c). ESP (Electrostatic Potential) interpretations are crucial in understanding the interactions at the molecular level and are often represented through color codes. Red indicates negative charges, blue signifies positive charges and white represents neutral residues. In the context of the catalytic site, Asp98, identified as a key amino acid, has shown interactions with all ligand molecules, underlining its importance in binding. The ligands Mol1 and Mol4, in particular, have exhibited interactions with both Asn97 and Asp98. The tetramine groups present in Mol1 and Mol4 are positively charged, facilitating strong electrostatic interactions with the negatively charged Asp98, which contains a sulphur atom. This electrostatic interaction significantly reinforces the binding affinity between the ligands and the receptor. Additionally, Mol1 and Mol4 have established three hydrogen bonds with Asp98, further strengthening the ligand binding at the catalytic site. These hydrogen bonds are crucial as they enhance the stability and specificity of the interaction, which is visually represented in Figure (d).
Table 3. The binding affinity for the ligands showing interaction at the binding pocket 1 and residues involved in interactions.
The Residue Solvent Accessibility (RSA) analysis provides further insights into the binding dynamics. RSA measures the extent to which residues are buried or exposed in the protein structure. For the protein–ligand complex involving Mol1, the total solvent-excluded surface area was calculated to be 14557.2, while the total solvent-accessible surface area was 16312.1. In comparison, the Mol4 complex exhibited a total solvent-excluded surface area of 15902.8 and a total solvent-accessible surface area of 17679.3. These values indicate that both compounds have a high level of accessible character, which supports effective binding affinity with the protein. The high solvent-accessible surface areas suggest that the ligands are well-exposed to the solvent, allowing for better interaction with the protein’s catalytic site. This exposure is beneficial for the binding affinity as it increases the probability of interactions between the ligands and the active site residues. The combination of electrostatic interactions, hydrogen bonding and favourable solvent accessibility underscores the potential of Mol1 and Mol4 as effective binders, enhancing their overall binding affinity and stability within the protein’s catalytic site. The structural insights from ESP interpretations, combined with RSA analysis, provide a comprehensive understanding of the molecular interactions at play, highlighting the critical role of Asp98 and the binding efficiency of Mol1 and Mol4 in the catalytic mechanism. These findings can be pivotal for designing more effective ligands in future studies.
3.4. PPI network analysis
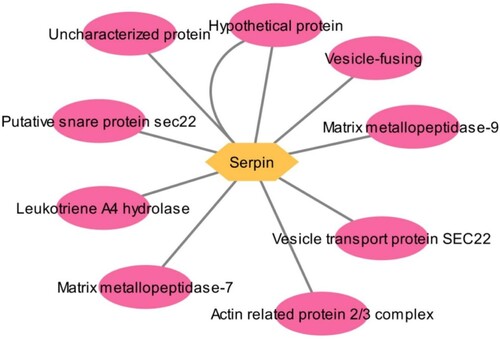
Network analysis revealed the number of nodes as 11 and number of edges as 27 and average node degree as 4.91, the average local clustering coefficient was determined as 0.838, the total expected number of edges was 30, the p-value of the PPI enrichment is 0.753. The network has shown known interaction from curated database and experimentally determined. The network has shown predicted interactions such as gene neighbourhood, fusion and co-occurrence. The other interaction observed are textmining, co-expression and protein homology. Serpin has shown gene fusion interaction with Smp_010690, which corresponds to Arp2/3 complex of 34 kDa subunit. The gene functions as actin-binding component of the Arp2/3 complex, which is involved in regulation of actin polymerization and together with an activating nucleation-promoting factor (NPF) that mediates the formation of branched actin networks. Interaction type from the network it was observed all the proteins have experimentally determined interactions. From the network, serpin has shown interaction with 10 proteins such as Putative snare protein sec22, Vesicle transport protein SEC22, Matrix metallopeptidase-7, Matrix metallopeptidase-9, Uncharacterized protein, 2 different Hypothetical proteins, Leukotriene A4 hydrolase, Actin related protein 2/3 complex and Vesicle-fusing (Figure ). From the GO prediction, the protein cellular components are determined as Endoplasmic reticulum, pigment granule, Golgi apparatus, distal axon and snare complex. The Reactome pathway for the involvement of proteins was determined as COPII-mediated vesicle transport and COPI-dependent Golgi-to-ER retrograde traffic. The protein have regulated SNARE like domain. Additionally, the clustering of the network has shown three groups. Serpin, Arp2/3 complex, Leukotriene A4 hydrolase and an uncharacterized complex forms a separate cluster. The Matrix metallopeptidase-9 occupies cluster 2 and SNARE proteins have occupied cluster 3.
3.5. C-T-N analysis
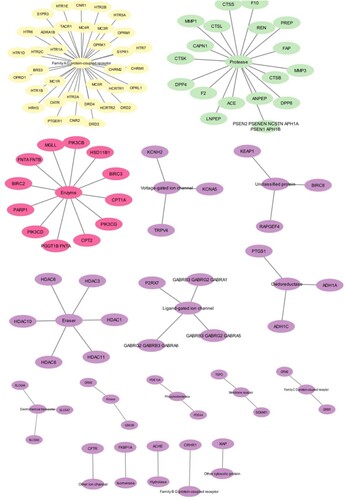
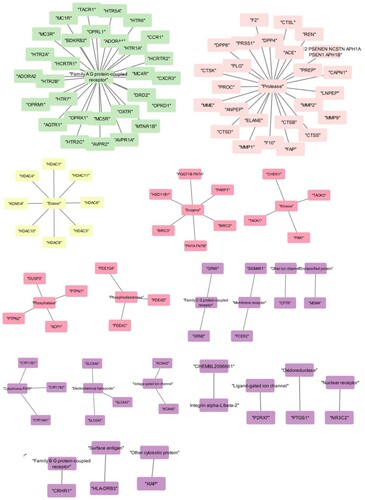
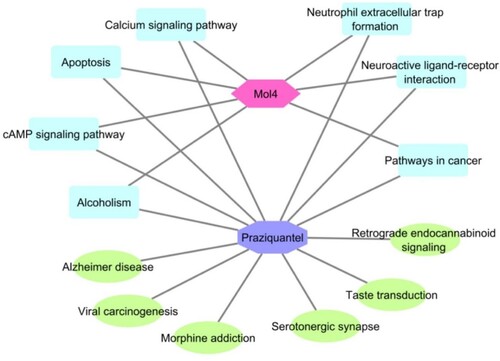
Praziquantel interaction with human resulted with 100 proteins, the network based on the class of the target was categorized into “ Family A G-protein-coupled receptor”, “protease”, “ Enzyme”, “Voltage-gated ion channel”, “Unclassified protein”, “Eraser”, “ ligand-gated ion channel”, “oxidoreductase”, “electrochemical transporter”, “kinase”, “Phospho esterase”, “membrane receptor”, “Family C G-protein-coupled receptor”, “ other ion channel”, “Isomerase”, “hydrolase” and “Family B G-protein-coupled receptor” (Figure a). The network obtained for Mol4 was resulted in 100 proteins and most of the targets categorized similarly to the praziquantel network. However, a few other classes were observed such as “Cytochrome P450”, “Family B G-protein-coupled receptor”, “surface antigen” and “nuclear receptor” (Figure b). Further, the networks were subjected to enrichment analysis and it was observed that praziquantel was able to interact with the proteins involved in diseases such as psych, pharmacogenomics, neurological, metabolic, chem-dependency, cancer, immune, developmental, reproduction, vision, aging and infection. Similarly, the Mol4 interacting network revealed the proteins involved in the disease class were similar to the standard drug. Mol4 targeted 51 proteins involved in the immune whereas praziquantel targeted 40 proteins (Figure a,b).
Figure 3. Network showing the targeted proteins where grouped into subnetwork based on the protein classification: (a) Praziquantel network and (b) Mol4 network.
Figure 4. Enrichment analysis for the Praziquantel (A, C, E and G) and Mol4 (B, D, F and H) network proteins. A and B reveal the disease class; C and D reveal the biological process; E and F reveal the cellular component; G and H reveal the molecular function.
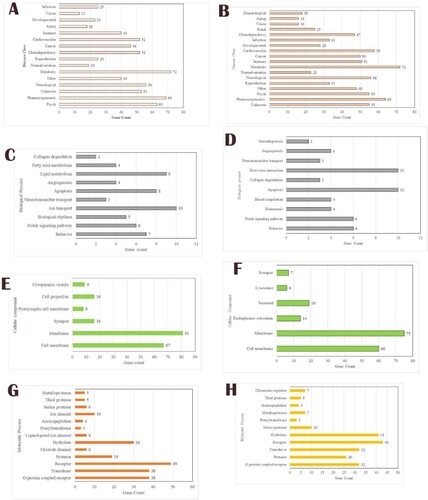
The biological process of praziquantel network revealed the proteins involved in the process such as “behaviour”, “Notch signalling pathway”, “Biological rhythms”, “Ion transport”, “Neurotransmitter transport”, “Apoptosis”, “Angiogenesis”, “Lipid metabolism”, “Fatty acid metabolism” and “Collagen degradation”. The Mol4 network revealed protein involved in proteins involved in similar processes except for a few processes such as “Haemostasis”, “Blood coagulation”, “Host–virus interaction” and “Steroidogenesis” (Figure c,d). Cellular components of the protein involved in the praziquantel network are “Cell membrane”, “Membrane Synapse”, “Postsynaptic cell membrane”, “Cell projection” and “Cytoplasmic vesicle”. For the Mol4 network, the cellular components are “Cell membrane”, “Membrane”, “Endoplasmic reticulum”, “Secreted”, “Lysosome” and “Synapse” (Figure e,f). The molecular process of the praziquantel network of the proteins was revealed as “G-protein-coupled receptor”, “Transducer”, “Receptor”, “Protease”, “Chloride channel”, “Hydrolase”, “Ligand-gated ion channel”, “Prenyltransferase”, “Aminopeptidase”, “Ion channel”, “Serine protease”, “Thiol protease” and “Metalloprotease”. The Mol4 network has similarly involved in molecular processes only a few of the proteins are involved in “Thiol protease” and “Chromatin regulator” (Figure g,h).
3.6. Gene ontology analysis
The network obtained for the standard drug revealed in the biological process the major proteins are involved “G-protein-coupled receptor signalling pathway”, “immune response” and “signal transduction”. The network obtained for Mol4 shows the derivatives can target protein involved in several other biological process compared to the standard drug. Interestingly, “proteolysis”, “inflammatory response”, “negative regulation of apoptotic process”, “apoptotic process”, “positive regulation of cell proliferation” and “negative regulation of transcription from RNA polymerase II promoter” (Table ).
Table 4. Gene ontology analysis for the Praziquantel and protein targets from human.
Gene ontology of cellular components of the networks of Mol4 was to be present majorly in the “membrane”, “integral component of the membrane”, “plasma membrane” and “cytoplasm”. From the molecular function of the networks, it was observed the proteins have role in the “protein binding”, G-protein-coupled receptor binding and “neurotransmitter receptor activity”. Additionally, the Mol4-target network has “zinc binding” and “endopeptidase activity” (Table ).
Table 5. Gene ontology analysis for the Mol4 and protein targets from human.
3.7. KEGG analysis
The network of Praziquantel and Mol4 revealed the drugs can significantly target the pathway such as “Neuroactive ligand–receptor interaction”, “Serotonergic synapse”, “cAMP signalling pathway”, “Apoptosis”, “Calcium signalling pathway”, “Alzheimer disease” and “Pathways in cancer” (Figure ). Totally 46 proteins were involved in Neuroactive ligand–receptor interaction pathways are targeted by Praziquantel. However, Mol4 has targeted 34 proteins in the network. Praziquantel and Mol4 have targeted 16 and 14 proteins respectively of cAMP signalling pathway. Excitingly, Praziquantel and Mol4 ligand interact with proteins and inhibit the Neutrophil extracellular trap formation pathway and inflammatory response pathway. Praziquantel has shown to interact with 8 proteins of the network and Mol4 has shown to interact with 10 proteins of the network. The proteins involved in the NET pathway are ELANE, HDAC1, HDAC10, HDAC11, HDAC3, HDAC4, HDAC6, HDAC8, ITGB1 and ITGB2. The proteins mainly support the inflammatory responses.
4. Discussion
S. mansoni causing schistosomiasis infection in humans is critical for global public health. Schistosomiasis affects millions of people worldwide, resulting in significant morbidity and mortality [Citation29]. Hence understanding S. mansoni and the disease is critical for managing and reducing its impact on global health [Citation3]. The present study network-based pharmacological approach provides insights into parasite biology, disease mechanisms and treatment strategies. The data is essential for understanding the details of public health policies aimed at reducing the disease burden and improving the overall well-being of affected populations. Investigating parasite biology is essential in identifying potential drug targets and aids in the development of new, more effective drugs to treat schistosomiasis and reduce drug resistance [Citation30]. From the PPI network of serpin, it was observed the proteins were showing interaction with 10 other proteins that can help in the biological function of the parasite. Thus targeting the PPI network proteins will be promising approach for drug discovery.
The network pharmacology approach provides insight into the human immune system response to S. mansoni infection and enlightens the development of therapies that can augment the immune response to combat the parasite [Citation31]. Additionally, understanding the interaction of disease networks with other diseases and conditions is essential for comprehensive healthcare and addressing health issues in endemic regions.
For the present study, Praziquantel was the drug of choice for investigation in similarity search, molecular docking as well as network pharmacology. Praziquantel is widely used in medication and is primarily used to treat infections schistosomiasis caused by Schistosoma parasites [Citation32]. The administration of Praziquantel disrupts the integrity of the parasite cell membrane, leading to an influx of calcium ions and muscle contraction resulting in paralysis and death of the parasite [Citation33]. Praziquantel has high efficacy and is administered as a single-dose treatment, which enhances compliance and simplifies treatment strategies in endemic areas [Citation34]. Hence using the standard drug already in use was used for performing similarity search. Based on the highest score, five molecules were selected for further evaluation.
ADMET analysis was performed for the compounds using the standard drug as control. In pharmacology, the water solubility of a drug is critical for its effective delivery and absorption in the body [Citation35]. Highly soluble drugs are more likely to be absorbed efficiently into the bloodstream, ensuring the bioavailability of the drugs. LogP influences bioavailability of drugs [Citation36], which is the fraction of the administered dose that reaches the systemic circulation. A balanced LogP is essential to ensure that the drug can be effectively absorbed and distributed throughout the body. Additionally, LogP affects metabolism and excretion of drug administered [Citation37]. Highly lipophilic compounds may be metabolized more slowly and can accumulate in fatty tissues, impacting the overall pharmacokinetic properties of the drug. Except for Mol5 all the test compounds have significant LogP values representing effective absorption, distribution, metabolism and excretion.
Caco2 permeability provides insights into the absorption of drugs in the gastrointestinal tract [Citation38]. CNS permeability assessment is essential for drugs targeting the central nervous system, as it determines their ability to reach the brain and exert their intended effects [Citation39]. Assessing the permeability of drugs through the skin is crucial for developing transdermal or topical drug delivery systems [Citation40]. It allows for non-invasive drug administration and sustained release of medications. Subsequently, Schistosomiasis occurs when individuals come into contact with water contaminated with Schistosoma larvae (cercariae) [Citation41]. The infection commonly happens during activities like bathing, swimming, washing clothes or doing household chores in contaminated water. Hence, developing ointments and creams for topical application can prevent the ingestion of the larvae into the blood stream of human. All the permeability assessments (CNS, skin permeability and Caco2) play significant roles in drug discovery and development, enabling to design of drugs that can effectively reach their target sites and produce the desired therapeutic effects during Schistosomiasis. From the investigation, all the molecules except Mol5 have excellent absorption index. Similarly, Mol5 was predicted with poor BBB permeability.
From the study, the role of ligands with cytochrome p450 has shown the ligands have a specific role in each cytochromes. Targeting specific cytochrome P450 enzymes for inhibition can increase the bioavailability and efficacy during drug development [Citation42]. This is particularly important if the therapeutic effect of the drug is enhanced by inhibiting CYP2C9 and CYP3A4 [Citation43]. Praziquantel and five compounds do not act as CYP2D6 substrates but act as inhibitors. However, all the compounds act as a substrate for CYP3A4. CYP2C9 and CYP3A4 are involved in metabolizing a significant number of drugs. By selectively inhibiting these enzymes, the drug candidate reduces the risk of unwanted side effects caused by interactions with other medications metabolized by these enzymes [Citation44]. Mol 4 is the only compound predicted as a CYP1A2 inhibitor. Targeting specific cytochrome P450 enzymes for inhibition can increase the bioavailability and efficacy of the drug being developed [Citation42]. From the total clearance, Mol1 has determined to be poor compared to the others. Also from AMES toxicity, all the compounds were determined as non-mutagens [Citation45]. From minnow toxicity, all compounds can target the flat head worms. The hepatotoxicity proved the Mol3 and Mol4 do not interfere with the normal liver function. Thus from the overall ADMET prediction, Mol 4 was predicted as excellent. However, Mol 5 has shown a violation of ADMET rules and hence was not taken for further molecular docking studies.
Serpin (serine protease inhibitor) proteins are indeed present in Schistosoma, the parasite. Serpins have been identified and studied for their involvement in evading the host immune response. These serpins promote the modulation of the host immune system and protect themselves from immune attacks [Citation46]. By inhibiting host proteases, they interfere with the host’s ability to mount an effective immune defence against the parasite. Serpin plays an essential role in modulating the host inflammatory responses, aiding in the survival of the parasite within the host. Hence understanding the functions and mechanisms of serpins in Schistosoma infection is essential for developing strategies to combat schistosomiasis effectively [Citation47]. The standard drug and test compounds (Mol1–4) were docked into the binding pocket 1 and it was observed Mol 1 and Mol4 were observed with high binding affinity with the active site residues predicted in the binding pocket. The interaction of Mol1 and Mol4 was observed in Leucine-rich binding pocket. Leucine in the binding pocket provides flexibility within the binding pocket, allowing it to accommodate ligands with varying sizes and conformations [Citation48]. This versatility is crucial for interacting with ligands of diverse chemical structures. Interestingly Leucine can form stabilizing interactions, such as hydrophobic and π–π interactions, with aromatic ligands or other hydrophobic parts of ligands, reinforcing the binding affinity [Citation49]. Leucine-rich regions can participate in signalling pathways and cellular regulation by interacting with other molecules, such as kinases, in a ligand-like manner [Citation50]. Hence it is hypothesized from the study that serpin on interaction with human proteins of different classes can be inhibited by interaction of ligands into the binding pocket.
From the network, pharmacology-based approach the network for standard drug and Mol4 was compared. It was observed the compound Mol4 has similar interactions compared to the standard drug. However, the few target proteins from Mol4 network have different disease classes, biological processes, cellular components and molecular functions. From the Gene ontology analysis, the network was observed with proteins having an essential role in “protein binding”, G-protein-coupled receptor binding, and “neurotransmitter receptor activity”. The neurotransmitter receptor activity plays a crucial role in the parasite–host interaction. However, it is important to note that S. mansoni is a complex parasitic organism, and the understanding of its neurotransmitter receptor activity as well their role in host system is crucial [Citation51]. Mol4-target network has “zinc binding” and “endopeptidase activity”. Zinc ions play a crucial role in stabilizing the structure of proteins by coordinating with specific amino acid residues. Proteins containing zinc-binding domains contribute to the structural integrity and function of the host–parasite interaction and survival [Citation52]. Zinc-binding proteins in S. mansoni may play a role in immune evasion. They can interfere with the host immune response by modulating immune cell activity or cytokine production. Zinc-binding proteins are involved in various metabolic and enzymatic processes crucial for the survival and virulence of S. mansoni [Citation53]. These processes contribute to the S. mansoni ability to establish infection within the host. Consequently, it is hypothesized that S. mansoni parasite might exploit zinc ions from the host environment or host tissues to fulfil its metabolic needs and sustain itself during infection. Similarly, endopeptidases produced by the S. mansoni are critical for breaking down host tissue barriers. They assist in tissue invasion, allowing the parasite to penetrate and migrate through host tissues. Endopeptidases produced by S. mansoni can digest host proteins to obtain essential amino acids and nutrients, supporting the growth and development of the S. mansoni [Citation47]. In addition, endopeptidases can modulate the host immune response by degrading host immune factors, cytokines, or antibodies, thereby aiding the S. mansoni in evading the host immune system [Citation54]. Hence, it is envisaged that the endopeptidase activity of S. mansoni on host proteins can influence the local inflammatory response and immune cell activation, potentially facilitating immune evasion and allowing the parasite to persist within the host [Citation55]. From the KEGG analysis, compounds significantly inhibit neuroactive ligand–receptor interaction pathways. It is envisaged that the neuroactive ligand–receptor interaction pathway can also represent a site of cross-talk between the host nervous system and the parasite, potentially influencing the progression of infection and response to the host. Interestingly Mol4 has a role in the neutrophil extracellular trap formation pathway and inflammatory responses pathway. Schistosoma parasites can trigger NET formation by neutrophils. The recognition of neutrophils in the presence of Schistosoma can release NETs to entrap the parasites [Citation56]. Therefore, the network and the proteins that are targeted by Praziquantel and Mol4 are crucial targets for drug discovery.
5. Conclusion
The present study underscores the critical importance of comprehending S. mansoni infection and associated diseases within the context of global public health. Employing a network-based pharmacological approach has proven invaluable in elucidating parasite biology, disease mechanisms and potential treatment avenues. The study accentuates the role of Praziquantel, a key drug in schistosomiasis treatment. The thorough evaluation of drug candidates, notably Mol4, using a range of ADMET parameters like water solubility, LogP and permeability, provides essential insights into their absorption, bioavailability and potential candidate for drug discovery. The study emphasizes the significance of understanding the intricate interplay of disease networks with other conditions, highlighting the need for a comprehensive healthcare approach, especially in endemic regions. The in-depth molecular docking and network pharmacology analyses shed light on the potential inhibitory roles of the test compounds, particularly Mol4, in critical pathways and binding pockets. Furthermore, the involvement of zinc-binding proteins and endopeptidases in the survival and virulence of Schistosoma reinforces their potential as promising drug targets. Overall, this study not only enriches our understanding of S. mansoni in schistosomiasis but also holds promise for future drug discovery endeavours. The approach undertaken, integrating molecular docking and network pharmacology, provides a robust platform for advancing drug development and devising effective treatment strategies against this debilitating parasitic infection, ultimately contributing to the enhancement of global public health.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data from this work is available on request and can be get by email from the corresponding author.
Additional information
Funding
References
- Verjee MA. Schistosomiasis: still a cause of significant morbidity and mortality. Res Rep Trop Med. 2019;10:153–163. doi:10.2147/RRTM.S204345
- McManus DP, Dunne DW, Sacko M, et al. Schistosomiasis. Nat Rev Dis Primers. 2018;4(1):13. doi:10.1038/s41572-018-0013-8
- Nelwan ML. Schistosomiasis: life cycle, diagnosis, and control. Curr Ther Res Clin Exp. 2019;91:5–9. doi:10.1016/j.curtheres.2019.06.001
- Mouahid G, Rognon A, de Carvalho Augusto R, et al. Transplantation of schistosome sporocysts between host snails: a video guide. Wellcome Open Res. 2018;3:3. doi:10.12688/wellcomeopenres.13488.1
- Wang T, Zhao M, Rotgans BA, et al. Proteomic analysis of the Schistosoma mansoni Miracidium. PLoS One. 2016;11(1):e0147247.
- Braun L, Sylivester YD, Zerefa MD, et al. Chlorination of Schistosoma mansoni cercariae. PLoS Negl Trop Dis. 2020;14(8):e0008665. doi:10.1371/journal.pntd.0008665
- El Ridi RA, Tallima HA. Novel therapeutic and prevention approaches for schistosomiasis: review. J Adv Res. 2013;4(5):467–478. doi:10.1016/j.jare.2012.05.002
- McManus DP, Bergquist R, Cai P, et al. Schistosomiasis—from immunopathology to vaccines. Semin Immunopathol. 2020;42(3):355–371. doi:10.1007/s00281-020-00789-x
- Mbabazi PS, Andan O, Fitzgerald DW, et al. Examining the relationship between urogenital schistosomiasis and HIV infection. PLoS Negl Trop Dis. 2011;5(12):e1396. doi:10.1371/journal.pntd.0001396
- Sarvel AK, Oliveira AA, Silva AR, et al. Evaluation of a 25-year-program for the control of Schistosomiasis mansoni in an endemic area in Brazil. PLoS Negl Trop Dis. 2011;5(3):e990. doi:10.1371/journal.pntd.0000990
- Njiokou E, Onguene Onguene AR, Tchuem Tchuente LA, et al. Urban schistosomiasis in Cameroon: a longitudinal study of its transmission in a new site of an extension of the intestinal schistosomiasis focus in Melen, Yaounde. Bull Soc Pathol Exot. 2004;97(1):37–40.
- Haas W, Haeberlein S. Penetration of cercariae into the living human skin: Schistosoma mansoni vs. Trichobilharzia szidati. Parasitol Res. 2009;105(4):1061–1066. doi:10.1007/s00436-009-1516-8
- Gryseels B, Polman K, Clerinx J, et al. Human schistosomiasis. Lancet. 2006;368(9541):1106–1118. doi:10.1016/S0140-6736(06)69440-3
- Doenhoff MJ, Pica-Mattoccia L. Praziquantel for the treatment of schistosomiasis: its use for control in areas with endemic disease and prospects for drug resistance. Expert Rev Anti Infect Ther. 2006;4(2):199–210. doi:10.1586/14787210.4.2.199
- Cheuka PM. Drug discovery and target identification against Schistosomiasis: a reality check on progress and future prospects. Curr Top Med Chem. 2022;22(19):1595–1610. doi:10.2174/1568026621666210924101805
- Zoete V, Daina A, Bovigny C, et al. SwissSimilarity: a web tool for low to ultra high throughput ligand-based virtual screening. J Chem Inf Model. 2016;56(8):1399–1404. doi:10.1021/acs.jcim.6b00174
- Bragina ME, Daina A, Perez MAS, et al. The SwissSimilarity 2021 web tool: novel chemical libraries and additional methods for an enhanced ligand-based virtual screening experience. Int J Mol Sci. 2022;23(2):811. doi:10.3390/ijms23020811
- Xiong G, Wu Z, Yi J, et al. ADMETlab 2.0: an integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 2021;49(W1):W5–W14. doi:10.1093/nar/gkab255
- Zhong HA, Mashinson V, Woolman TA, et al. Understanding the molecular properties and metabolism of top prescribed drugs. Curr Top Med Chem. 2013;13(11):1290–1307. doi:10.2174/15680266113139990034
- Alsawalha M, Bolla R, Kandakatla S, et al. Molecular docking and ADMET analysis of hydroxamic acids as HDAC2 inhibitors. Bioinformation. 2019;15(6):380–387. doi:10.6026/97320630015380
- Badraoui R, Bardakci AM, Alreshidi F, et al. Chloroquine and hydroxychloroquine interact differently with ACE2 domains reported to bind with the coronavirus spike protein: mediation by ACE2 polymorphism. Molecules. 2021;26(3):673. doi:10.3390/molecules26030673
- Badraoui R, Saoudi M, Hamadou WS, et al. Antiviral effects of artemisinin and its derivatives against SARS-CoV-2 main protease: computational evidences and interactions with ACE2 allelic variants. Pharmaceuticals (Basel). 2022;15(2):129. doi:10.3390/ph15020129
- Adasme MF, Linnemann KL, Bolz SN, et al. PLIP 2021: expanding the scope of the protein-ligand interaction profiler to DNA and RNA. Nucleic Acids Res. 2021;49(W1):W530–W534. doi:10.1093/nar/gkab294
- Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31(2):455–461. doi:10.1002/jcc.21334
- Szklarczyk D, Gable AL, Nastou KC, et al. The STRING database in 2021: customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021;49(D1):D605–D612. doi:10.1093/nar/gkaa1074
- Otasek D, Morris JH, Boucas J, et al. Cytoscape automation: empowering workflow-based network analysis. Genome Biol. 2019;20(1):185. doi:10.1186/s13059-019-1758-4
- Sherman BT, Hao M, Qiu J, et al. DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022;50(W1):W216–W221. doi:10.1093/nar/gkac194
- Kanehisa M, Furumichi M, Tanabe M, et al. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017;45(D1):D353–D361. doi:10.1093/nar/gkw1092
- Wu GY, Halim MH. Schistosomiasis: progress and problems. World J Gastroenterol. 2000;6(1):12–19. doi:10.3748/wjg.v6.i1.12
- Huang HH, Rigouin C, Williams DL. The redox biology of schistosome parasites and applications for drug development. Curr Pharm Des. 2012;18(24):3595–3611.
- Colley DG, Secor WE. Immunology of human schistosomiasis. Parasite Immunol. 2014;36(8):347–357. doi:10.1111/pim.12087
- Vale N, Gouveia MJ, Rinaldi G, et al. Praziquantel for Schistosomiasis: single-drug metabolism revisited, mode of action, and resistance. Antimicrob Agents Chemother. 2017;61(5):82–98. doi:10.1128/AAC.02582-1
- Chai JY. Praziquantel treatment in trematode and cestode infections: an update. Infect Chemother. 2013;45(1):32–43. doi:10.3947/ic.2013.45.1.32
- You H, McManus DP, Hu W, et al. Transcriptional responses of in vivo praziquantel exposure in schistosomes identifies a functional role for calcium signalling pathway member CamKII. PLoS Pathog. 2013;9(3):e1003254. doi:10.1371/journal.ppat.1003254
- Alsenz J, Kansy M. High throughput solubility measurement in drug discovery and development. Adv Drug Deliv Rev. 2007;59(7):546–567. doi:10.1016/j.addr.2007.05.007
- Veber DF, Johnson SR, Cheng HY, et al. Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem. 2002;45(12):2615–2623. doi:10.1021/jm020017n
- Koch CJ, Hahn SM, Rockwell K Jr, et al. Pharmacokinetics of EF5 [2-(2-nitro-1- H -imidazol-1-yl)- N -(2,2,3,3,3-pentafluoropropyl) acetamide] in human patients: implications for hypoxia measurements in vivo by 2-nitroimidazoles. Cancer Chemother Pharmacol. 2001;48(3):177–187. doi:10.1007/s002800100324
- Press B. Optimization of the Caco-2 permeability assay to screen drug compounds for intestinal absorption and efflux. Methods Mol Biol. 2011;763:139–154. doi:10.1007/978-1-61779-191-8_9
- Upadhyay RK. Drug delivery systems, CNS protection, and the blood brain barrier. Biomed Res Int. 2014;2014:869269. doi:10.1155/2014/869269
- Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26(11):1261–1268. doi:10.1038/nbt.1504
- Evan Secor W. Water-based interventions for schistosomiasis control. Pathog Glob Health. 2014;108(5):246–254. doi:10.1179/2047773214Y.0000000149
- Zhao M, Ma J, Li M, et al. Cytochrome P450 enzymes and drug metabolism in humans. Int J Mol Sci. 2021;22(23):12808, doi:10.3390/ijms222312808
- Van Booven D, Marsh S, McLeod H, et al. Cytochrome P450 2C9-CYP2C9. Pharmacogenet Genomics. 2010;20(4):277–281. doi:10.1097/FPC.0b013e3283349e84
- Subramanian M, Tam H, Zheng H, et al. CYP2C9–CYP3A4 protein–protein interactions: role of the hydrophobic N terminus. Drug Metab Dispos. 2010;38(6):1003–1009. doi:10.1124/dmd.109.030155
- Samiei M, Asgary S, Farajzadeh M, et al. Investigating the mutagenic effects of three commonly used pulpotomy agents using the Ames test. Adv Pharm Bull. 2015;5(1):121–125. doi:10.5681/apb.2015.017
- Granzin J, Huang Y, Topbas C, et al. Three-dimensional structure of a schistosome serpin revealing an unusual configuration of the helical subdomain. Acta Crystallogr D Biol Crystallogr. 2012;68(Pt 6):686–694. doi:10.1107/S0907444912008372
- Qokoyi NK, Masamba P, Kappo AP. Proteins as targets in anti-Schistosoma drug discovery and vaccine development. Vaccines (Basel). 2021;9(7):762. doi:10.3390/vaccines9070762
- Kobe B, Deisenhofer J. A structural basis of the interactions between leucine-rich repeats and protein ligands. Nature. 1995;374(6518):183–186. doi:10.1038/374183a0
- Platzer G, Mayer M, Beier A, et al. PI by NMR: probing CH–π interactions in protein–ligand complexes by NMR spectroscopy. Angew Chem Int Ed Engl. 2020;59(35):14861–8. doi:10.1002/anie.202003732
- Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141(7):1117–1134. doi:10.1016/j.cell.2010.06.011
- El-Shehabi F, Taman A, Moali LS, et al. A novel G protein-coupled receptor of Schistosoma mansoni (SmGPR-3) is activated by dopamine and is widely expressed in the nervous system. PLoS Negl Trop Dis. 2012;6(2):e1523. doi:10.1371/journal.pntd.0001523
- Baglivo I, Russo L, Esposito S, et al. The structural role of the zinc ion can be dispensable in prokaryotic zinc-finger domains. Proc Natl Acad Sci USA. 2009;106(17):6933–6938. doi:10.1073/pnas.0810003106
- Hambrook JR, Hanington PC. Immune evasion strategies of Schistosomes. Front Immunol. 2021;11:624178. doi:10.3389/fimmu.2020.624178
- Jenkins SJ, Hewitson JP, Jenkins GR, et al. Modulation of the host’s immune response by schistosome larvae. Parasite Immunol. 2005;27(10–11):385–393. doi:10.1111/j.1365-3024.2005.00789.x
- Hambrook JR, Hanington PC. A cercarial invadolysin interferes with the host immune response and facilitates infection establishment of Schistosoma mansoni. PLoS Pathog. 2023;19(2):e1010884. doi:10.1371/journal.ppat.1010884
- Diaz-Godinez C, Carrero JC. The state of art of neutrophil extracellular traps in protozoan and helminthic infections. Biosci Rep. 2019;39(1):BSR20180916. doi:10.1042/BSR20180916