ABSTRACT
To address climate change and promote sustainable development in the building materials industry, this study aimed to develop eco-friendly geopolymer products to replace the high carbon footprint cement industry. The study evaluated the production of geopolymer by partially substituting slag with sewage sludge ash (SSA) and testing different alkaline activator ratios. The ratio of GGBS to SSA was 70:20% by weight, and Na2SiO3 and NaOH were the utilized activators with varying percentages by weight. The geopolymer pastes were cured by using a thermal bath with a temperature of 38°C at 100% relative humidity in a controlled humidity chamber for 3 months. The chemical composition, the mechanical strength at specific testing times, and the microstructure of the materials and the pastes have been analyzed through XRD, XRF, FTIR and SEM. The valuable results have been achieved at a ratio of (10:10%) Na2SiO3 to NaOH, with a strength of 285.6 kg/cm2 at 90 days. XRD and FTIR of the mix A1 showed the formation of amorphous phases of geopolymer gel. Also, a dense microstructure was observed in the SEM micrograph of the mix A1. Consequently, the study results demonstrated that sewage sludge ash can be reused as a value-added product through the potential use in the synthesis of geopolymer products with an optimum alkaline activator of (10:10%) sodium silicate to sodium hydroxide. SSA – geopolymer products will help in the reduction of the carbon footprint and maintain the natural resources in addition to the achievement of climate goals.
Introduction
Cement is a crucial material in infrastructure establishments, such as tall buildings, bridges, subways and dams, due to its high strength, durability and versatility. However, the increasing need for new infrastructure has led to an increased cement output which is associated with an environmental footprint. The cement output contributes to very high levels of CO2 emissions into the atmosphere, as well as the consumption of high amounts of energy and the use of raw materials extracted from quarries such as limestone and clay [Citation1]. These environmental concerns require the development of alternative materials and practices that are more sustainable and eco-friendlier [Citation2].
One ton of cement requires approximately two tons of raw materials to be produced which is accompanied by a release of 0.9 tons of CO2, 3 kg of nitrogen oxides (NOx) and 0.4 kg of PM10. Global CO2 emissions reached approximately 23 billion tons annually, 7–8% of them were resultant from OPC production only [Citation3,Citation4]. The energy requirements for its production come at significant costs. Therefore, the cement industry is one of the industries with a high carbon footprint and is accompanied by negative environmental impacts such as greenhouse gas emissions, in addition to the depletion of natural resources, causing potential environmental risks [Citation5]. Accordingly, many countries in the world have begun to develop plans to reduce the carbon footprint, such as the International Energy Organization, which aims to reduce carbon dioxide emitted from the cement industry to 24% by the year 2100, and the European Union initiative, which hopes that Europe will become carbon neutral in the year 2050. In line with facing climate changes [Citation4].
To address these concerns, there is a growing need for the development of cementitious products with reduced environmental impacts and increased economic benefits [Citation6,Citation7].
Geopolymers have shown great potential as a new alternative to cement in building materials, as some have shown high strength, durability and superior high-temperature properties compared to concrete [Citation8]. Geopolymer is a novel type of building matter which was produced by the reaction between a material containing Si and Al and an alkaline solution. It is preferred as a building material in construction due to its high compressive strength characteristics and robustness, performing the same role as ordinary Portland cement (OPC) [Citation9].
Geopolymers have two main components: material and alkaline solution. Raw materials for aluminum silicate-based geopolymers must have high concentrations of silicon and aluminum and may contain natural minerals such as kaolinite and clay or some solid wastes such as fly ash, silica dust, slag and other ashes. The solubilization of sodium or potassium produces the required alkaline solution [Citation10].
Geopolymerization is a process of synthesizing minerals through a chemical reaction. It involves exposing aluminosilicate materials to an alkaline environment. This leads to the production of geopolymers, which are marked by a two- to three-dimensional Silica–O–Alumina composition. Geopolymer is a unique matter in that they offer ceramic and zeolite properties that are not typically found in traditional cement materials.
The final geopolymer produced is an amorphous, semi-crystalline material that possesses cementitious properties. The material contains both amorphous and crystalline phases, with the amorphous phase consisting of a network of Si–O–Al bonds and the crystalline phase consisting of various aluminosilicate species, which can include zeolites. The specific characteristics of the produced geopolymer rely on the composition of the starting components, the activating solution used and the curing conditions [Citation6].
SSA is a byproduct formed due to the firing of sewage sludge (SS), which is characterized by its high content of silicon oxide, aluminum oxide, ferric oxide and quicklime representing the most essential components for geopolymerization [Citation8]. Sewage sludge quantities increase dramatically over time because of the growing population and new regulations established for the improvement of the wastewater quality. It has been found that SSA can be represented as an appropriate material for geopolymer concrete because of its high reactivity and also its pozzolanic properties [Citation11]. The SS production is approximately 1,700,000 tons annually, with a dramatic increase. The behavior of the generated sludge varies depending on the composition and organic loads of the raw wastewater and the treatment technology used [Citation12].
Also, any variation in the operation of the treatment method applied will directly affect the quality of the produced primary or secondary sludge. The produced sludge has variable contents from organic and inorganic substances such as heavy metals, organic contaminants and pathogens which are very complex and difficult to be removed. Beneficial ingredients in sludge will be reused after the relevant steps of treatment [Citation13].
The use of SSA has several advantages, including reduction of greenhouse gas emissions and carbon footprint at wastewater treatment plants [Citation14]. SSA was utilized as a filler in the construction sector [Citation15,Citation16], mortar [Citation17–19] blocks [Citation20] and bricks [Citation21].
The SSA utilization in geopolymer concrete not only reduces waste disposal problems but also provides a more sustainable and environmentally friendly alternative to traditional building materials. Furthermore, the utilization of SSA in geopolymerization will decrease the use of fossil energy in the manufacture of construction materials [Citation2].
The most commonly used aluminosilicate sources for geopolymer production are slag, fly ash and metakaolin. Many researchers mainly prefer the utilization of GGBS in geopolymerization reactions for its great mechanical efficiency on concrete, availability and high pozzolanic activity [Citation22–24]. The evaluation of the environmental impacts of geopolymers with other precursors such as SSA besides common precursors such as GGBS leads to the development of geopolymers with a better environmental profile. The preparation of geopolymer via using these industrial wastes will relieve disposal fees and guide the cost of building materials production [Citation25].
This study aims to use supplementary cementing materials such as sewage sludge ash (SSA) and slag instead of cement to produce green eco-friendly geopolymer composites using different percentages of alkali activators for environmental development, enhancing and converting materials to be smart, more effective and sustainable. It mainly aims to evaluate the effect of alkaline activator concentration on the properties and microstructure of geopolymer specimens. The fulfillment of these goals will help in reducing the carbon footprint, achieving plans to confront climate change and protecting the natural resources used in the cement industry from depletion.
Experimental
Materials
SS was brought from the Abu Rawash wastewater plant (ARWWTP) which lies in Kerdasa city, Giza governorate, Egypt. Sewage sludge was incinerated at 800°C for 2 hours producing Sewage Sludge Ash (SSA) which mainly was composed of SiO2, CaO, Fe2O3 and Al2O3 of 34.02%, 20.02%, 20.3% and 8.78%, respectively. Slag was brought from the Iron and Steel factory, in Helwan, Egypt. It contains a high amount of barium oxide, magnesium oxide, and manganese oxide. Quicklime, silicon oxide and aluminum oxide of 40.1%, 33.08% and 9.96%, respectively. shows the oxide structure of SSA and GGBS.
Table 1. Chemical oxide composition for SSA and GGBS.
Techniques
The chemical oxide composition of the used specimens was measured by X-ray fluorescence (XRF) by using the PANalytical model, the Netherlands. The used method was Rh-kα (rubidium) radiation tube at 50 Kv and 50 mA. The XRF was conducted according to ASTM D8064-16 [Citation26].
The XRD technique was used to determine the mineralogical composition of the different samples, by using XRD equipment of model (PANalytical) diffractometer. A continuous collection of data was done at the 2θ range from 5–50º at a scanning speed of 2o/min.
Compressive strength is the amount of load per unit area that a cube can withstand before breaking. The strength of each specimen was done using the American Standard Test Methods ASTM C109-13 [Citation27].
FTIR of alkali-activated specimens was analyzed using Jasco 6100 FTIR spectrometer. The range of wave numbers was from 400 to 4,000 cm−1. FTIR was tested according to ASTM E1252-98 [Citation28].
The morphology and microstructure of geopolymer specimens were studied, by SEM technique. The specimens were dried at 60°C until a constant weight has been obtained. The samples were then examined using SEM Inspect S of the model (FEI Company, Holland). The SEM technique was conducted according to ASTM E986-04 [Citation29].
Geopolymer preparation
The geopolymer mixes were prepared from different ground ingredients with different activators. The activator solutions were composed of NaOH and Na2SiO3 which were added to the binding materials in different ratios 10:10, 5:10, 10:5 and 5:5, respectively, to examine the highest efficiency of the produced geopolymers with calcium hydroxide in a constant percent of 10%. The ratio of GGBS to SSA remained constant (70%:20%) at all mixes. Mixes were cured by using a thermal bath (38°C and 100% humidity). Finally, the strength was measured at specific testing times of 0, 7, 28 and 90 days [Citation30].
The resulting parts were treated to stop the hydration process [Citation31], then preserved until the date of testing. The studied mixes design is shown in .
Table 2. The studied mixes composition containing GGBS and SSA materials.
Results and discussion
X-ray diffraction analysis (XRD)
The XRD results of mixes A1, A2, A3 and A4 of GGBS and SSA geopolymer specimens’ alkali activated by different alkaline activator percentages cured at 90 days are shown in
Figure 1. The X-ray diffraction patterns of slag and SSA materials and geopolymer specimens. (Q: Quartz, C–S–H: CaH2O4Si, N: Anhydrite, AK: Akermanite, A: Albite, H: Hematite and Z: Zeolite)
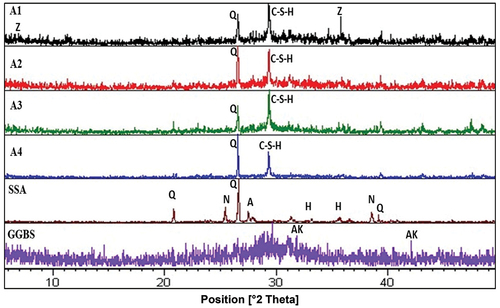
shows the mineralogy of raw materials of SSA and GGBS. The raw SSA pattern showed a slight deviation in the peak baseline from 20° to 40° (2Ө), relating to the transformation of SSA from a crystalline phase to an amorphous phase due to burning it up to 800°C. The observed crystalline phases make it hard to identify the amorphous phases in SSA. The crystalline peaks of SSA mainly indicated the presence of quartz (SiO2), anhydrite (CaSO4) and albite (NaAlSi3O8). Hematite (Fe2O3) and Rutile (TiO2) were also found.
Whereas, raw GGBS exhibited almost a broad amorphous hump with a minor amount of akermanite phase (Ca2MgSi2O7). Regarding the XRD of raw materials, the crystalline peaks were attributed to SSA. The XRD of geopolymer specimens showed broad diffuse scattering humps at 21° to 39° (2Ө) observed at all the geopolymer specimens. These humps indicated the amorphous geopolymer gels formation.
Compared with the precursors, the XRD of the geopolymer pastes showed a decrease in the intensities of quartz peaks which indicated that they have been used in the geopolymerization synthesis.
The hematite peaks which were found in raw materials GGBS and SSA (at angles (2Ө) of 33, 35 and 41) become not found in XRD patterns of all geopolymer pastes. The peaks which belong to anhydrite were not observed in the geopolymer pastes which indicates the dissolving of it. The observed bands around 2Ө degrees of 24°, 29°, 38°, 44° and 49° in all alkali-activated mixes were related to the production of C–S–H gel due to the alkaline activation of GGBS [Citation32].
Also, Ca–O bond is weaker than the Al–O and Si–O bonds so additional calcium was dissolved and then reacted with the present Si to form C–S–H with denser bonds and faster precipitation of geopolymer products [Citation2,Citation33]. Calcite (CaCO3) crystalline phases were detected in geopolymer pastes, while other crystalline phases such as pirssonite, a sodium carbonate compound, were detected as a result of the interaction of NaOH with CO2 in the atmosphere in the presence of Ca [Citation32].
The zeolite phases were formed in A1 and A4 geopolymer paste at angles (2Ө) of 12, 18, 23 and 36). The formation of zeolite phases is preferable due to the reduction of the calcium: sodium ratio where the formation of zeolites during the activation of GGBS leads to a higher mechanical strength for the produced geopolymer [Citation32].
(A1) geopolymer paste which formed with 20% SSA, 70% GGBS and sodium hydroxide:sodium silicate (10:10) showed the lowest intensities of peaks contributing to an increase in amorphous phase so it has the greatest amounts of geopolymer gel. On the other hand, the lower alkaline activator percentages led to a decrease in the glassy phase of the geopolymer component for A2, A3 and A4 mixes, respectively.
Fourier transform infrared spectroscopy (FTIR)
shows the IR of SSA, GGBS, and hardened geopolymer specimens mixed with different alkaline activator percentages.
Figure 2. FTIR spectra of GGBS and SSA raw materials and geopolymer specimens (cured at 90 days) with different percentages of alkaline activator [1: Stretching vibration of O–H bond, 2: Bending vibration of H–O–H, 3: Stretching vibration of CO2, 4: Asymmetric stretching vibration of (T–O–Si), 5: Symmetric stretching vibration of (Si–O–Si), 6: Stretching vibration of Carbonate 7: stretching vibration of (Si–O), 8: stretching vibration of (Fe–O), 9: stretching vibration of [(N, C)–A–S–H], 10: stretching vibration of (C–S–H) and 11: Zeolite formation].
![Figure 2. FTIR spectra of GGBS and SSA raw materials and geopolymer specimens (cured at 90 days) with different percentages of alkaline activator [1: Stretching vibration of O–H bond, 2: Bending vibration of H–O–H, 3: Stretching vibration of CO2, 4: Asymmetric stretching vibration of (T–O–Si), 5: Symmetric stretching vibration of (Si–O–Si), 6: Stretching vibration of Carbonate 7: stretching vibration of (Si–O), 8: stretching vibration of (Fe–O), 9: stretching vibration of [(N, C)–A–S–H], 10: stretching vibration of (C–S–H) and 11: Zeolite formation].](/cms/asset/97add3eb-e4c3-4c76-a384-95793889c51b/thbr_a_2252231_f0002_b.gif)
FTIR spectrum of GGBS and SSA showed a beak between 3700 and 3200 cm−1 contributed to the stretching vibration of O–H and another band at 1625, 1630 cm−1 attributed to stretching bending of O–H. For GGBS, a stretching vibration of O–C–O was found at 1432 cm−1. The band 971 cm−1 related to stretching vibrations of T–O–Si bonds where T = (Al, Si) and a stretching bending of Al–O–Si and Si–O–Si bonds is observed at 503 cm−1. SSA spectra showed a stretching vibration of CO3−2 at 1440 cm−1. Also, the Si–O bond appeared at 1127 cm−1. The peak at 460 cm−1 is corresponding to Fe–O stretching vibrations of hematite. FTIR for geopolymer pastes showed a shift of bands to reduce energy which had an indication of the formation of a new phase of C–A–S–H gel [Citation32,Citation34].
The peak formed at 1420 cm−1 is related to calcite formation. The band at 977 cm−1 related to hydrated products (N, C)–A–S–H gel for mixes A1, A2, A3 and A4. C–S–H which was formed at 866 cm−1, agreement with XRD patterns. The replacement of Na by Ca in the (N, C)–A–S–H gels did not change the bands but changed their shape giving an indication of the formation of new phases of C–S–H gels [Citation32]. Secondary products appeared at 700, 630 cm−1 related to zeolite formation which matched with XRD results.
Scanning electron microscopy (SEM)
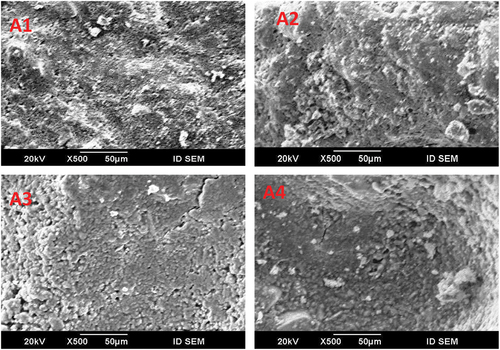
The SEM of the hardened GGBS-SSA geopolymer specimens was activated by using different percentages of NaOH + Na2SiO3 solution for 90 days are shown in .
Geopolymer pastes A1 and A4 are shown in . It showed that GGBS and SSA are affected by alkaline activators and geopolymer gels are formed. A typical compact microstructure was obtained at A1 and C–S–H gel appeared, indicating the geopolymerization process due to the presence of calcium in GGBS [Citation35]. A4 showed unreacted particles of GGBS and SSA and some crystalline phases were observed. The SEM micrographs were matching with XRD and strength values which indicated the highest strength of (A1) paste under the effect of higher alkaline activator percentages where larger SSA particles transformed into geopolymer gels more than A4 geopolymer paste.
Compressive strength
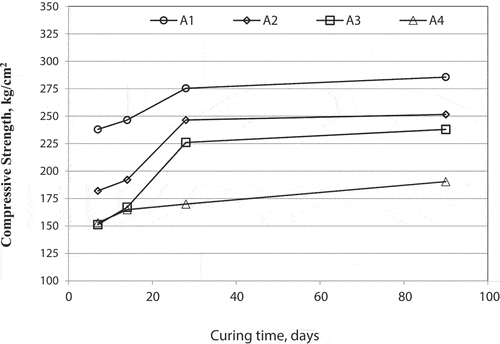
The strength of the geopolymer specimens with different alkaline activator percentages cured at 100% relative humidity and 38°C up to 90 days are shown in .
shows that the decrease of alkaline activator percentage lead to a decrease of the compressive strength in mix A4 because of low alkali activator percentage generates low-dissolved ions which hinder the geopolymerization then increased again when the sodium hydroxide percent increase in mix A3. The mix A3 has more dissolved ions produced which achieves the process. The increase of Na2SiO3 percentage more than the NaOH percentage of the alkaline activator that used in mix A2 let the reaction activated with Ca led to the production of C–S–H gel which is also observed in the XRD and FTIR results. This increase prevents the removal of air bubbles and water which affect inversely the compressive strength. Regarding mix A1, its compressive strength was 285.6 kg/cm2 which was achieved by adjusting the ratio of Na2SiO3 to NaOH at 1 [Citation36,Citation37]. A1 mix showed the best compressive strength value at the optimum alkaline activator percentages of 10%:10%. The strength is strongly affected by the amount of alkaline activator and also affected by an increase in curing time. The molarity increase has a major role in increasing strength leading to good dissolution of Si and Al from raw materials sources [Citation38].
Depending on the above, many potential environmental benefits will be achieved through the use of SSA in geopolymer production, such as the reduction in carbon dioxide emissions which is measured using a life cycle assessment tool (LCA). LCA found that the replacement of ordinary Portland cement with supplementary cementitious materials as GGBS reduces emission footprints by 15%–88% [Citation4]. Also, 73% of the energy used in the concrete industry was reduced when other supplementary materials were used [Citation39].
Conclusion
Relying on the study results, it was concluded that SSA is converted to be an added-value product material through a geopolymerization reaction. The highest compressive strength of 285.6 kg/cm2 was obtained at 90 days in mix A1 with an optimum alkaline activator ratio of 10%:10% for Na2SiO3 to NaOH. The main products that appeared in mix A1 after geopolymerization were C–S–H and C–A–S–H gels with a dense and compact microstructure that appeared in mix A1 SEM micrograph. The valuable characteristics of geopolymer pastes produced make them preferable to be utilized in building materials applications as a load-bearing building material. SSA recommended being reused in geopolymer synthesis as a green building material to overcome environmental, economic and social impacts, more consistent resource availability, lowering carbon footprint and less energy consumption.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Granados NB, Restrepo JC, Tobón JI. Alternative cement production methods would reduce its environmental footprint. Material Sci & Eng. 2021;5(4):100–101. doi: 10.15406/mseij.2021.05.00164
- Lagrani S, Aziz A, Bellil A, et al. Synthesis and characterization of slag-sludge-based eco-friendly materials–industrial implications. J Ecol Eng. 2023;24(1):227–238. doi: 10.12911/22998993/156078
- Rajamane NP, Nataraja MC, Lakshmanan N. An introduction to geopolymer concrete. Indian Concr J. 2011;85(11):25–28.
- Georgiades M, Shah IH, Steubing B, et al. Prospective life cycle assessment of European cement production. ResouConserv Recycl. 2023;194:106998. doi: 10.1016/j.resconrec.2023.106998
- Bhagat GV, Savoikar PP (2021, November). Auditing carbon reduction potential of green concrete using life cycle assessment methodology. IOP Conference Series: Earth and Environmental Science (Vol. 850, No. 1, p. 012002). IOP Publishing.
- Petermann JC, Saeed A, Hammons MI. Alkali-activated geopolymers: a literature review. Air Force Res. Lab. Mater and Manuf. Directorate Airbase Tech. Div. 2010.
- El-Sockary MA, Hasballah AF, El-Batrawy OA, et al. Construction rubble, foundry sand and marble waste powder; as raw materials on concrete bricks production. Sci J Damietta Faculty Sci. 2018;8(1):76–83. doi: 10.21608/sjdfs.2018.194810
- Chen Z, Poon CS, Li JS, et al. Design optimization and characterization of a green product by combined geopolymerization of sewage sludge ash with metakaolin. Appl Clay Sci. 2021;214:106271. doi: 10.1016/j.clay.2021.106271
- Istuque DB, Reig L, Moraes JCB, et al. Behavior of metakaolin-based geopolymers incorporating sewage sludge ash (SSA). Mater Lett. 2016;180:192–195. doi: 10.1016/j.matlet.2016.05.137
- Paulnath RCM, Aski AMM, Hamsath F, et al. Suitability of Incinerated sewage sludge ash to produce geopolymer concrete. 2016.
- Tarpani RRZ, Alfonsin C, Hospido A, et al. Life cycle environmental impacts of sewage sludge treatment methods for resource recovery considering ecotoxicity of heavy metals and pharmaceutical and personal care products. J Environ Manage. 2020;260:109643. doi: 10.1016/j.jenvman.2019.109643
- Istuque D, Soriano L, Borrachero MV, et al. Evaluation of the long-term compressive strength development of the sewage sludge ash/metakaolin-based geopolymer. Materiales de Construcción. 2021;71(343):e254–e254. doi: 10.3989/mc.2021.13220
- Erik A, Vedran M 2004. Dewatering of sludge, Lund University, P.O. Box 124, SE-22100, Lund, Sweden, 1–3.
- Wahaab RA, Mahmoud M, van Lier JB. Toward achieving sustainable management of municipal wastewater sludge in Egypt: the current status and future prospective. Renew Sust Energ Rev. 2020;127:109880. doi: 10.1016/j.rser.2020.109880
- Cyr M, Coutand M, Clastres P. Technological and environmental behavior of sewage sludge ash (SSA) in cement-based materials. Cement Concr Res. 2007;37(8):1278–1289. doi: 10.1016/j.cemconres.2007.04.003
- Garcés P, Carrión MP, García-Alcocel E, et al. Mechanical and physical properties of cement blended with sewage sludge ash. Waste Manage. 2008;28(12):2495–2502. doi: 10.1016/j.wasman.2008.02.019
- Agrawal D, Hinge P, Waghe UP, et al. Utilization of industrial waste in construction material – a review. Int J Innov Res Technol Sci Eng Technol. 2014;3(1):8390–8397.
- Lin KL, Chang WC, Lin DF, et al. Effects of nano-SiO 2 and different ash particle sizes on sludge ash – cement mortar. J Environ Manage. 2008;88(4):708–714. doi: 10.1016/j.jenvman.2007.03.036
- Monzó J, Paya J, Borrachero MV, et al. Reuse of sewage sludge ashes (SSA) in cement mixtures: the effect of SSA on the workability of cement mortars. Waste Manage. 2003;23(4):373–381. doi: 10.1016/S0956-053X(03)00034-5
- Baeza-Brotons F, Garces P, Paya J, et al. Portland cement systems with addition of sewage sludge ash. Application in concretes for the manufacture of blocks. J Clean Prod. 2014;82:112–124. doi: 10.1016/j.jclepro.2014.06.072
- Cusidó JA, Cremades LV. Environmental effects of using clay bricks produced with sewage sludge: Leachability and toxicity studies. Waste Manage. 2012;32(6):1202–1208. doi: 10.1016/j.wasman.2011.12.024
- Mayhoub OA, Nasr ESA, Ali Y, et al. Properties of slag-based geopolymer reactive powder concrete. Ain Shams Eng J. 2021;12(1):99–105. doi: 10.1016/j.asej.2020.08.013
- Istuque DB, Payá J, Soriano L, et al. The role of dissolved rice husk ash in the development of binary blast furnace slag-sewage sludge ash alkali-activated mortars. J Buil Eng. 2022;52:104472. doi: 10.1016/j.jobe.2022.104472
- Jegan M, Annadurai R, Rajkumar PK. A state of the art on the effect of alkali activator, precursor, and fibers on properties of geopolymer composites. Case Studies Construction Mater. 2023;18:e01891. doi: 10.1016/j.cscm.2023.e01891
- Abdulkareem M, Komkova A, Havukainen J, et al. Identifying optimal precursors for geopolymer composite mix design for different regional settings: a multi-objective optimization study. Recycling. 2023;8(2):32. doi: 10.3390/recycling8020032
- ASTM D8064-16. “Standard test method for elemental analysis of soil and solid waste by monochromatic energy dispersive x-ray fluorescence spectrometry using multiple monochromatic excitation beams”. West Conshohocken, PA, USA: ASTM International; 2016.
- ASTM C109. “Standard test method for compressive strength of hydraulic cement mortars”. West Conshohocken, PA, USA: ASTM International; 2012.
- ASTM E1252-98. Standard practice for general techniques for obtaining infrared spectra for qualitative analysis. West Conshohocken, PA, USA: ASTM International; 2021.
- ASTM E986-04. Standard practice for scanning electron microscope beam size characterization. West Conshohocken, PA, USA: ASTM International; 2017.
- Hassaan MM, Khater HM, El-Mahllawy MS, et al. Production of geopolymer composites enhanced by nano-kaolin material. J Adv Ceram. 2015;4(4):245–252. doi: 10.1007/s40145-015-0156-y
- Khater HM, El-Sabbagh BA, Fanny M, et al. Effect of nano-clay on alkali activated water-cooled slag. Geopolymer British J Appl Sci Technol. 2013;3(4):764–776. doi: 10.9734/BJAST/2013/2690
- Tashima MM, Reig L, Santini MA, et al. Compressive strength and microstructure of alkali-activated blast furnace slag/sewage sludge ash (GGBS/SSA) blends cured at room temperature. Waste Biomass Valorization. 2017;8(5):1441–1451. doi: 10.1007/s12649-016-9659-1
- Chen Z, Li JS, Zhan BJ, et al. Compressive strength and microstructural properties of dry-mixed geopolymer pastes synthesized from GGBS and sewage sludge ash. Constr Build Mater. 2018;182:597–607. doi: 10.1016/j.conbuildmat.2018.06.159
- Ma Z, Dan H, Tan J, et al. Optimization design of MK-GGBS based geopolymer repairing mortar based on response surface methodology. Materials. 2023;16(5):1889. doi: 10.3390/ma16051889
- Bhina MR, Liu KY, Hu JEHY, et al. Investigation of the mechanical properties of quick-strength geopolymer material considering preheated-to-Room temperature ratio of Sand, Na2SiO3-to-NaOH ratio, and fly ash-to-GGBS ratio. Polymers. 2023;15(5):1084. doi: 10.3390/polym15051084
- Morsy MS, Alsayed SH, Al-Salloum Y, et al. Effect of sodium silicate to sodium hydroxide ratios on strength and microstructure of fly ash geopolymer binder. Arab J Sci Eng. 2014;39(6):4333–4339. doi: 10.1007/s13369-014-1093-8
- Jan A, Pu Z, Khan KA, et al. A review on the effect of silica to alumina ratio, alkaline solution to binder ratio, calcium oxide+ ferric oxide, molar concentration of sodium hydroxide and sodium silicate to sodium hydroxide ratio on the compressive strength of geopolymer concrete. Silicon. 2022;14(7):3147–3162. doi: 10.1007/s12633-021-01130-3
- Gopalakrishna B, Dinakar P. Mix design development of fly ash-GGBS based recycled aggregate geopolymer concrete. J Buil Eng. 2023;63:105551. doi: 10.1016/j.jobe.2022.105551
- Manjunath A, Patil NN (2021, July). Effect of sustainable materials on embodied energy, carbon footprint and cost for a proposed conventional apartment. In IOP Conference Series: Materials Science and Engineering (Vol. 1166, No. 1, p. 012037). IOP Publishing.