Abstract
The genus Lacerta, particularly the green lizard (Lacerta viridis), is a significant lizard group in the Western Palearctic. This study aims to predict its geographic range in Northern Türkiye and identify key habitat types based on the CORINE Land Cover classification. Occurrence records, gathered from published sources and on-site investigations, were analyzed using a 30 arc-second resolution layer and bioclimatic parameters from WorldClim v.2.1. The distribution was modeled in kuenm, and habitat use was assessed with generalized linear models. Results show that the green lizard’s occurrence is strongly influenced by precipitation dynamics and it prefers areas with little to no shade. These findings highlight the importance of precipitation in determining the species’ distribution and suggest further research on local dynamics for effective conservation.
1. Introduction
Environmental conditions have extensive effects on the organization of biodiversity in terrestrial and aquatic ecosystems. These conditions, especially abiotic elements such as temperature and precipitation, have a high influence on shaping not only ecological patterns, but also evolutionary processes [Citation1]. Ecological niche modelling (ENM) is a useful tool for determining species distributions via the statistical analysis of environmental variables. Because ENM is strongly related to geospatiall data of species and environmental data, it may reveal potential geographic distribution of the species of interest [Citation2].
Medium-sized, green lizards (Lacerta viridis complex) can reach a maximum body length of about 40 cm and brightly green dorsal colours [Citation3]. These lizards are diurnally active and distributed across Northern Spain, France, and Italy, through the Balkans and southeastern East Europe, to Western Ukraine and Northern Anatolia [Citation4]. Despite its wide range distribution, debates on the taxonomic status of this species have continued for the past three decades [Citation4–8].
The Western Asian plate exhibits an intricate geological configuration, leading to notable vegetation diversity and variations in topography [Citation9]. In addition to that, Northern Anatolia and Black Sea Basin have been affected by many environmental dynamics (i.e. land use and cover, hydrologic modelling, sea level changes, and tectonic evolution) [Citation10,Citation11].
The herpetofaunal diversity of Anatolian Peninsula has been becoming richer and richer with new expeditions continuously [Citation12–15]. This situation might be the result of its intersectional position of three global-wide biodiversity hotspots: Irano-Anatolian, Caucasus and Mediterranean [Citation16]. Furthermore, this extensive range of diversity also allows researchers to evaluate numerous biogeographic or phylogeographic hypotheses [Citation17–22]. In addition to that, ecological analysis of species has been getting a rise in herptile studies in this peninsula and adjacent areas [Citation23–27].
On the other hand, the scale selected for the Coordination of Information on the Environment (CORINE hereafter) land cover classification was evaluated for habitat use of lizards. It provides information on the biophysical characteristics of the Earth’s surface; therefore, it is the same as that generally adopted by other European-scale typologies, for example, by the European land cover classification [Citation28,Citation29] and is comparable to the scale applied to the classification of vegetation. Nevertheless, there has been no research conducted on the assessment of habitat utilization and potential distribution of L. viridis in Anatolia and Eastern Thrace by considering ecological (abiotic) parameters.
Therefore, the aims of this study are (i) to predict the habitat suitability of green lizards in Northern Anatolia and Thrace under current climatic scenarios, (ii) to examine its habitat use via CORINE and (iii) to evaluate the abiotic parameters which have an influence on habitat differentiation of this species.
2. Materials and methods
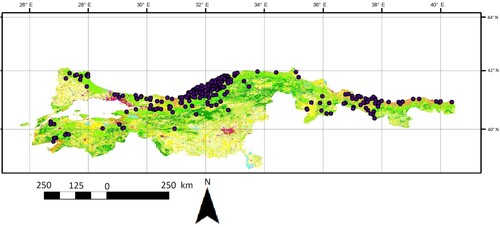
This study was performed between 25.6 and 40.5 eastern longitudes and 38.6 and 42.1 northern latitudes, including the northern part of the Anatolian Peninsula, and Thrace Region of Türkiye. A compilation of 330 raw presence records of the species was gathered from both published sources [Citation30–52] and our field investigations spanning the years 2015–2022 (Appendix I, Figure ). When GPS format information was not provided explicitly, the most precise location was determined using Google Earth, an online geographic system application. A total of 1 topographic (elevation) parameter and 19 bioclimatic parameters were obtained from WorldClim in version 2.1 [Citation53] in 30 arc second spatial resolution (∼1 km at the equator) (Appendix II). In addition to them, Corine Land Cover for 2018 was integrated into model construction [Citation54]. The environmental variables were trimmed for the research region using ArcGIS 10.3 (ESRI). The data for this species underwent a two-step process of error-checking and improvement to ensure compliance with the necessary requirements for ENM and habitat utilization. Initially, the georeferenced data underwent a thorough examination to identify any errors and ensure the consistency of the geographic coordinates [Citation55]. Furthermore, to prevent biases in spatial sampling and misunderstanding of the study on the suitability of the habitat, the occurrence data for the species were geographically rarefied. This was done by retaining only one location within every 20 km using the SDM Toolbox 2.0 [Citation56]. A Pearson correlation coefficient (with a value between 0.75 and −0.75) was calculated using R 4.04 for all variables. Variables that showed a significant correlation were excluded from the analysis for the distribution modelling of L. viridis (see Appendix III). Logistic, Random Forest and MaxEnt algorithms and the kuenm package in R [Citation57] to assess the candidate models. In order to assess the significance of environmental parameters, the jack-knife test was implemented in MaxEnt.
This test allows for a thorough analysis using presence data [Citation58]. Thanks to recent advancements in the modelling process, we utilized both the MaxEnt, The candidate models were constructed using 80% of the occurrence records, with the remaining 20% designated for the independent presence of test data. A total of 1054 candidate models were evaluated in order to construct the models for the species. These models comprised parameters that represented all possible combinations of 17 regularization multiplier settings (0.1, 0.2, 0.3, … 6, 8, 10), 31 class combinations of feature classes (hinge, threshold, product, quadratic, linear), and a distinct set of environmental variables. Model performance was examined by two criteria, AUC and AICc, respectively [Citation59,Citation60]. The area under the receiver-operator (ROC) curve (AUC) values was utilized to assess the predictive capability and precision of the model during the validation process. (−0.5: severely discouraged from running; >0.6: acceptable; >0.7: moderately favourable; >0.8: satisfactory; >0.9: exceptional; = 1: outstanding) [Citation61]. In addition to AUC, Akaike Information Criteria (AICc) was calculated to determine the best model in each examination for limited sample sizes [Citation59].
In order to evaluate the quality of fit of all models, sensitivity, specificity, and concordance indices were computed. The validation sample, which was segregated for validation purposes and was independent of the training set, was used to calculate these metrics. Subsequently, the aggregate accuracy metric was calculated by analysing the confusion matrix of each model. Map binarization was done by using the average logistic thresholds of maximum training sensitivity plus specificity of 10 replicates [Citation62].
After ENM analysis, the general linear model (GLM) approach was applied to the rarefied and relatively recent occurrence records (last decade) to determine the habitat use of lizards via CORINE Land Cover for 2018 in two steps. The high rarefaction rate helped us to decrease the intensive field survey biases from the analysis. In addition to the rarefaction of occurrence records, each analysed locality was weighted by inverse proportion of the distance of this point to the dense zones [Citation63–65]. The parameters, that are examined with CORINE Land Cover 2018 of its 100 m resolution are obtained from a study of [Citation66], Copernicus Climate Data Store [Citation54], French Republic Library [Citation67] and WorldClim [Citation68]: Dominant leaf type (DLT), tree cover density (TCD), elevation, slope, distance to the river (river_dst), mean of monthly precipitation, mean of monthly temperature and mean of monthly solar radiation rate. Additionally, monthly values for precipitation for lizard’s active period (March–October) were evaluated in another GLM to better understand the precipitation effects on habitat use of lizards. As a total of 720 GLM were constructed to examine the effects of parameters, highly correlated variables were discarded, and the best model was selected by AIC scores [Citation59].
3. Results
The predicted distribution range of green lizards within Northern Anatolia is given in Figure . After eliminating the highly correlated variables as a result of Pearson correlation analysis (Appendix III), the selection process for a total of six bioclimatic layers (Bio 2: Mean Diurnal Range, Bio 5: Maximum Temperature of Warmest Quarter, Bio 8: Mean Temperature of Wettest Quarter, Bio 11: Mean Temperature of Coldest Quarter, Bio 12: Annual Precipitation, Bio 15: Precipitation Seasonality) was based on the Pearson correlation coefficient. Their contributions to predicted distribution is given with Corine Land Cover parameter in Table . The mean AUC value for the species was computed as 0.879 ± 0.01274, with the best MaxEnt feature of linear and product indicating that this value is sufficiently high to be retained for further investigation (Table ). The AUC values of 0.831, 0.852, and 0.879 for logistic regression, random forests and MaxEnt, respectively, were indicative of reliable modelling in the context of L. viridis, as derived by the various approaches employed in this study (Table ). The Maxent modelling approach was employed to obtain the highest performance, as shown in Table . According to this map, all suitable estimated areas are thought to cover the northern shores of Black Sea Basin, especially eastern and central regions compared to the inner areas. The presence of L. viridis has a close association with annual precipitation – Bio 12 – (30.5%), mean diurnal range – Bio 2 – (22.7%), precipitation seasonality – Bio 15 – (21.5%) and maximum temperature of the warmest quarter – Bio 5 – (19%).
Figure 2. Habitat suitability predictions of Lacerta viridis (warmer colours refer to the high suitability level).
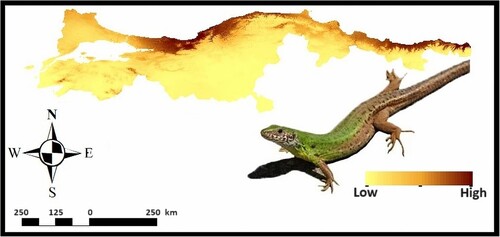
Table 1. Contribution of low correlated environmental variables in ENM of Lacerta viridis.
Table 2. Summary statistics for the best models selected for species distribution map of Lacerta viridis via kuenm package.
Table 3. Validation metrics used for comparison purposes in ENM of Lacerta viridis.
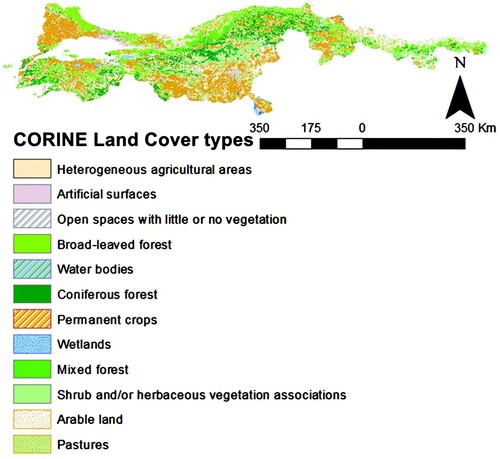
On the other hand, the study area was determined to be covered with 12 identified land cover habitat types according to CORINE Land Cover [Citation69] (Figure ). The green lizard was detected in 11 of these land cover types, except wetlands. The most used habitat type is the heterogeneous agricultural areas (47.3%), and permanent crops (13.8%) followed it. Thereafter, the influence of environmental factors on the habitat use was examined by GLM in two steps. Firstly, it was found that the annual mean of precipitation shows a slight influence on the habitat use (AIC: 793.50, Table ). Therefore, we needed to apply a second GLM to examine the effect of precipitation in the monthly period. The second GLM was built with the weighted monthly precipitation of the active season of the lizards. The results of this GLM showed that precipitation in June has an influence on the habitat use and precipitation between March and May followed it with a relatively slight effect (AIC: 798.28, Table ).
Table 4. GLM of environmental factors on the habitat use of Lacerta viridis.
Table 5. GLM of environmental factors on the habitat use of Lacerta viridis (focused on monthly precipitation in the active season).
4. Discussion
The distribution of species is influenced by many biotic and abiotic factors [Citation70]. Bioclimatic conditions play a crucial influence in determining biodiversity patterns at both global and local scales [Citation71]. ENM is a practical approach to provide a view about the species distribution range. The general distribution trend was significantly influenced by the precipitation dynamics, as demonstrated by our findings. This situation is compatible with some lacertid’s diurnal life cycle traits [Citation72].
The ENM projections for the current distribution of L. viridis were mostly consistent with its known distribution in Northern Türkiye. Furthermore, these findings have demonstrated that the range of Lacerta viridis is limited by a certain set of environmental factors, primarily associated with precipitation. The prolonged duration of precipitation can potentially impact the vegetation composition of the narrow sections located in the northern region of the Anatolian Peninsula [Citation73]. Moreover, although the rarefication method was strictly applied in the distribution analysis, it was observed that the Western Black Sea region provides more suitable habitats for L. viridis. Additionally, this could provide green lizards with alternative favourable microhabitats.
According to GLM analysis on habitat use of green lizards, it is clear that the lizards have a strong tendency to inhabit non-canopied habitats, such as agricultural sites and adjacent areas (Table ; Figure ). Even in these areas most likely to receive solar radiation higher than forestry ones, shaping patterns of the open areas are depending on precipitation [Citation74]. This might be the consequence of relatively low precipitation rates in non-canopied habitats. Our results partially supported this argument via June precipitations in the study area [Citation75]. Due to microhabitat requirements playing a critical role via affecting the local dynamics, it is recommended that actual data obtained from locality-specific parameters would enlighten us on how to understand these habitat patterns (Table ).
For instance, distance to the river is also considered to be a good predictor for habitat use of green lizards. Because rivers not only provide a consistent and reliable water sources for drinking, thermoregulation of lizards, but also riparian zones along rivers provide important resources such as foraging opportunity, shelter and suitable microhabitats [Citation76]. A recent study on the niche differentiation dynamics of Anatololacerta species in the Anatolian Peninsula presented the importance of “distance to river” as shaping the species distribution patterns [Citation77].
The study area, which is one of the 34 major biodiversity hotspots [Citation16] is under several regional eco-crisis [Citation78]. Although the effects of climatological dynamics on species distribution and habitat use have been discussed above, the anthropogenic pressure on habitat modification and fragmentation should be immediately assessed. Because this habitat loss threat was recently shown in Czechia [Citation79].
On the other hand, the microhabitat selection of these green lizards was studied in Bulgaria [Citation80]. Moreover, it was found that the green lizards' occupancy probability was mainly defined by landscape configuration parameters and patch geometric characteristics [Citation79]. However, there is no ecological data to display the distribution pattern of L. viridis in its Anatolian and Eastern Thrace populations. In addition to them, it is known that the ecosystem services of lacertids are also crucial for ecosystem sustainability [Citation81]. Therefore, because of land use transformations, ecotoxicological polluted patchy areas close to agricultural sites might damage the suitability of the green lizard’s survival [Citation82]. This is especially important, because almost half (47.3%) of the occurrence records in the last decade were obtained from several agricultural sites (Figure ).
Therefore, instead of putting forward the results of habitat loss, focusing on valuable and/or threatened habitat patches might contribute to conservation action for many aspects. For instance, identifying and prioritizing the conservation of valuable habitat patches are remarkably important for the survival and welfare of lizard populations [Citation83]. Because these patches might provide foraging areas, breeding sites and shelters under continuously increasing anthropogenic pressure on natural biotopes.
As a result of all these threats, further studies depending on the data related to local environments might provide us with some conservation plans, such as species monitoring research, public awareness and education, and fostering collaboration at local, national and international levels.
TUSC2378548_Supplementary Appendice.docx
Download MS Word (28.6 KB)Acknowledgments
We would like to thank the anonymous reviewers and the editor for improving the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Kozak KH, Graham CH, Wiens JJ. Integrating GIS-based environmental data into evolutionary biology. Trends Ecol. Evol. (Amst.). 2008;23(3):141–148. doi:10.1016/j.tree.2008.02.001
- Guisan A, Thuiller W. Predicting species distribution: offering more than simple habitat models. Ecol Lett 2005;8:993–1009. doi:10.1111/j.1461-0248.2005.00792.x
- Nettmann HK, Rykena S. Lacerta viridis (Laurenti, 1768) Smaragdeidechse. In: Böhme W, editor. Handbuch der Reptilien und Amphibien Europas. Band 2/I. Echsen (Sauria) II. (Lacertidae II: Lacerta). Wiesbaden: Aula-Verlag; 1984. p. 129–180.
- Nettmann HK. Die Smaragdeidechsen (Lacerta s. Str.)–Eine Übersicht über Verwandtschaft und Formenvielfalt. Mertensiella. 2001;13:11–32.
- Rykena S. Kreuzungsexperimente zur Prüfung der Artgrenzen im Genus Lacerta sensu stricto. Mitteilungen Aus Dem Museum Für Naturkunde in Berlin. Zool Museum Institut Zoo (Berlin). 1991;67(1):55–68. doi:10.1002/mmnz.19910670108
- Brückner M, Klein B, Düring A, et al. Phylogeographische Analyse des Lacerta viridis/bilineata Komplexes: Molekulare Muster und Verbreitung. Mertensiella. 2001;13:45–51.
- Godinho R, Crespo E, Ferrand N, et al. Phylogeny and evolution of the green lizards, Lacerta spp. (Squamata: Lacertidae) based on mitochondrial and nuclear DNA sequences. Amphib-Reptilia. 2005;26:271–285. doi:10.1163/156853805774408667
- Marzahn E, Mayer W, Joger U, et al. Phylogeography of the Lacerta viridis complex: mitochondrial and nuclear markers provide taxonomic insights. J Zool Syst Evol Res. 2016;54(2):85–105. doi:10.1111/jzs.12115
- Rajabizadeh M, Nagy ZT, Adriaens D, et al. Alpine-Himalayan orogeny drove correlated morphological, molecular, and ecological diversification in the Persian dwarf snake (Squamata: Serpentes:Eirenis persicus). Zool J Linn Soc 2016;176(4):878–913. doi:10.1111/zoj.12342
- Buynevich IV. Geology and geoarchaeology of the Black Sea Region: beyond the flood hypothesis. Boulder: Geological Society of America; 2011. p. 189–196.
- Okay AI, Nikishin AM. Tectonic evolution of the southern margin of Laurasia in the Black Sea region. Int Geol Rev. 2015;57(5–8):1051–1076. doi:10.1080/00206814.2015.1010609
- Kurnaz M. Species list of amphibians and reptiles from Turkey. J Anim Diversity. 2020;2(4):10–32. doi:10.52547/JAD.2020.2.4.2
- Kurnaz M, Şahin MK. Contribution to the taxonomic knowledge of Acanthodactylus (Squamata, Lacertidae): description of a new lacertid lizard species from Eastern Anatolia, Turkey. J Wildl Biodivers. 2021;5(3):100–119.
- Yilmaz C, Ilgaz Ç, Üzüm N, et al. First record of Jan’s Cliff Racer, Platyceps rhodorachis (Jan, 1863) (Serpentes: Colubridae) in Turkey. Zool Middle East. 2021;67(1):92–94.
- Kurnaz M, Şahin MK, Eroğlu Aİ. Hidden diversity in a narrow valley: description of new endemic Palearctic Rock Lizard Darevskia (Squamata: Lacertidae) species from Northeastern Turkey. Zool Stud. 2022;61:44.
- Mittermeier RA, Turner WR, Larsen FW, et al. Global biodiversity conservation: the critical role of hotspots. In: Zachos F, Habel J, editors. Biodiversity hotspots. Berlin, Heidelberg: Springer; 2011. p. 3–22.
- Ahmadzadeh F, Flecks M, Carretero MA, et al. Rapid lizard radiation lacking niche conservatism: Ecological diversification within a complex landscape. J Biogeogr. 2013;40(9):1807–1818. doi:10.1111/jbi.12121
- Tamar K, Carranza S, in Den Bosch H, et al. Hidden relationships and genetic diversity: molecular phylogeny and phylogeography of the Levantine lizards of the genus Phoenicolacerta (Squamata: Lacertidae). Mol Phylogenet Evol. 2015;91:86–97. doi:10.1016/j.ympev.2015.05.002
- Kornilios P, Jablonski D, Sadek RA, et al. Multilocus species-delimitation in the Xerotyphlops vermicularis (Reptilia: Typhlopidae) species complex. Mol Phylogenet Evol. 2020;152:106922. doi:10.1016/j.ympev.2020.106922
- Candan K, Kornilios P, Ayaz D, et al. Cryptic genetic structure within Valentin’s Lizard, Darevskia valentini (Boettger, 1892) (Squamata, Lacertidae), with implications for systematics and origins of parthenogenesis. Syst Biodivers. 2021;19:665–681. doi:10.1080/14772000.2021.1909171
- Karakasi D, Ilgaz Ç, Kumlutaş Y, et al. More evidence of cryptic diversity in Anatololacerta species complex Arnold, Arribas and Carranza, 2007 (Squamata: Lacertidae) and re-evaluation of its current taxonomy. Amphib-Reptilia. 2021;42(2):201–216. doi:10.1163/15685381-bja10045
- Arribas O, Candan K, Kornilios P, et al. Revising the taxonomy of Darevskia valentini (Boettger, 1892) and Darevskia rudis (Bedriaga, 1886) (Squamata, Lacertidae): a morpho-phylogenetic integrated study in a complex Anatolian scenario. Zootaxa. 2022;5224:1–68. doi:10.11646/zootaxa.5224.1.1
- Fattahi R, Ficetola GF, Rastegar-Pouyani N, et al. Modelling the potential distribution of the Bridled Skink, Trachylepis vittata (Olivier, 1804), in the Middle East. Zool Middle East. 2014;60:208–216. doi:10.1080/09397140.2014.944428
- Gül S, Kumlutaş Y, Ilgaz Ç. Predicted distribution patterns of Pelias kaznakovi (Nikolsky, 1909) in the Caucasus hotspot with a new locality record from Turkey. Russ J Herpetol. 2016;23:224–230.
- Kurnaz M, Eroğlu Aİ, Koç H, et al. The potential distribution and morphological data of Podarcis siculus (Rafinesque-Schmalstz, 1810) with new locality records from Turkey (Squamata: Sauria: Lacertidae). Russ J Herpetol. 2019;26(2):77–86. doi:10.30906/1026-2296-2019-26-2-77-86
- Kurnaz M, Şahin MK. A contribution to the biogeography and taxonomy of two Anatolian mountain brook newts, Neurergusbarani and N. strauchii (Amphibia: Salamandridae) using ecological niche modeling. Turk J Zool. 2021b;45(1):54–64. doi:10.3906/zoo-2007-37
- Gül S, Kumlutaş Y, Ilgaz Ç, et al. Climatic envelopes of the genus Lacerta Linnaeus, 1758 in Türkiye: an application of ecological niche modeling. Environ Sci Pollut Res. 2023;30(19):1–16.
- Bossard M, Feranec J, Otahel J. CORINE land cover technical guide: Addendum 2000. Vol. 40. Copenhagen: European Environment Agency; 2000.
- EEA. Corine land cover (CLC) 2018, Version 20b2. Release Date: 21-12-2018. European Environment Agency. Available from: https://land.copernicus.eu/pan-european/corine-land-cover/clc2018
- Andren C, Nilson G. Observations on the herpetofauna of Turkey in 1968–1973. Br J Herpetol. 1976;5:575–584.
- Arikan H, Atatür MK, Çevik İE, et al. A serological investigation of Lacerta viridis (Laurenti, 1768) (Sauria: Lacertidae) populations in Turkey. Turk J Zool. 1999;23(3):227–230.
- Bird C. XXXIII. – The distribution of reptiles and amphibians in Asiatic Turkey, with notes on a collection from the Vilayets of Adana, Gaziantep, and Malatya. Ann Mag Nat Hist. 1936;18(104):257–281. doi:10.1080/00222933608655191
- Bodenheimer FS. Introduction into the knowledge of the amphibia and Reptilia of Turkey. İstanbul Üniversitesi Fen Fakültesi Mecmuası: Revue de la Faculté Des Sciences de L'Université D'Istanbul. Série B, Sciences Naturelles. Tabii ilimler. Seri B. 1944;9:1–78.
- Bülbül U, Kurnaz M, Eroğlu Aİ, et al. New locality record of Podarcis tauricus tauricus (Pallas, 1814) (Squamata: Lacertidae) from the western Black Sea region of Turkey. Turk J Zool. 2015;39(5):981–986. doi:10.3906/zoo-1411-25
- Clark RJ. Notes on a third collection of reptiles made in Turkey. Br J Herpetol. 1972;4:258–262.
- Clark RJ, Clark ED. Report on a collection of amphibians and reptiles from Turkey. Calif Acad Sci. 1973;104:1–62.
- Çakmak M, Akman B, Yıldız MZ. Herpetofauna of Bartın Province (Northwest Blacksea Region, Turkey). South West J Hortic Biol Environ. 2017;8(2):89–102.
- Çevik İE, Kumlutaş Y. Taxonomical states of Lacerta viridis (Lacertidae) populations from Turkey. Turk J Zool. 1999;23(5):37–46.
- Mulder J. Herpetological observations in Turkey (1987-1995). Deinsea. 1995;2(1):51–66.
- Kumlutaş Y. Karadeniz Bölgesi Lacerta viridis (Sauria: Lacertidae) populasyonları üzerinde taksonomik araştırmalar. Turk J Zool. 1996;20:223–247. Turkish.
- Hür H, Uğurtaş İH, İşbilir A. The amphibian and reptile species of Kazdağı National Park. Turk J Zool. 2008;32(3):359–362.
- Ilgaz Ç, Kumlutaş Y. The amphibian and reptile species of Igneada (Kirklareli) and its Vicinity. Pak J Biol Sci. 2005;8:558–560. doi:10.3923/pjbs.2005.558.560
- Jablonski D, Stloukal E. Supplementary amphibian and reptilian records from European Turkey. Herpetozoa. 2012;25(1–2):59–65.
- Mertens R. Amphibien und Reptilien aus der Türkei. İstanbul Üniversitesi Fen Fakültesi Mecmuası. 1952;18:41–75.
- Kumlutas Y, Ilgaz Ç, Yakar O. Herpetofauna of Karabük Province. Acta Biologica Turcica. 2017;30(4):102–107.
- Schmidtler JF. Orientalische Smaragdeidechsen. II: Über Systematik und Synökologie von Lacerta trilineata, L. media und L. pamphylica (Sauria: Lacertidae). Salamandra (Frankfurt am Main). 1986;22(2–3):126–146.
- Sahın MK, Afsar M. Evaluation of the reptilian fauna in Amasya province, Turkey with new locality records. Gazi Univ J Sci. 2018;31(4):1007–1020.
- Teynié A. Observations herpétologiques en Turquie. I. Bull Soc Herpétol France. 1987;43:9–18.
- Teynié A. Observations herpétologiques en Turquie. 2éme partie. Bull Soc Herpétol France. 1991;58:21–30.
- Tok CV, Çiçek K. Amphibians and reptiles in the Province of Canakkale (Marmara Region, Turkey) (Amphibia; Reptilia). Herpetozoa. 2014;27(1-2):65–76.
- Baran İ, Kumlutaş Y, Ilgaz Ç. On the Amphibians and Reptiles of İzmit-Bolu region: results of field survey. Anadolu Univ J Sci Technol. 2001;2(1):57–62.
- Kumlutaş Y, Tok CV, Türkozan O. The herpetofauna of the Ordu-Giresun region. Turk J Zool. 1998;22(3):199–202.
- Fick SE, Hijmans RJ. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int J Climatol. 2017;37:4302–4315. doi:10.1002/joc.5086
- Copernicus. n.d. [cited March 14, 2023]. Available from: https://cds.climate.copernicus.eu/#!/home
- Chapman AD. Principles and methods of data cleaning. Copenhagen: GBIF; 2005.
- Brown JL. SDMtoolbox: a python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods Ecol Evol. 2014;5:694–700. doi:10.1111/2041-210X.12200
- Cobos ME, Peterson AT, Barve N, et al. kuenm: an R package for detailed development of ecological niche models using Maxent. PeerJ. 2019;7:e6281. doi:10.7717/peerj.6281
- Elith J, Phillips SJ, Hastie T, et al. A statistical explanation of MaxEnt for ecologists. Divers Distrib. 2011;17:43–57. doi:10.1111/j.1472-4642.2010.00725.x
- Hurvich CM, Tsai CL. Regression and time series model selection in small samples. Biometrika. 1989;76(2):297–307. doi:10.1093/biomet/76.2.297
- Phillips SJ, Dudík M, Schapire RE. Maxent software for modeling species niches and distributions. Version 3.4. 1. New York: Biodiversity Informatics; 2017.
- Manel S, Williams HC, Ormerod SJ. Evaluating presence–absence models in ecology: the need to account for prevalence. J Appl Ecol. 2001;38:921–931. doi:10.1046/j.1365-2664.2001.00647.x
- Liu C, White M, Newell G. Selecting thresholds for the prediction of species occurrence with presence-only data. J Biogeogr 2013;40:778–789. doi:10.1111/jbi.12058
- Lütkenhöner B, Hoke M, Pantev C. Possibilities and limitations of weighted averaging. Biol Cybern. 1985;52(6):409–416. doi:10.1007/BF00449599
- Foss AH. Clustering methods for mixed-type data. Buffalo: State University of New York; 2017.
- Andreopoulos B. Clustering categorical data. In: Aggarwal CC, Reddy CK, editors. Data clustering. Palm Beach Country, Florida: Chapman and Hall/CRC; 2018. p. 277–304.
- Gavashelishvili A, Tarkhnishvili D. Biomes and human distribution during the last ice age. Glob Ecol Biogeogr. 2016;25:563–574. doi:10.1111/geb.12437
- French Republic Library. n.d. [cited July 4, 2023]. Available from: https://geo.data.gouv.fr/en/datasets/5272890d5e34c7e22c0fc5dcadffa0aad954e6c7
- Worldclim. n.d. [cited 24 Jan 2023]. Available from: https://www.worldclim.org/
- Evans D. The EUNIS habitats classification - past, present and future. Rev Investig Mar. 2012;19:28–29.
- Peterson AT, Soberón J, Pearson RG, et al. Ecological niches and geographic distributions (MPB-49). Princeton, New Jersey: Princeton University Press; 2011.
- Rissler LJ, Apodaca JJ. Adding more ecology into species delimitation: ecological niche models and phylogeography help define cryptic species in the black salamander (Aneides flavipunctatus). Syst Biol. 2007;56(6):924–942. doi:10.1080/10635150701703063
- Gaceu O, Josan I. Note on the occurrence of Darevskia pontica (Reptilia) north of the Mureş River, in Metaliferi Mountains, western Romania. North West J Zool. 2013;9:450–452.
- Shumilovskikh LS, Arz HW, Wegwerth A, et al. Vegetation and environmental changes in Northern Anatolia between 134 and 119 ka recorded in Black Sea sediments. Quat Res. 2013;80(3):349–360. doi:10.1016/j.yqres.2013.07.005
- Anderson RC, Loucks OL, Swain AM. Herbaceous response to canopy cover, light intensity, and throughfall precipitation in coniferous forests. Ecology. 1969;50:255–263. doi:10.2307/1934853
- Akkemik Ü, Dağdeviren N, Aras A. A preliminary reconstruction (A.D. 1635–2000) of spring precipitation using oak tree rings in the western Black Sea region of Turkey. Int J Biometeorol. 2005;49:297–302. doi:10.1007/s00484-004-0249-8
- Sabo JL, Power ME. River–watershed exchange: effects of riverine subsidies on riparian lizards and their terrestrial prey. Ecology. 2002;83(7):1860–1869.
- Şahin MK, Candan K, Karakasi D, et al. Ecological niche differentiation in the Anatolian rock lizards (Genus: Anatololacerta) (Reptilia: Lacertidae) of the Anatolian Peninsula and Aegean Islands. Acta Herpetol. 2022;17(2):165–175. doi:10.36253/a_h-13089
- Şekercioğlu ÇH, Anderson S, Akçay E, et al. Turkey’s globally important biodiversity in crisis. Biol Conserv. 2011;144(12):2752–2769. doi:10.1016/j.biocon.2011.06.025
- Prieto-Ramirez AM, Röhler L, Cord AF, et al. Differential effects of habitat loss on occupancy patterns of the eastern green lizard Lacerta viridis at the core and periphery of its distribution range. PLoS One. 2020;15(3):e0229600.
- Prieto-Ramirez AM, Pe’er G, Rödder D, et al. Realized niche and microhabitat selection of the eastern green lizard (Lacerta viridis) at the core and periphery of its distribution range. Ecol Evol. 2018;8(22):11322–11336. doi:10.1002/ece3.4612
- Martínez-López J, Teixeira H, Morgado M, et al. Participatory coastal management through elicitation of ecosystem service preferences and modelling driven by “coastal squeeze”. Sci Total Environ. 2019;652:1113–1128. doi:10.1016/j.scitotenv.2018.10.309
- Freitas LM, Paranaíba J, Peréz APS, et al. Toxicity of pesticides in lizards. Hum Exp Toxicol. 2020;39:596–604. doi:10.1177/0960327119899980
- Ryberg WA, Hill MT, Painter CW, et al. Landscape pattern determines neighborhood size and structure within a lizard population. PLoS One. 2013;8(2):e56856. doi:10.1371/journal.pone.0056856